Back to Journals » OncoTargets and Therapy » Volume 10
New developments in the treatment of advanced squamous cell lung cancer: focus on afatinib
Authors Hirsh V
Received 1 October 2016
Accepted for publication 24 November 2016
Published 11 May 2017 Volume 2017:10 Pages 2513—2526
DOI https://doi.org/10.2147/OTT.S104177
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chiung-Kuei Huang
Video abstract presented by Vera Hirsh
Views: 3127
Vera Hirsh
McGill Department of Oncology, Royal Victoria Hospital, Montreal, QC, Canada
Abstract: Until recently, few treatment options existed for the treatment of squamous cell carcinoma (SqCC) of the lung, especially in the second-line setting following platinum-based chemotherapy. Accordingly, outcomes in this subtype of non-small-cell lung cancer (NSCLC) were generally poor. In this context, the recent availability of the checkpoint inhibitors nivolumab and pembrolizumab, the anti-VEGFR2 antibody ramucirumab (combined with docetaxel), and the ErbB-family blocker afatinib for the treatment of relapsed/refractory SqCC of the lung represent major advances. However, the rapid expansion of the treatment armamentarium invites many questions regarding optimal treatment choice and sequence in individual patients. This review focuses on the biologic rationale and clinical evidence to support the use of afatinib in this treatment setting, highlighting the prominent role of the ErbB-signaling cascade in SqCC tumors. The seminal Phase III LUX-Lung 8 study, on which the approval of afatinib is based, is discussed and contextualized with the emergence of immunotherapies. Finally, criteria are explored that might drive physicians’ treatment decisions when considering the use of afatinib based on individual patient characteristics. Other ongoing developments in the treatment of SqCC of the lung that will lead to further options and welcome improvements in the management of this difficult-to-treat disease are summarized.
Keywords: squamous cell carcinoma of the lung, afatinib, LUX-Lung 8, EGFR, ErbB
Introduction
Non-small-cell lung cancer (NSCLC) is a common malignancy that traditionally had a poor prognosis, due to disease diagnosis at an advanced stage (when locoregional therapies are unfeasible) and a lack of effective systemic treatments.1 However, recent advances in the treatment of NSCLC have served as part of a paradigm of “personalized” medicine in oncology, at least in a subset of patients with molecular drivers; examples include mutations in the EGFR gene and rearrangements of the ALK gene.2 In patients with EGFR mutations, first and later lines of treatment can be tailored to target the underlying biology and molecular evolution of the tumor, in terms of both the overall EGFR-mutation status and specific EGFR-resistance mutations, such as T790M, acquired during progression on EGFR tyrosine-kinase inhibitors (TKIs). Currently, first- (gefitinib, erlotinib), second- (afatinib), and third- (osimertinib) generation EGFR-targeted agents are well established in NSCLC-treatment algorithms, based on their favorable efficacy and toxicity profiles; therefore, many patients never receive cytotoxic drugs, and thus avoid their associated toxicity burden.3,4
Unfortunately, progress in the treatment of squamous cell carcinoma (SqCC), which accounts for 20%–30% of cases of NSCLC,5 lags behind that in EGFR mutation-positive NSCLC (usually adenocarcinoma) because less is known about its underlying molecular pathogenesis. While progress is being made with regard to the identification of potential oncogenic drivers of SqCC of the lung (eg, FGFR1 amplification, PI3K abnormalities, DDR mutations),6 none has led to the development of approved therapies to date. Moreover, EGFR mutations and ALK aberrations are rare in SqCC of the lung.7 Nevertheless, it should be noted that SqCC of the lung is generally associated with tobacco smoking, which even within this histological subtype is associated with a lower frequency of EGFR mutations.8,9
Because of the association of SqCC with tobacco smoking, its anatomical characteristics can complicate treatment, as squamous tumors are usually located within the main airways. Consequently, patients are particularly prone to such symptoms as dyspnea, cough, obstructive pneumonia, and hemoptysis.10 The predisposition to hemoptysis in particular limits the use of antiangiogenic agents, such as bevacizumab, in this setting.10–12
Owing to these factors, first-line standard of care for SqCC of the lung usually comprises platinum-doublet chemotherapy with at-best modest outcomes. Unfortunately, despite the development of doublet regimes incorporating third-generation agents, such as gemcitabine, taxanes, or vinorelbine, chemotoxic chemotherapy for NSCLC has reached a plateau of therapeutic efficacy, with no significant difference overall in efficacy among regimens.13 According to an analysis of four different platinum-based doublets encompassing 1,155 patients with NSCLC, contemporary chemotherapy elicits a response rate of only ~20%, with a median overall survival (OS) of less than 8 months.14 Despite some recent developments (eg, albumin-based paclitaxel regimens have shown promise),15 available data indicate that outcomes in patients with SqCC following frontline chemotherapy may be even worse than those in patients with adenocarcinoma. For example, outcomes with pemetrexed-based chemotherapy regimens are particularly poor in patients with SqCC of the lung;16 therefore, pemetrexed is contraindicated in this setting. This lack of effectiveness is thought to be related to overexpression of thymidylate synthase expression in squamous tumors, which confers reduced sensitivity to pemetrexed.17,18 Second-line chemotherapy options for patients with SqCC of the lung are also extremely limited. Currently, the only chemotherapy options in this setting are docetaxel and possibly gemcitabine.19
Overall, therefore, improved treatment options for SqCC of the lung, both in first-line and relapsed/refractory settings have remained a huge unmet medical need. This has driven extensive research into novel treatment strategies, and such efforts are beginning to bear fruit. In the last few years, several new drugs have been approved for the treatment of patients with SqCC of the lung; these include anti-EGFR monoclonal antibodies (necitumumab and cetuximab) in combination with standard frontline chemotherapy. The immune-checkpoint inhibitors nivolumab and pembrolizumab, the anti-VEGFR2 antibody ramucirumab (combined with docetaxel), and the ErbB-family blocker afatinib are all approved in a second-line setting.
The focus of this review is the development of afatinib for the treatment of SqCC of the lung. The rationale and available clinical evidence that support the targeting of EGFR in this setting are summarized, and the benefits of targeting the whole ErbB-receptor family, rather than EGFR only, are discussed. The seminal LUX-Lung 8 trial, on which the approval of afatinib was based, is reviewed and contextualized with the recent emergence of immunotherapy drugs. Likely further developments in the treatment of SqCC of the lung in coming years are discussed. Finally, factors that are likely to be important when physicians consider how best to integrate afatinib into optimal treatment strategies for their patients are explored.
