Back to Journals » Infection and Drug Resistance » Volume 12
New Delhi metallo-β-lactamase-producing Acinetobacter isolates among late-onset VAP patients: multidrug-resistant pathogen and poor outcome
Authors Elbrolosy AM, Labeeb AZ , Hassan DM
Received 8 September 2018
Accepted for publication 12 December 2018
Published 11 February 2019 Volume 2019:12 Pages 373—384
DOI https://doi.org/10.2147/IDR.S186924
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Asmaa M Elbrolosy,1 Azza Z Labeeb,1 Dina M Hassan2
1Department of Medical Microbiology and Immunology, Faculty of Medicine, Menoufia University, Shebin El-Kom, Egypt; 2Department of Clinical Pathology, Faculty of Medicine, Cairo University, Cairo, Egypt
Background: Acinetobacter spp. are increasingly important microbes involved in late-onset ventilator-associated pneumonia (VAP).
Purpose: The aims of this study were to determine the prevalence of New Delhi metallo-β-lactamase (MβL) (blaNDM-1)-producing Acinetobacter spp. among late-onset VAP patients in different intensive care units (ICUs) of Menoufia and Kasr Al Ainy University Hospitals, to investigate the possible risk factors contributing to the acquisition of blaNDM-1-producing Acinetobacter infection, and to correlate between antimicrobial resistance pattern and therapeutic efficacy as well as clinical outcomes of these patients.
Materials and methods: Sixty-four Acinetobacter isolates were collected from mechanically ventilated patients with suspected late-onset VAP and subjected to antimicrobial susceptibility testing. The modified Hodge test (MHT) and combined disk tests (CDT) were applied for blaNDM-1 MβL detection. Acinetobacter isolates with phenotypically confirmed MβLs production were subjected to a PCR assay to verify the presence of blaNDM-1 gene. The most obvious risk factors for acquisition of carbapenem resistance in VAP patients and treatment outcomes were also analyzed.
Results: Out of 64 Acinetobacter isolates, 42 (65.6%) proved to be blaNDM-1 positive. The sensitivity and specificity of MHT were 52.38% and 41.67%, while for CDT they were 92.86% and 83.33%, respectively. Acinetobacter isolates showed high susceptibility to colistin (85.7%). The clinical response was better among VAP patients who received combined carbapenem plus colistin therapy than those who received colistin alone. Relapse of infection was detected in 12.5% (8/64) of VAP cases. The reported mortality reached 46.8% (30/64) of which 27 (64.3%) were infected with blaNDM-1-positive isolates. Prolonged duration of mechanical ventilation, longer hospital and ICU stays, and prior exposure to antibiotic therapy were by far the most important factors predisposing to carbapenem resistance among VAP patients.
Conclusion: A worldwide spread of Acinetobacter spp. expressing carbapenemases represents a significant threat to the medical community. The current study addressed the high prevalence of blaNDM-1-producing Acinetobacter isolates among late-onset VAP patients.
Keywords: Acinetobacter spp., blaNDM-1, late-onset ventilator-associated pneumonia
Introduction
Acinetobacter species have now emerged as one of the most important pathogens in nosocomial infections, particularly in intensive care settings. Although considered as opportunistic organisms, they often pose high-level resistance to a diverse array of antimicrobial agents1 and are involved in different types of hospital-acquired infections, eg, ventilator-associated pneumonia (VAP), urinary tract infections, and bloodstream and wound infections with high mortality rates.2
VAP continues to complicate the course of 8%–28% of all mechanically ventilated patients and accounts for 15%–25% of all types of intensive care unit (ICU)-acquired infections.3 The causative agents of VAP may be a part of the patient’s endogenous flora or may be acquired from an external environment including other patients, health care providers, and medical devices.4
VAP is of two types: early onset and late onset. Early-onset VAP is usually caused by Staphylococcus aureus, Streptococcus pneumoniae, or Haemophilus influenzae, whereas late-onset VAP is often caused by multidrug-resistant (MDR) pathogens of which Pseudomonas aeruginosa, Acinetobacter spp., and methicillin-resistant S. aureus are the most prevalent.4
The antimicrobial resistance mechanisms among Acinetobacter spp. are variable. Different mechanisms conferring resistance to β-lactam drugs have been documented involving the production of β-lactamases, overexpression of drug efflux pumps, and decreased outer membrane permeability. However, the production of carbapenem hydrolyzing enzymes is of great concern.5
Most Acinetobacter spp. develop carbapenem resistance by the production of metallo-β-lactamases (MβLs) and oxacillinase-type carbapenemase-type carbapenemase.6 New Delhi MβL (blaNDM-1) is a novel MβL with great ability to provide resistance to almost all the β-lactams as well as carbapenems. This is the most critical feature because carbapenems are among the few backup agents for use against MβL producers.7
Plasmids carrying the blaNDM-1 gene are diverse, highly transmissible, and can harbor a large number of resistance genes associated with other carbapenemase genes, aminoglycosides, macrolides, rifampin, and even sulfamethoxazole resistance genes resulting in MDR or extremely drug-resistant (XDR) phenotypes.8 The blaNDM-1 type is becoming the most threatening carbapenemase.9
VAP mortality rate was reported to be high and may reach up to 70% of patients. Treatment usually starts before the result of antibiotic resistance pattern and includes colistin alone or with another antibiotic belonging to another group as imipenem or meropenem in order to obtain synergistic effects and to prevent the selection of hetero-resistant strains.10
The objective of this study was to determine the prevalence of blaNDM-1-producing Acinetobacter spp. among patients with late-onset VAP admitted to different ICUs of both Menoufia and Kasr Al Ainy University Hospitals by both phenotypic and molecular characterization methods, to investigate the possible risk factors for acquisition of blaNDM-1-producing Acinetobacter infection, and to correlate between the presence of the blaNDM-1 gene and therapeutic efficacy as well as patient outcomes.