EGFR is a validated drug target in patients with SqCC of the lung
Although EGFR mutations are rare, SqCC tumors are often characterized by high levels of EGFR-protein expression.20 EGFR overexpression is observed in 60%–80% of SqCC tumors; moreover, some tumors (7%–10%) also demonstrate EGFR gene copy-number alterations.21 Based on these findings, several studies have assessed first-line EGFR-targeted agents in patients with SqCC of the lung, with particular focus on the anti-EGFR antibodies necitumumab and cetuximab, in combination with chemotherapy (Table 1). The Phase III SQUIRE trial assessed necitumumab, a second-generation anti-EGFR monoclonal antibody, in combination with gemcitabine/cisplatin vs chemotherapy alone in 1,093 patients with SqCC of the lung.22 Addition of necitumumab significantly improved OS vs the chemotherapy arm (median 11.5 months vs 9.9 months, hazard ratio [HR] 0.84, 95% confidence interval [CI] 0.74–0.96; P=0.01). Progression-free survival (PFS) was also significantly improved (median 5.7 months vs 5.5 months, HR 0.85, 95% CI 0.74–0.98; P=0.02). However, this improved efficacy must be considered in the context of the additional toxicity burden associated with the addition of necitumumab vs chemotherapy alone, as there was a higher rate of grade ≥3 adverse events (AEs; 72.1% vs 61.6%) and treatment discontinuations due to AEs (31.2% vs 24.6%) in the combination arm. Specific grade ≥3 AEs that were elevated including rash (7.1% vs 0.4%), hypomagnesemia (9.3% vs 1.1%), and venous thromboembolic events (5% vs 2.6%).22 Nevertheless, a recent analysis of patient-reported outcomes indicated that addition of necitumumab and its associated AEs did not negatively impact on patients’ health-related quality of life (HRQoL).23 No Phase III trials have specifically assessed cetuximab plus chemotherapy in patients with SqCC of the lung. However, subanalysis of four randomized trials undertaken in histologically unselected NSCLC patients24–27 suggested this regimen particularly benefits those with SqCC.28
Taken together, these data, with both necitumumab and cetuximab, validate EGFR as a therapeutic target in patients with SqCC of the lung, although the clinical benefit of these agents must be balanced with their toxicity and costs. Furthermore, the clinical relevance of the observed modest efficacy benefits with these drugs is open to debate. This question has focused efforts on the identification of biomarkers that could be used to identify a subgroup of patients who may particularly benefit from EGFR blockade. The obvious choice for a biomarker would seem to be the level of EGFR expression, as quantified by immunohistochemistry, although data are inconsistent. In the Phase III FLEX trial (in histologically unselected patients), high EGFR-expression levels appeared to predict superior OS with cetuximab.26 However, in a prespecified analysis of the SQUIRE trial, high levels of EGFR expression did not predict improved survival with necitumumab.22
Emerging evidence suggests that analyzing EGFR copy number using a fluorescence in situ hybridization (FISH) assay could have promise as a predictive biomarker and may be applicable to “real-world” clinical practice. In a recent subanalysis of SQUIRE, elevated copy number of EGFR detected by FISH in 37% of patients, was associated with a trend toward improved OS with necitumumab.29 Similar findings were observed in the Phase III SWOG 0819 trial that assessed cetuximab plus chemotherapy (± bevacizumab) vs chemotherapy (with or without bevacizumab) in patients with NSCLC. In patients with SqCC who had elevated EGFR copy number and were not suitable for bevacizumab, cetuximab conferred a strong OS benefit over the control arm (median OS 11.8 months vs 6.4 months; HR 0.56).30 Nevertheless, the potential for EGFR FISH as a predictive biomarker requires validation in prospective trials.
In contrast to anti-EGFR antibodies, first-line EGFR TKI monotherapy is not recommended in patients with SqCC without known EGFR mutations if they are eligible for chemotherapy, and several prospective trials have demonstrated that EGFR TKIs are inferior to chemotherapy in patients with unselected or EGFR wild-type NSCLC.31 Furthermore, first-line combinations of EGFR TKIs with chemotherapy have failed to demonstrate clinical benefit over chemotherapy alone in patients with NSCLC, with no significant difference in efficacy between histological subtypes.32–35
Despite these data, experience with EGFR TKIs in a second-line setting indicates that they have clinical applicability in some patients with SqCC, thus providing further evidence that the EGFR-signaling pathway is a bona fide therapeutic target in this setting. For example, in subanalyses of the Phase III BR.21 trial, erlotinib significantly improved OS vs placebo in patients with SqCC of the lung.36,37 In addition, although the Phase III TAILOR trial demonstrated that docetaxel was superior to erlotinib in NSCLC patients with wild-type EGFR, OS did not differ between the two treatment arms in patients with SqCC.38
EGFR TKIs have also shown promise as maintenance therapy in patients with advanced NSCLC. The Phase III SATURN trial assessed the impact of erlotinib as maintenance therapy in histologically unselected patients who responded (or had stable disease) following platinum-doublet chemotherapy. Erlotinib delayed disease progression in patients with squamous histology, and improved OS in those patients who achieved stable disease.39
Overall, these studies indicate a potential role for EGFR TKIs in patients with relapsed/refractory SqCC; however, improvements in clinical outcomes are modest at best. Indeed, in contrast with erlotinib, gefitinib has not demonstrated any benefit over placebo in this setting in either histologically unselected patients or patients with nonadenocarcinoma histology.40
ErbB-family inhibition in SqCC of the lung: rationale for afatinib
The limited efficacy benefits observed with EGFR-specific inhibitors (necitumumab, cetuximab, erlotinib) likely reflect the fact that EGFR is just one of several related receptors (within the ErbB family) that cooperate via a network of interconnected intracellular pathways to regulate cellular proliferation.41 As such, inhibition of just one branch within a complex web of degenerate pathways may be a suboptimal means of targeting squamous tumors.
In addition to EGFR, the ErbB family comprises the structurally related receptor tyrosine kinases, HER2 (Neu, ErbB2), HER3 (ErbB3; no intrinsic kinase activity), and HER4 (ErbB4). When ligands bind to these receptors (known ligands include EGF, TGFα, and neuregulin 1/2/3/4) they undergo a conformational change, leading to the formation of homo- and/or heterodimers at the cell surface. These dimers (including those that contain HER3) then undergo autophosphorylation, leading to the activation of a myriad of downstream pathways, including the PI3K–Akt pathway, the Ras–Raf–MEK–ERK1/2 pathway, and the phospholipase C pathway.41,42
Afatinib is an irreversible ErbB-family blocker that inhibits signaling from all ErbB hetero- and homodimers (Figure 1).43,44 Afatinib was originally developed with the aim of improving clinical outcomes vs first-generation EGFR inhibitors, with the hope that its broader inhibitory pathway could delay acquired resistance compared with erlotinib and gefitinib.45 Emerging clinical data indicate that this may well be the case. For example, a recent randomized Phase IIB trial (LUX-Lung 7) was undertaken to compare afatinib vs gefitinib head to head in 319 patients with EGFR mutation-positive NSCLC.46 In this trial, PFS by blinded independent assessment was significantly improved with afatinib vs gefitinib (median PFS 11 months vs 10.9 months, HR 0.73, 95% CI 0.57–0.95; P=0.017). Intriguingly, the PFS curves were nearly identical at the median but separated thereafter, such that the 2-year PFS rate was markedly higher with afatinib than gefitinib (17.6% vs 7.6%, respectively). The authors of the study attributed this observation to the broader and more durable inhibitory profile of afatinib and its potential to delay possible resistance mechanisms when compared with gefitinib. Other clinical end points, including time to treatment failure and objective response rate, also favored afatinib vs gefitinib, and efficacy benefits were largely consistent across patient subgroups, including age, race, Eastern Cooperative Oncology Group performance status, and EGFR-mutation type (del19 or L858R).46 Mature OS data have not yet been reported.
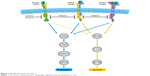  | Figure 1 Afatinib mechanism of action. |
Other trials in the EGFR mutation-positive setting also suggest that afatinib may afford efficacy benefits vs first-generation EGFR TKIs. In two Phase III studies that compared afatinib vs chemotherapy (LUX-Lung 3 and LUX-Lung 6), afatinib conferred an OS advantage vs chemotherapy in patients harboring del19 mutations (LUX-Lung 3 median OS 33.3 months vs 21.1 months, HR 0.54, 95% CI 0.36–0.79; P=0.0015; LUX-Lung 6 median OS 31.4 months vs 18.4 months, HR 0.64, 95% CI 0.44–0.94; P=0.0229).47–49 By comparison, in a recent meta-analysis, neither erlotinib nor gefitinib demonstrated a survival advantage vs chemotherapy in del19 patients.50
There is a biologic rationale for assessing afatinib in patients with SqCC of the lung. As well as EGFR, other members of the ErbB family are overexpressed in SqCC tumors, implying a role in the pathogenesis of the disease; eg, HER2 and HER3 are overexpressed in up to 20%–30% of SqCC cases.51–54 Furthermore, genomic analysis of 178 SqCC tumor samples identified genetic aberrations in HER2 (4%) and HER3 (2%), as well as in several signaling molecules within the ErbB intracellular signaling network, such as KRAS (3%), HRAS (3%), BRAF (4%), and RASA1 (4%).7 Other studies have identified HER4 mutations in SqCC tumors,55,56 and another study demonstrated that genomic aberrations in NRG1, one of the cognate ligands of the ErbB family of receptors, are a recurrent feature of squamous lung tumors.57
The results of several clinical studies have suggested that afatinib is active in patients with squamous tumors. In the Phase III LUX-Lung 5 trial, patients with NSCLC who had progressed on chemotherapy and subsequently received at least 12 weeks of erlotinib or gefitinib were treated with afatinib monotherapy (50 mg/day). Among 90 patients with SqCC, the disease-control rate (DCR) was approximately 60%, with a median PFS of 3.7 months in this heavily pretreated group of patients.58 In the Phase III LUX-Head and Neck 1 trial, afatinib monotherapy (40 mg/day) conferred superior PFS (median 2.6 months vs 1.7 months, HR 0.8, 95% CI 0.65–0.98; P=0.03) and DCR (49% vs 39%, P=0.035) vs methotrexate in patients with recurrent or metastatic (R/M) SqCC of the head and neck (HNSCC).59 Based on this trial, afatinib is recommended in NCCN Clinical Practice Guidelines In Oncology (NCCN Guidelines®) for Head and Neck Cancers Version.2.2016, as a category 2B second-line therapy option for unresectable R/M HNSCC (non-nasopharygeal).60
LUX-Lung 8: second-line afatinib vs erlotinib in patients with SqCC of the lung
Based on the aforementioned rationale, the Phase III LUX-Lung 8 trial was undertaken. This open-label randomized trial compared afatinib (40 mg/day, n=398) and erlotinib (150 mg/day, n=397) in patients with relapsed/refractory stage IIIB/IV SqCC of the lung.61 The primary end point (PFS) and key secondary end point (OS) were both reached, thus confirming the superiority of afatinib over erlotinib in this setting (Figure 2). Median PFS was 2.6 months vs 1.9 months (HR 0.81, 95% CI 0.69–0.96; P=0.01). Median OS was 7.9 months vs 6.8 months (HR 0.81, 95% CI 0.69–0.95; P=0.008). Although the difference in median survival between the two treatment arms was small (and thus of debatable clinical significance), it is noteworthy that OS curves separated more substantially with time: survival rates at 12 months (36.4% vs 28.2%, P=0.016) and 18 months (22.0% vs 14.4%, P=0.013) were both significantly better with afatinib than erlotinib. DCR was also significantly improved with afatinib vs erlotinib (50.5% vs 39.5%, P=0.002).
  | Figure 2 OS and PFS in the overall LUX-Lung 8 population (n=795). |
The improvements in survival end points observed with afatinib in LUX-Lung 8 were not attributable to imbalances in ErbB molecular aberrations across treatment arms.61 In a post hoc genomic analysis of archival tumor samples from 238 patients, only 14 patients (6%) had EGFR mutations (eight in the afatinib arm and six in the erlotinib arm). Of these mutations, ten were novel with unknown clinical significance. The frequency of EGFR copy-number amplifications was also low, being detected in only 15 patients. Overall, the cumulative frequency of ErbB aberrations (mutations and copy-number alterations) was 29%, but the presence of aberrations was neither prognostic nor predictive of improved PFS or OS with afatinib.62
The AE profile of afatinib in LUX-Lung 8 was consistent with previous studies, with no unexpected safety concerns. Overall, there was no difference in the frequency of grade ≥3 AEs between the afatinib and erlotinib arms (57.1% and 57.5% of patients, respectively). As expected, certain treatment-related grade ≥3 AEs, such as diarrhea (10.4% vs 2.6%) and stomatitis (4.1% vs 0%), were more frequent with afatinib than erlotinib. However, some AEs, such as grade 3 rash/acne, were more frequent with erlotinib vs afatinib (10.4% vs 5.9%).61
Afatinib has a well-defined dose-reduction protocol, and is available in several dose formulations. The frequency of dose reductions due to AEs in LUX-Lung 8 was higher with afatinib than erlotinib (27% vs 14%), possibly due to the high percentage of current smokers participating in the study (smoking reduces the bioavailability of erlotinib and reduces the likelihood of AEs).63 Notably, there was little difference between the two treatment arms in the frequency of treatment discontinuations due to AEs (20% vs 17%), indicating that the dose-reduction scheme is effective in the management of AEs. Of note, discontinuation due to diarrhea (the most prevalent treatment-related AE with afatinib) was necessary in 15 (4%) patients.62
Given that NSCLC symptoms, including cough, dyspnea, and pain, have a profound impact on HRQoL,64 LUX-Lung 8 also included end points to evaluate patient-report outcomes. Patients completed the multidimensional, cancer-specific European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 and its lung cancer-specific module (QLQ-LC13) at the first visit of each treatment course and at the end of treatment. Questionnaires were completed independently by the patients prior to clinical assessment to ensure that responses were a true reflection of how they felt about their condition without any influence from their treating physician. Compliance with the questionnaires was high (68.7%–99%). Significantly more patients in the afatinib arm reported improved global health status/QoL compared with the erlotinib arm (36% vs 28%, P=0.041). Furthermore, changes in mean scores for the key symptoms of dyspnea, cough, and pain all favored afatinib.61
In summary, LUX-Lung 8 demonstrated that afatinib improved PFS, OS, and DCR vs erlotinib in patients with relapsed/refractory SqCC. These efficacy benefits with afatinib were complemented by HRQoL improvements. Furthermore, afatinib was associated with a predictable and manageable AE profile, consistent with the mechanistic profile of EGFR inhibition. On the basis of LUX-Lung 8, afatinib has recently been approved by the US Food and Drug Administration (FDA) and European Medicines Agency for the treatment of locally advanced or metastatic SqCC of the lung progressing on or after platinum-based chemotherapy.