Materials and methods
Study population
A total of 64 Acinetobacter isolates were collected from 312 mechanically ventilated patients (143 males and 169 females with a mean age of 59 years ± SD) admitted to different ICUs of both Menoufia and Kasr Al Ainy University Hospitals and suspected to have late-onset VAP in the period from September 2017 to August 2018. VAP was defined as pneumonia occurring >48 hours following endotracheal intubation, with the following diagnostic criteria: new or progressive lung infiltrates, consolidation, or cavitations on chest X-ray, with one of the following criteria – new onset of purulent bronchial secretions with leukopenia (white blood cells <4×109/L) or leukocytosis (>12×109/L), or core temperature ≥38.5°C or ≤36°C without other cause, significant positive culture from blood, or endotracheal aspirate, bronchoalveolar lavage (BAL), or culture from another relevant site of infection.3 However, late-onset VAP was defined as pneumonia occurring on the fifth and subsequent days following endotracheal intubation.11 The Clinical Pulmonary Infection Score (CPIS), based on six clinical assessments, was calculated for patients on the same day of the endotracheal secretions collection, and all those with CPIS ≥6 were included in this study.12 All VAP events enrolled in the study were primary episodes. Recurrent infection occurred during patients follow-up in the form of relapse (pure growth of the organism causing the initial infection typically with worsening of symptoms after an evidence of clearance of the primary VAP with at least 5 days therapy).3
Ethics statement
The study protocol conformed to the ethical guidelines of the 2013 “Helsinki Declaration” and was approved by the ethical committee of the Faculty of Medicine, Cairo University and Faculty of Medicine, Menoufia University. All patients, or family members, signed the written consent form to participate in the study.
Clinical specimens collection and clinical isolates identification
Both endotracheal and mini-BAL samples were collected from all participants13,14 and inoculated onto the corresponding culture media (MacConkey’s agar; Thermo Fisher Scientific, Waltham, MA, USA). Acinetobacter isolates were identified by the standard microbiological methods; the genus Acinetobacter comprises Gram-negative, aerobic, glucose-non-fermenting, non-fastidious, nonmotile, catalase-positive, and oxidase-negative bacteria.15 Species identification was carried out by the Vitek-2 system (BioMerieux, Marcy-l’Étoile, France). The isolates were suspended in nutrient broth supplemented with 16% glycerol and stored frozen at −80°C.