Emergence of immunotherapy for treatment of lung SqCC
In addition to the emergence of afatinib as a new treatment option, there has been remarkable recent progress in the development of immunotherapies for the treatment of SqCC of the lung. Such therapies work by stimulating T-cell responses against tumor antigens in order to potentiate the body’s own anticancer defenses. T-cells are the gatekeepers of cell-mediated immunity, and following interaction with ‘foreign’ antigens found on the surface of specialist cells (antigen-presenting cells), are primed to initiate immune responses against those cells. However, in order to prevent aberrant immune responses against ‘self’ tissues (autoimmunity), a safety mechanism exists in the form of costimulatory pathways that must also be activated to drive T-cell-mediated immune responses.65 The two key costimulatory immune pathways are the CTLA4–CD28 pathway and the PD1–PDL1 and/or PDL2 pathway.
Although these pathways have evolved to ensure that self-tissues are not attacked by the immune system, developing tumors are able to hijack these mechanisms to evade immune detection by overexpressing PDL1, CTLA4, and other “checkpoint” molecules.66,67 There is evidence that immunoescape is a particularly important feature in the development of SqCC of the lung.5 Given such observations, along with the success of immunotherapies in treating other tumor types68 and the shortage of effective treatment options in SqCC of lung, there has been a great deal of interest in the development of immunotherapies in this clinical setting.
Eight checkpoint inhibitors are currently in clinical development for NSCLC, and two agents – the PD1 inhibitors nivolumab and pembrolizumab – are approved for use in patients with advanced NSCLC. Nivolumab is approved for the treatment of both squamous and nonsquamous tumors that have progressed during or after platinum-based chemotherapy. This approval is based on results from two pivotal Phase III studies – CheckMate 017 (squamous)69 and CheckMate 057 (nonsquamous)70 – following promising data (in patients with SqCC of the lung) from a Phase II trial – CheckMate 063.71 CheckMate 017 was undertaken in 272 patients with previously treated SqCC of the lung randomized to nivolumab (3 mg/kg every 2 weeks) or docetaxel (75 mg/m2 every 3 weeks). The primary end point of OS was significantly improved with nivolumab (median 9.2 months vs 6 months, HR 0.59, 95% CI 0.44–0.79; P<0.001). Moreover, PFS (median 3.5 months vs 2.8 months, HR 0.62, 95% CI 0.47–0.81; P<0.001) and overall response rate (20% vs 9%, P=0.008) also favored nivolumab. Importantly, levels of PDL1 expression were neither prognostic nor predictive of nivolumab activity.69 Generally, nivolumab had a favorable tolerability profile, and the occurrence of treatment-related grade ≥3 AEs was only 7% compared with 55% in the docetaxel arm. The most common AEs were fatigue (16%), decreased appetite (11%), and asthenia (10%). Permanent discontinuation of nivolumab due to treatment-related AEs was required in 3.1% of patients.69
Pembrolizumab is approved for the treatment of patients with pretreated advanced NSCLC (any histology) whose tumors express PDL1, as detected by an FDA-approved test. This approval was supported by the Phase II/III KEYNOTE-010 study, in which 1,034 patients with previously treated NSCLC (with PDL1 expression on at least 1% of tumor cells) were randomized to receive pembrolizumab (2 mg/kg or 10 mg/kg) or docetaxel (75 mg/m2), every 3 weeks.72 OS was significantly improved with both pembrolizumab 2 mg/kg (median 10.4 months vs 8.5 months, HR 0.71, 95% CI 0.58–0.88; P=0.0008) and 10 mg/kg (median 12.7 months vs 8.5 months, HR 0.61, 95% CI 0.49–0.75; P<0.0001) vs docetaxel. Consistent with a previous Phase IB study,73 OS benefit with pembrolizumab was particularly striking in those patients with high levels of PDL1 expression (over 50% of tumor cells). In these patients, median OS in the pembrolizumab 10 mg/kg group was 17.3 months vs 8.2 months in the docetaxel group (HR 0.54, 95% CI 0.38–0.77; P=0.0008). Interestingly, there was no significant difference in PFS among the three treatment arms.72
Unlike nivolumab, pembrolizumab has not been tested specifically in patients with SqCC of the lung. Prespecified subanalysis of KEYNOTE-010 suggested that pembrolizumab (pooled analysis of both dose groups) benefits patients with SqCC vs docetaxel, although statistical significance was not reached (HR for OS 0.74, 95% CI 0.5–1.09).72 Pembrolizumab was well tolerated in the overall KEYNOTE-010 population. Grade ≥3 treatment-related AEs occurred in 13% and 16% of patients in the 2 mg/kg and 10 mg/kg groups, respectively; permanent discontinuation of pembrolizumab due to treatment-related AEs was required in 4.4% and 5% of patients, respectively.72
Despite the approval of checkpoint inhibitors, it should be noted that their safety in patients with underlying autoimmune conditions, such as autoimmune pneumonitis and colitis, is unclear, as patients with these disorders were excluded from pivotal studies of nivolumab and pembrolizumab in NSCLC.69,70,72 In addition, nivolumab is administered as an intravenous infusion over 60 minutes every 2 weeks. Therefore, this treatment may not be suitable for elderly patients or those with certain comorbidities, who may need to be accompanied by relatives or carers during treatment visits.
Ongoing developments in treatment of lung SqCC
As summarized, over just a few years second-line treatment options for patients with SqCC of the lung have expanded from only docetaxel (or erlotinib in some patients) to docetaxel, afatinib, nivolumab, and pembrolizumab. Furthermore, in addition to these treatments, the anti-VEGFR2 antibody ramucirumab has recently been approved in combination with docetaxel for the second-line treatment of NSCLC, including SqCC of the lung, based on the Phase III REVEL trial.74 Further developments in the treatment of SqCC of the lung (both in frontline and relapsed/refractory settings) can be expected in coming years. At present, there are three key areas of development: 1) further immunotherapeutic drugs, and potential expansion of immunotherapy into a frontline setting; 2) combination regimens incorporating immunotherapies with other immune-checkpoint inhibitors or chemotherapy; and 3) agents directed against novel drug targets. Progress in these areas is now summarized.
Immunotherapeutic drugs
Four PDL1 checkpoint inhibitors are currently in development for the treatment of NSCLC: BMS-936559, atezolizumab, durvalumab, and avelumab.65 It is hypothesized that PDL1 inhibitors may have a lower immunorelated AE burden compared with pembrolizumab and nivolumab,65 and there are emerging clinical data that support this hypothesis. In the randomized Phase II POPLAR study, atezolizumab significantly improved OS vs docetaxel in 287 patients with pretreated squamous or nonsquamous NSCLC (median OS 12.6 months vs 9.7 months, HR 0.73, 95% CI 0.53–0.99; P=0.04).75 Among 97 patients with SqCC, median OS with atezolizumab was 10.1 months vs 8.6 months with docetaxel (HR 0.8, 95% CI 0.49–1.3; P=0.04). In this study, OS was correlated with increasing levels of PDL1 expression (on both tumor cells and tumor-infiltrating immune cells). Immunorelated AEs, including pneumonitis, colitis, and hepatitis, all occurred at low frequencies (<5%). An ongoing randomized Phase III trial (vs docetaxel) will provide further insight into the role of atezolizumab in nonsquamous NSCLC (NCT02008227). Other ongoing randomized trials are assessing the role of PDL1 inhibitors in the second-line setting in patients with PDL1-positive disease. For example, the Phase III JAVELIN Lung 200 trial is evaluating avelumab vs docetaxel in patients with PDL1-positive NSCLC (squamous or nonsquamous) after failure of a platinum-based doublet (NCT02395172).
In addition to PDL1 inhibitors, the anti-CTLA4 monoclonal antibodies ipilimumab and tremelimumab are currently in development for the treatment of NSCLC, largely in a first-line setting (either as monotherapy or in combination with other immunotherapies). In one randomized Phase II trial, addition of ipilimumab to paclitaxel/carboplatin improved immunorelated PFS vs chemotherapy alone (median PFS 5.7 months vs 4.6 months, HR 0.72, 95% CI 0.5–1.06; P=0.05).76 Outcomes were better in patients with SqCC compared with nonsquamous NSCLC, and accordingly ongoing Phase III trials are assessing ipilimumab plus paclitaxel/carboplatin specifically in patients with SqCC of the lung (NCT02279732, NCT01285609).
Other Phase III trials are ongoing to assess nivolumab (CheckMate 026; NCT02041533) and pembrolizumab (KEYNOTE 042/024, NCT02220894/NCT02142738) vs chemotherapy in a frontline setting in patients with PDL1-positive tumors (squamous and nonsquamous NSCLC).77 Initial press releases indicate that CheckMate 026 did not achieve its primary end point of PFS, whereas KEYNOTE-024 did. At the time of writing, full data from these trials have not been published.
Immunotherapy-combination regimens
Despite the success with immunotherapies, not all patients will respond to these treatments. Based on preclinical evidence of synergism78 and striking results in other tumor types, such as melanoma,79 there is rationale for assessing combinations of PD1/PDL1 inhibitors and CTLA4 inhibitors in patients with NSCLC, including SqCC, to improve response rates. Clinical evidence to date suggests that such an approach is promising. For example, interim results from the Phase I KEYNOTE-021 trial demonstrated that ipilimumab plus pembrolizumab had acceptable toxicity and robust clinical activity in a small cohort of patients with recurrent NSCLC.80 In another Phase I study, second-line durvalumab plus tremelimumab conferred an overall response rate of 27% in 102 patients with NSCLC (squamous and nonsquamous).81 Ongoing Phase III trials assessing combination-immunotherapy regimens in both squamous and nonsquamous NSCLC include CheckMate 227 (NCT02477826; nivolumab plus ipilimumab, or nivolumab plus standard chemotherapy, vs standard chemotherapy), NEPTUNE (NCT02542293; durvalumab plus tremelimumab vs standard chemotherapy), and ARTIC (NCT02352948; durvalumab plus tremelimumab vs standard chemotherapy in patients with PDL1-positive tumors). It is hoped that such trials will demonstrate strong antitumor activity (as has been the case in other tumor types), and that immunotherapy-combination regimens will be integrated into standard treatment algorithms for NSCLC, including SqCC, in the future. However, it must be remembered that these regimens will not be without challenges, not least due to the potential for considerably higher toxicity burden than monotherapy;82 the additional financial costs will also need to be considered.
In addition to the combination of multiple immunotherapies, other trials are assessing the combination of immune-checkpoint inhibitors with contemporary chemotherapy regimens. For example, given the promise of nanoparticle albumin-bound (NAB) paclitaxel in SqCC of the lung in a first-line setting,15 an ongoing Phase III trial is assessing atezolizumab plus NAB paclitaxel/carboplatin vs atezolizumab plus paclitaxel/carboplatin, vs NAB paclitaxel/carboplatin (NCT02367794).
Novel drug targets
The large number of recurrent aberrations in certain genes and pathways identified in a Cancer Genome Atlas squamous lung cancer study has led to renewed interest in several putative drug targets.7 These targets include FGFR, the PI3K pathway, the IGF pathway, and DDRs.83,84 A number of agents that target these pathways are in early clinical development. For example, inhibitors of FGFR1, which is overexpressed in 21%–22% of SqCC tumors, have shown some early promise in patients with SqCC.85 Several early phase clinical trials have also assessed or are assessing PI3Kα-specific inhibitors, such as GDC0032 and BKM120, or PI3Kα/mTOR inhibitors (LY3023414) in patients with advanced SqCC. However, it will be several years before any of these novel agents are approved.
Second-line treatment of SqCC of the lung: which patients should be treated with afatinib?
Given the previous lack of second-line treatment options and poor outcomes in patients with SqCC of the lung, the recent approvals of afatinib, nivolumab, pembrolizumab, and ramucirumab are a massive fillip for oncologists and patients. However, this recent expansion of the treatment armamentarium inevitably invites questions regarding optimal treatment choice and sequence. As with any clinical decision process, selection of treatment depends on a balance of various factors, including efficacy, safety, patient-reported outcomes, physician experience and preference, patient comorbidities, cost and reimbursement issues, and other factors.
Efficacy
Although cross-trial comparisons are not possible, it is interesting to note that survival outcomes with nivolumab and afatinib appear to be largely similar. For instance, the 95% CIs for median OS in CheckMate 017 (9.2 months, 95% CI 7.3–13.3) and LUX-Lung 8 (7.9 months, 95% CI 7.2–8.7) overlap, as do those for 1-year OS rates (42%, 95% CI 34%–50%, and 36%, 95% CI 32%–41%, respectively).61,69 However, head-to-head trials are required to determine which treatment is most appropriate for which patient. Even within individual trials, it is important to note that statistically significant differences between treatment arms do not necessarily indicate clinical relevance, as the observed efficacy benefits with available treatments can be modest.