Antibiotic susceptibility testing and screening for carbapenem resistance
All Acinetobacter isolates were tested by the Kirby–Bauer disk diffusion method on Mueller Hinton (MH) agar (HiMedia Laboratories, India) against different antibiotics (Thermo Fisher Scientific) as per Clinical and Laboratory Standards Institute (CLSI, Waltham, MA, USA), 2017 and zone diameters were interpreted according to the CLSI guidelines.16 MDR was defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories; XDR was defined as non-susceptibility to at least one agent in all but two or fewer antimicrobial types (ie, bacterial isolates remain susceptible to only one or two categories, most often polymyxins, eg, colistin); and pan-drug resistance (PDR) was defined as non-susceptibility to all agents in all antimicrobial categories as per the European Committee on Antimicrobial Susceptibility Testing and the US Food and Drug Administration.17,18
Minimum inhibitory concentration (MIC) determination by agar dilution method
The MICs of imipenem were determined for all imipenem non-susceptible Acinetobacter isolates using the agar dilution method. The isolates were categorized as susceptible and resistant according to MIC interpretive criteria for Acinetobacter spp. (CLSI, 2017 guidelines).16
Phenotypic characterization of blaNDM-1 production
This was performed by the following methods:
Modified Hodge test (MHT)
All the carbapenem-resistant strains were subjected to MHT for detection of blaNDM-1 carbapenemase. A 0.5 McFarland standard suspension of Escherichia coli ATCC 25922 (the indicator organism) was prepared and diluted 1:10 in saline or broth. An MH agar plate was inoculated with the indicator strain as per the routine disk diffusion method. An imipenem disk (10 µg) was placed in the center of the plate. The test organism, positive control (Klebsiella pneumoniae ATCC BAA-1706), and negative control (K. pneumoniae ATCC BAA-1705) were streaked in straight lines starting from the edge of the imipenem disk to the edge of the plate. The plate was incubated at 35±2°C for 24 hours. MβLs production was detected by the appearance of the enhanced growth of the carbapenem-susceptible ATCC E. coli 25922 along the test organism giving a clover leaf-like indentation that indicated positive MβL production (Figure 1).19
Combined disk test (CDT)
This test was applied for the phenotypic confirmation of blaNDM-1 production in carbapenem-resistant isolates. An EDTA solution of 0.5 M concentration was prepared by dissolving 46.53 g of disodium EDTA2H2O in 250 mL of distilled water and adjusting the pH to 8.0 by using NaOH. The mixture was sterilized by autoclaving. Two imipenem disks (10 µg) were placed on the surface of the MH agar plate inoculated with 0.5 McFarland standard suspension of the test organism at a suitable distance and 4 µL of EDTA solution was added to one of them to get the desired concentration. After 24 hours incubation at 37°C, an increase of ≥7 mm in the zone diameter of the EDTA-containing imipenem disk compared to the imipenem disk alone was considered positive for the production of MβL among carbapenem-resistant strains.20
Genotypic characterization of blaNDM-1 gene by conventional PCR
All MDR Acinetobacter isolates were subjected to conventional PCR assays to verify the presence of the blaNDM-1 gene. Plasmid DNA was extracted via the DNA extraction kit (Qiagen NV, Venlo, the Netherlands) according to the manufacturer’s instructions. Specific primers for blaNDM-1, forward (5′-GGGCAGTCGCTTCCAACGGT) and reverse (5′-GCTCAGTGTCGGCAT), that amplified 475-bp internal fragment of the gene were used. The cycling protocol involved an initial DNA release and denaturation step at 94°C for 5 minutes, followed by 36 cycles at 94°C for 30 seconds, 52°C for 40 seconds, and 72°C for 50 seconds, with a subsequent single elongation step at 72°C for 5 minutes. The PCR products were then detected by agarose gel electrophoresis using 1% agarose (Figure 2).21
Patients treatment and outcome assessment
All VAP patients enrolled in the study had received intravenous (iv) colistin (colistimethate sodium), as a dose of 1–2 million IU every 8 hours (62,500 IU/kg/day) according to body weight with normal renal function. Treatment continued according to clinical response with close monitoring for the possibilities of nephrotoxicity and neurotoxicity; dosages were adjusted for patients with renal impairment. Imipenem 500 mg iv four times per day was used in combination with colistin in 28/64 of VAP cases.10,22 Effective antibiotic therapy was determined by complete clinical cure, ie improvement of all symptoms, signs and laboratory values related to the VAP infection, while failed treatment was defined as persistence of symptoms and signs of VAP despite antimicrobial treatment.23 Patients were followed up until 30 days after onset of VAP or until death.
Statistical analysis
Continuous variables were described as median and categorical variables were described as n (%). For comparative tests on continuous variables, Mann–Whitney U test was applied. For categorical variables, Pearson’s chi-squared test or Fisher’s exact test was used as appropriate. Statistical analysis was performed using the SPSS-17 software (SPSS Inc., Chicago, IL, USA). P<0.05 was considered significant. Accuracy was represented using the terms sensitivity and specificity.
Results
During the period from September 2017 to August 2018, a total of 312 late-onset VAP events were diagnosed in both Menoufia and Kasr Al Ainy University Hospitals. As per the aim of our study, only 64 patients (41 males and 23 females with a mean age of 59 years ± SD) with microbiologically confirmed Acinetobacter VAP infections were enrolled in this study. All patients were contracting primary VAP episodes. The recurrence rate in Acinetobacter VAP episodes was 12.5% (8/64), and all were in the form of relapse i.e. re-isolation of Acinetobacter spp. About 10.2% (32/312) of the total VAP events were diagnosed clinically, as no pathogen was identified. The majority of pathogens associated with VAP infection were Gram-negative species. Acinetobacter isolates (64/280 [22.8%]; 37 Acinetobacter baumannii, 15 Acinetobacter calcoaceticus, and 12 A. baumannii/A. calcoaceticus complex) were the most frequent pathogens collected from VAP patients.
Carbapenem resistance was observed in 54/64 (84.4%) of Acinetobacter isolates by both the screening disk diffusion and MIC determination methods. Genotypic confirmation by PCR assay revealed that 42/54 isolates (65.6%) were positive for the blaNDM-1 gene; such isolates exhibited higher imipenem MIC values than blaNDM-1-negative isolates (Table 1). In relation to PCR results, the sensitivity and specificity of MHT were 52.38% and 41.67%, respectively, while for CDT they were 92.86% and 83.33%, respectively (Table 2).
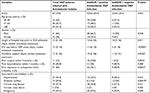
Most Acinetobacter isolates displayed extreme resistance against most of the tested antibiotics except for colistin. All Acinetobacter isolates were 100% resistant to cefotaxime, ceftriaxone, ceftazidime, cefepime, and trimethoprim/sulfamethoxazole, and >90% resistance rates against piperacillin/tazobactam, tetracycline, aztreonam, ciprofloxacin, and amikacin were detected. Moreover, 54/64 (84.4%) of the total Acinetobacter isolates were resistant to both imipenem and meropenem. All blaNDM-1-positive Acinetobacter isolates (42/42; 100%) were resistant to imipenem and meropenem compared to only a 54.5% (12/22) resistance rate among blaNDM-1-negative isolates. However, colistin was the most effective tested agent displaying high bactericidal activity against Acinetobacter isolates, as only 6/64 (9.3%) of the total isolates were colistin-resistant. Interestingly, all colistin-resistant isolates were blaNDM-1-positive (Figure 3 and Table 3).
  | Table 3 Comparative antibiotic resistance patterns in blaNDM-1-positive and blaNDM-1-negative Acinetobacter isolates Abbreviation: blaNDM, New Delhi metallo-β-lactamase. |
The number of VAP patients infected with blaNDM-1-positive Acinetobacter isolates exceeded those infected with blaNDM-1-negative isolates (42/64, 65.6%; 12/64, 34.4%). Demographic data regarding age and sex were comparable among both blaNDM-1-positive and blaNDM-1-negative VAP groups. There was a significant positive correlation (P<0.0001) regarding longer ICU stay (median ICU stay =11 days) and prolonged ventilator support days (median ventilator support days =19) and the occurrence of VAP due to blaNDM-1-positive Acinetobacter isolates (Table 4).
Self-observation in this work also confirmed that prior exposure to antimicrobial agents, particularly carbapenems, before admission to hospital is very likely to be significantly associated with the acquisition of VAP infection due to blaNDM-1-positive Acinetobacter isolates (76.1% vs 40.9%; P<0.01). Comorbidities, such as malignancy, hypertension, and chronic lung disease, were independent risk factors associated with VAP caused by MDR Acinetobacter isolates (Table 4).
Colistin was used as a single empirical monotherapy in 36/64 (56.2%) of VAP cases, and the commonly prescribed combination therapy was colistin plus imipenem in 28/64 (43.8%) cases. Although 93.7% of all VAP patients received early treatment, complete clinical response was achieved in only 40/64 (62.5%) of them. A low rate of relapse (8/64; 12.5%) was detected during the course of treatment; it was higher in patients infected with blaNDM-1-positive Acinetobacter isolates (6/42; 14.2%) compared with those infected with blaNDM-1-negative isolates (2/22; 9%) but with no statistically significant difference (Table 5).
  | Table 5 Treatment modalities and patient outcomes in both VAP groups Note: *Statistical significance. Abbreviations: blaNDM, New Delhi metallo-β-lactamase; VAP, ventilator-associated pneumonia. |
Regarding treatment complications, ie, failure of therapy (24/64; 37.5%) or relapse (8/64; 12.5%), they were higher among patients infected with blaNDM-1-positive Acinetobacter isolates; those complications were associated with poor patients’ survival and death. The overall mortality of VAP patients was found to be high (30/64; 46.8%); of these, 27/42 (64.3%) patients were infected with blaNDM-1-positive Acinetobacter isolates, and only three patients (3/22; 13.6%) were infected with blaNDM-1-negative isolates with a statistically significant difference (P=0.0001). Upon comparing the two treatment modalities, the clinical response was found to be better among VAP patients who received combined carbapenem plus colistin therapy than those who received colistin alone (Table 5).