From an efficacy standpoint, further research into biomarkers could also be instructive in driving treatment decisions. As discussed, pembrolizumab is more active in patients with PDL1 expression (which is not surprising, given pembrolizumab’s mechanism of action),72,73 although outcomes with respect to PDL1 expression have not been assessed specifically in patients with SqCC. In CheckMate 017, the activity of nivolumab appeared to be independent of PDL1 expression.69 However, these data are contradictory with other studies, albeit in a range of tumor types. Meta-analyses across 12 studies in patients with lung, melanoma, and genitourinary cancers indicate that nivolumab generally has differential activity according to PDL1-expression levels.86 Variances across studies could reflect differences in immunohistochemical methodologies (as multiple proprietary assays exist) and/or inconsistency in cutoffs used to define tumor positivity.86 Based on the currently available data, it may be that immune-checkpoint inhibitors should be the treatment of choice for tumors with high PDL1 expression, whereas afatinib could be considered for tumors with low PDL1 expression. However, additional biomarker data from the LUX-Lung 8 trial would be important to explore this possibility further. Given the molecular heterogeneity of SqCC, other emerging biomarkers, such as blood-based protein assays like VeriStrat,87 mutational load analysis,88 and inflammatory gene signatures,89 could also be instructive in terms of who would benefit most from immunotherapy or afatinib.
Safety
Although immunotherapies are generally characterized by favorable safety profiles compared with docetaxel,69,72 they can be associated with immunologic AEs that are attributable to nonspecific immune activation, leading to increased production of inflammatory cytokines in healthy tissues.65 Immunorelated AEs can effect a number of organ systems, including the lungs (pneumonitis), gastrointestinal tract (eg, colitis), endocrine system, liver, skin, and eyes.78 In rare cases, immunorelated AEs, such as pneumonitis, can be potentially life-threatening, requiring hospitalization, and discontinuation of therapy. In the recent CheckMate 017 and CheckMate 057 trials, the incidence of pneumonitis ranged from 3% to 5%.69,70
In most cases, immunorelated AEs are manageable and reversible with steroids or other immunosuppressive drugs. However, it is important that physicians consider the risk of immunorelated AEs when choosing treatment, particularly in patients with underlying immune disorders. For example, nivolumab would not be an appropriate therapy in patients with autoimmune disease, symptomatic interstitial lung disease, or those receiving systemic immunosuppression. There are also a number of patients for whom docetaxel would not be an appropriate therapy, due to various patient comorbidities, including neuropathies, pericardial effusion, left ventricular dysfunction, edema (including pulmonary edema), and neutropenia. In LUX-Lung 8, it was noteworthy that these comorbidities occurred in approximately 10%–15% of randomized patients.
The incidence of grade ≥3 treatment-related AEs in clinical trials with afatinib has been higher than experienced with nivolumab and pembrolizumab.61 However, AEs with afatinib have proven to be predictable (diarrhea, rash, stomatitis) and manageable, and have rarely led to treatment discontinuation. Given the different safety profiles of immune-checkpoint inhibitors and afatinib, a key factor in selecting treatment should be patients’ comorbidities and tolerance of expected toxicity.84
HRQoL
When several drugs are available for the treatment of a terminal cancer, a key (yet often undervalued) consideration is the effect of the treatment on a patient’s HRQoL, on which disease-related symptoms, treatment-related AEs, and treatment efficacy all have a profound influence.90 Indeed, a striking statistic, based on a patient survey, is that 68% of patients would prefer a therapy that would improve disease-related symptoms without prolonging life span, as opposed to treatment that prolonged survival without improving symptoms.91
Central to HRQoL in lung cancer is the control of the symptoms of cough, dyspnea, and pain. As discussed already, data from LUX-Lung 8 and other Phase III trials have shown that afatinib consistently delays deterioration of cough and dyspnea, leading to significant improvements in global health status/QoL.61,92,93 Although LUX-Lung 8 did not show any significant impact of afatinib on pain, fewer patients in the afatinib arm required pain medication than in the erlotinib arm (52% vs 59%, P=0.05). In contrast with LUX-Lung 8, no HRQoL outcomes were reported in the CheckMate 017 or KEYNOTE-010 trials. Consequently, at this stage, the impact of checkpoint inhibitors on disease-related symptoms and HRQoL is not known.
Other factors
Another factor that might support the use of afatinib over checkpoint inhibitors is the fact that it is orally available, rather than being administered as an intravenous infusion, as the latter may be inconvenient for some patients and increase the cost of treatment.84
Conclusion
The recent approvals of several molecularly targeted agents and immunotherapies have provided a new level of optimism for patients with SqCC of the lung. Most likely due to its broad mechanism of action, the ErbB-family blocker afatinib has demonstrated encouraging clinical activity in patients with squamous tumors, including SqCC of the lung. Afatinib demonstrated improved PFS, OS, and DCR vs erlotinib in the Phase III LUX-Lung 8 trial, leading to its approval for locally advanced or metastatic SqCC of the lung progressing on or after platinum-based chemotherapy. Immune-checkpoint inhibitors, such as nivolumab and pembrolizumab, have demonstrated durable tumor responses and encouraging survival improvements vs standard cytotoxic agents. However, immunotherapies may not benefit all patients, and are unsuitable for certain individuals, such as those with underlying autoimmune conditions or those who have trouble in attending regular treatment visits.
These recent additions to the treatment armamentarium for SqCC of the lung inevitably pose questions, mainly unanswered, regarding the best choice and sequence of treatment according to individual patient characteristics. Future prospective evaluation of clinical and molecular biomarkers is likely to be instrumental in this regard. Despite these outstanding questions, the future for the treatment of SqCC of the lung looks increasingly promising, and we can look forward with optimism to likely further developments in the coming years.
Acknowledgments
Medical writing assistance, financially supported by Boehringer Ingelheim, was provided by Lynn Pritchard of GeoMed, an Ashfield company, part of UDG Healthcare PLC, during the preparation of this manuscript. The author was fully responsible for all content and editorial decisions, was involved at all stages of manuscript development, and has approved the final version.
Disclosure
The author reports no conflicts of interest in this work.
References
Saintigny P, Burger JA. Recent advances in non-small cell lung cancer biology and clinical management. Discov Med. 2012;13(71):287–297. | ||
Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res. 2015;4(1):36–54. | ||
Russo A, Franchina T, Ricciardi GR, et al. A decade of EGFR inhibition in EGFR-mutated non small cell lung cancer (NSCLC): old successes and future perspectives. Oncotarget. 2015;6(29):26814–26825. | ||
Wang S, Cang S, Liu D. Third-generation inhibitors targeting EGFR T790M mutation in advanced non-small cell lung cancer. J Hematol Oncol. 2016;9:34. | ||
Stinchcombe TE. Unmet needs in squamous cell carcinoma of the lung: potential role for immunotherapy. Med Oncol. 2014;31(5):960. | ||
Drilon A, Rekhtman N, Ladanyi M, Paik P. Squamous-cell carcinomas of the lung: emerging biology, controversies, and the promise of targeted therapy. Lancet Oncol. 2012;13(10):e418–e426. | ||
Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489(7417):519–525. | ||
Liam CK, Leow HR, Pang YK. EGFR mutation testing for squamous cell lung carcinoma. J Thorac Oncol. 2013;8(12):e114. | ||
Zhang Q, Zhu L, Zhang J. Epidermal growth factor receptor gene mutation status in pure squamous-cell lung cancer in Chinese patients. BMC Cancer. 2015;15:88. | ||
Scagliotti GV, Novello S, Rapetti S, Papotti M. Current state-of-the-art therapy for advanced squamous cell lung cancer. Am Soc Clin Oncol Educ Book. 2013:354–358. | ||
Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22(11):2184–2191. | ||
Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27(8):1227–1234. | ||
Derman BA, Mileham KF, Bonomi PD, Batus M, Fidler MJ. Treatment of advanced squamous cell carcinoma of the lung: a review. Transl Lung Cancer Res. 2015;4(5):524–532. | ||
Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. | ||
Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30(17): 2055–2062. | ||
Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two phase III studies. Oncologist. 2009;14(3):253–263. | ||
Ceppi P, Volante M, Saviozzi S, et al. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer. 2006;107(7): 1589–1596. | ||
Takezawa K, Okamoto I, Okamoto W, et al. Thymidylate synthase as a determinant of pemetrexed sensitivity in non-small cell lung cancer. Br J Cancer. 2011;104(10):1594–1601. | ||
Ettinger DS, Wood DE, Akerley W, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 4.2016. J Natl Compr Canc Netw. 2016;14(3):255–264. | ||
Lee Y, Shim HS, Park MS, et al. High EGFR gene copy number and skin rash as predictive markers for EGFR tyrosine kinase inhibitors in patients with advanced squamous cell lung carcinoma. Clin Cancer Res. 2012;18(6):1760–1768. | ||
Zugazagoitia J, Ponce S, Paz-Ares L. Necitumumab for first-line treatment of advanced, squamous, non-small-cell lung cancer: a relevant step forward? Transl Lung Cancer Res. 2016;5(1):95–97. | ||
Thatcher N, Hirsch FR, Luft AV, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16(7):763–774. | ||
Reck M, Socinski MA, Luft A, et al. The effect of necitumumab in combination with gemcitabine plus cisplatin on tolerability and on quality of life: results from the phase 3 SQUIRE trial. J Thorac Oncol. 2016;11(6):808–818. | ||
Butts CA, Bodkin D, Middleman EL, et al. Randomized phase II study of gemcitabine plus cisplatin or carboplatin [corrected], with or without cetuximab, as first-line therapy for patients with advanced or metastatic non small-cell lung cancer. J Clin Oncol. 2007;25(36):5777–5784. | ||
Lynch TJ, Patel T, Dreisbach L, et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter phase III trial BMS099. J Clin Oncol. 2010;28(6):911–917. | ||
Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373(9674):1525–1531. | ||
Rosell R, Robinet G, Szczesna A, et al. Randomized phase II study of cetuximab plus cisplatin/vinorelbine compared with cisplatin/vinorelbine alone as first-line therapy in EGFR-expressing advanced non-small-cell lung cancer. Ann Oncol. 2008;19(2):362–369. | ||
Pujol JL, Pirker R, Lynch TJ, et al. Meta-analysis of individual patient data from randomized trials of chemotherapy plus cetuximab as first-line treatment for advanced non-small cell lung cancer. Lung Cancer. 2014;83(2):211–218. | ||
Hirsch FR, Boyle TA, Thatcher N, et al. EGFR IHC and FISH correlative analyses (SQUIRE trial): necitumumab + gemcitabine-cisplatin vs gemcitabine-cisplatin in 1st-line squamous NSCLC. Poster presented at: 16th World Conference on Lung Cancer (WCLC); September 6–9, 2015; Denver, CO. | ||
Herbst P. A randomized, phase III study comparing carboplatin/paclitaxel or carboplatin/paclitaxel/bevacizumab with or without concurrent cetuximab in patients with advanced non-small cell lung cancer (NSCLC): SWOG S0819. Poster presented at: 16th World Conference on Lung Cancer (WCLC); September 6–9, 2015; Denver, CO. | ||
Goss GD, Spaans JN. Epidermal growth factor receptor inhibition in the management of squamous cell carcinoma of the lung. Oncologist. 2016;21(2):205–213. | ||
Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation trial. J Clin Oncol. 2007;25(12):1545–1552. | ||
Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial – INTACT 1. J Clin Oncol. 2004;22(5):777–784. | ||
Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial – INTACT 2. J Clin Oncol. 2004;22(5):785–794. | ||
Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23(25):5892–5899. | ||
Clark GM, Zborowski DM, Santabarbara P, et al. Smoking history and epidermal growth factor receptor expression as predictors of survival benefit from erlotinib for patients with non-small-cell lung cancer in the National Cancer Institute of Canada Clinical Trials Group study BR.21. Clin Lung Cancer. 2006;7(6):389–394. | ||
Wojtowicz-Praga S, Leon L. Comparative efficacy and safety of erlotinib in non-small cell lung cancer (NSCLC) of squamous cell and adenocarcinoma histology in the phase III NCIC CTG BR.21 and Saturn (BO18192) trials. Ann Oncol. 2012;23(Suppl 9):ix419. | ||
Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol. 2013;14(10):981–988. | ||
Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11(6): 521–529. | ||
Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005;366(9496):1527–1537. | ||
Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer. 2012;12(8):553–563. | ||
Roskoski R Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res. 2014;79:34–74. | ||
Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27(34):4702–4711. | ||
Solca F, Dahl G, Zoephel A, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther. 2012;343(2):342–350. | ||
Hirsh V. Next-generation covalent irreversible kinase inhibitors in NSCLC: focus on afatinib. BioDrugs. 2015;29(3):167–183. | ||
Park K, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17(5):577–589. | ||
Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27): 3327–3334. | ||
Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–222. | ||
Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16(2):141–151. | ||
Kuan FC, Kuo LT, Chen MC, et al. Overall survival benefits of first-line EGFR tyrosine kinase inhibitors in EGFR-mutated non-small-cell lung cancers: a systematic review and meta-analysis. Br J Cancer. 2015; 113(10):1519–1528. | ||
Heinmöller P, Gross C, Beyser K, et al. HER2 status in non-small cell lung cancer: results from patient screening for enrollment to a phase II study of Herceptin. Clin Cancer Res. 2003;9(14):5238–5243. | ||
Hirsch FR, Franklin WA, Veve R, Varella-Garcia M, Bunn PA Jr. HER2/neu expression in malignant lung tumors. Semin Oncol. 2002;29(1 Suppl 4):51–58. | ||
Ugocsai K, Mandoky L, Tiszlavicz L, Molnar J. Investigation of HER2 overexpression in non-small cell lung cancer. Anticancer Res. 2005;25(4):3061–3066. | ||
Yi ES, Harclerode D, Gondo M, et al. High C-erbB-3 protein expression is associated with shorter survival in advanced non-small cell lung carcinomas. Mod Pathol. 1997;10(2):142–148. | ||
Kan Z, Jaiswal BS, Stinson J, et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466(7308): 869–873. | ||
Soung YH, Lee JW, Kim SY, et al. Somatic mutations of the ERBB4 kinase domain in human cancers. Int J Cancer. 2006;118(6):1426–1429. | ||
Dhanasekaran SM, Alejandro BO, Chen G, et al. Transcriptome meta-analysis of lung cancer reveals recurrent aberrations in NRG1 and Hippo pathway genes. Nat Commun. 2014;5:5893. | ||
Schuler M, Yang JC, Park K, et al. Afatinib beyond progression in patients with non-small-cell lung cancer following chemotherapy, erlotinib/gefitinib and afatinib: phase III randomized LUX-Lung 5 trial. Ann Oncol. 2016;27(3):417–423. | ||
Machiels JP, Haddad RI, Fayette J, et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trial. Lancet Oncol. 2015;16(5):583–594. | ||
Pfister DG, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Head and Neck Cancers Version 2.2016. © 2016 National Comprehensive Cancer Network, Inc. Available from: NCCN.org. Accessed December 21, 2016. | ||
Soria JC, Felip E, Cobo M, et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2015;16(8):897–907. | ||
Park K, Li W, Zhou C, et al. Phase III trial of afatinib vs erlotinib in patients (pts) with squamous cell carcinoma (SCC) of the lung (LUX-Lung 8): EGFR molecular aberrations and survival outcomes. Ann Oncol. 2015;26 Suppl 9:ix125–ix147. | ||
Hamilton M, Wolf JL, Rusk J, et al. Effects of smoking on the pharmacokinetics of erlotinib. Clin Cancer Res. 2006;12(7 Pt 1):2166–2171. | ||
Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Impact of dyspnea, pain, and fatigue on daily life activities in ambulatory patients with advanced lung cancer. J Pain Symptom Manage. 2002;23(5):417–423. | ||
Melosky B, Chu Q, Juergens R, Leighl N, McLeod D, Hirsh V. Pointed progress in second-line advanced non-small-cell lung cancer: the rapidly evolving field of checkpoint inhibition. J Clin Oncol. 2016;34(14): 1676–1688. | ||
Nirschl CJ, Drake CG. Molecular pathways: coexpression of immune checkpoint molecules – signaling pathways and implications for cancer immunotherapy. Clin Cancer Res. 2013;19(18):4917–4924. | ||
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. | ||
Ascierto ML, Melero I, Ascierto PA. Melanoma: from incurable beast to a curable bet – the success of immunotherapy. Front Oncol. 2015; 5:152. | ||
Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. | ||
Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. | ||
Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–265. | ||
Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016; 387(10027):1540–1550. | ||
Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. | ||
Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665–673. | ||
Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. | ||
Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30(17):2046–2054. | ||
Scarpace SL. Metastatic squamous cell non-small-cell lung cancer (NSCLC): disrupting the drug treatment paradigm with immunotherapies. Drugs Context. 2015;4:212289. | ||
Callahan MK, Postow MA, Wolchok JD. CTLA-4 and PD-1 pathway blockade: combinations in the clinic. Front Oncol. 2014;4:385. | ||
Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(13):1270–1271. | ||
Patnaik A, Socinski MA, Gubens MA, et al. Phase 1 study of pembrolizumab (pembro; MK-3475) plus ipilimumab (IPI) as second-line therapy for advanced non-small cell lung cancer (NSCLC): KEYNOTE-021 cohort D. J Clin Oncol. 2015;33 Suppl:8011. | ||
Antonia SJ, Goldberg SB, Balamanoukian AS, et al. Phase IB study of MEDI4736, a programmed cell death ligand-1 (PD-L1) antibody, in combination with tremelimumab, a cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) antibody, in patients (pts) with advanced NSCLC. J Clin Oncol. 2015;33 Suppl:3014. | ||
Orloff M, Weight R, Valsecchi ME, Sato T. Immune check point inhibitors combination in melanoma: worth the toxicity? Rev Recent Clin Trials. 2016;11(2):81–86. | ||
Gerber DE, Paik PK, Dowlati A. Beyond adenocarcinoma: current treatments and future directions for squamous, small cell, and rare lung cancer histologies. Am Soc Clin Oncol Educ Book. 2015:147–162. | ||
Hall PE, Spicer J, Popat S. Rationale for targeting the ErbB family of receptors in patients with advanced squamous cell carcinoma of the lung. Future Oncol. 2015;18:1–17. | ||
Dienstmann R, Rodon J, Prat A, et al. Genomic aberrations in the FGFR pathway: opportunities for targeted therapies in solid tumors. Ann Oncol. 2014;25(3):552–563. | ||
Carbognin L, Pilotto S, Milella M, et al. Differential activity of nivolumab, pembrolizumab and MPDL3280A according to the tumor expression of programmed death-ligand-1 (PD-L1): sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS One. 2015;10(6):e0130142. | ||
Carbone DP, Ding K, Roder H, et al. Prognostic and predictive role of the VeriStrat plasma test in patients with advanced non-small-cell lung cancer treated with erlotinib or placebo in the NCIC Clinical Trials Group BR.21 trial. J Thorac Oncol. 2012;7(11):1653–1660. | ||
Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. | ||
Ascierto PA, Capone M, Urba WJ, et al. The additional facet of immunoscore: immunoprofiling as a possible predictive tool for cancer treatment. J Transl Med. 2013;11:54. | ||
Hirsh V. Is the evaluation of quality of life in NSCLC trials important? Are the results to be trusted? Front Oncol. 2014;4:173. | ||
Hirsh V. Are the data on quality of life and patient reported outcomes from clinical trials of metastatic non-small-cell lung cancer important? World J Clin Oncol. 2013;4(4):82–84. | ||
Geater SL, Xu CR, Zhou C, et al. Symptom and quality of life improvement in LUX-Lung 6: an open-label phase III study of afatinib versus cisplatin/gemcitabine in Asian patients with EGFR mutation-positive advanced non-small-cell lung cancer. J Thorac Oncol. 2015;10(6): 883–889. | ||
Yang JC, Hirsh V, Schuler M, et al. Symptom control and quality of life in LUX-Lung 3: a phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3342–3350. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