Discussion
Acinetobacter infections are predominant in critically ill patients, particularly in ICUs. Acinetobacter is one of the leading causes of VAP in some countries as it is related to high morbidity and mortality. This notorious pathogen has extraordinary novel resistance mechanisms against many antibiotics including carbapenems and survives under harsh conditions on inanimate objects in the hospital environment.24
Comparing VAP-associated pathogens of the current study, Acinetobacter isolates were the most common pathogen detected (64/280; 22.8%). Consistent with our findings, several authors have declared Acinetobacter isolates as the most prevalent pathogen (21%–36%) in late-onset VAP patients.3,25 Previous studies in Egypt by Hasanin et al and Amr and Abdel Razek addressed the high prevalence of Acinetobacter spp. among VAP patients.26,27 Also, in a recent study of VAP in Asian countries by Chung et al, Acinetobacter spp. were the most frequent pathogen (36.5%).28 Our current finding was dramatically different from previous studies in Western countries and several developing countries where P. aeruginosa was the most commonly isolated pathogen (20%–52%).29 Acinetobacter spp. are now particularly known as the most prevalent cause of late-onset VAP among ICUs patients.3
The prevalence of carbapenem-resistant Acinetobacter has increased worldwide.24 In the current study, the majority of Acinetobacter isolates were carbapenem non-susceptible (54/64; 84.4%). High resistance to carbapenems in Acinetobacter isolates has been notable recently in Egypt, ranging from 75% to 100%.30 Al-Hassan et al reported that the Acinetobacter resistance rate to imipenem exceeded 70%.31 The resistance rate of Acinetobacter to imipenem was found to be 65% in Saudi Arabia and 45% in Turkey.3,32 Imipenem resistance reflects a problem that might be described as countrywide due to the uncontrolled extensive use of these drugs.30
The problem of carbapenem resistance recently reached a climax with the discovery of the blaNDM-1 gene that confers resistance to the last-resort antibiotics, carbapenems, and hampered the capability of all antibiotics of the β-lactam group to treat infections caused by microorganisms carrying such resistance determinants.33 The striking finding in our study was the high prevalence of blaNDM-1-positive Acinetobacter isolates (42/64; 65.6%) in VAP patients. Our finding agreed with previous studies which reported that blaNDM-1 was first described in Acinetobacter from Egypt with no obvious link with the Indian subcontinent.34
In Egypt since 2012, the blaNDM-1 gene has been described in Acinetobacter isolates by Abd El-Glil (19.2%) and El-Sayed-Ahmed et al (39.3%).35,36 Moreover, the blaNDM-1-carrying Acinetobacter isolates have emerged in many other countries, including Germany, Spain, Israel, Switzerland, Libya, India, Pakistan, and Nepal,37 and have already disseminated in the Middle East. In 2018 in Bangladesh, the authors reported that infection with imipenem-resistant blaNDM-1-producing Acinetobacter is increasing in VAP patients – 3.5% in 2011, 22% in 2013, and lastly in 2018 reaching up to 86%.38
Regarding the phenotypic detection of the blaNDM-1 gene among carbapenem-resistant isolates, the current study revealed that the sensitivity and specificity of the MHT were 52.38% and 41.67%, respectively, in relation to PCR results. Our results correlated with those of Bialvaei et al who reported that MHT suffers from poor sensitivity for MβL detection.39 In another study by Sun et al, the sensitivity of MHT was 75.5%, with 34.8% (16/46) of the blaNDM-1 producers being missed.40 Lifshitz et al also concluded that MHT is used as the earliest developed phenotypic method to detect carbapenemase; however, poor sensitivity and specificity in detecting the blaNDM carriers have hindered its wide clinical application.41
For CDT, the sensitivity and specificity were 92.86% and 83.33%, respectively, which were nearly comparable to those reported by Pandya et al who recommended imipenem-EDTA CDT for rapid MβL detection among Gram-negative bacilli42 and Omair et al who stated that the combined disk method is an economical and reliable method for MβL detection which can be used routinely in any laboratory.43
The current study revealed that all Acinetobacter isolates showed 100% resistance to cefotaxime, ceftriaxone, ceftazidime, cefepime, and trimethoprim/sulfamethoxazole with a resistance rate >90% to piperacillin/tazobactam, tetracycline, and aztreonam. Similar results were seen in studies performed by Muneeza et al wherein all Acinetobacter isolates showed very high resistance to all antimicrobials from 70% to 100%.44
The present study is consistent with a study conducted in Egypt by Al-Agamy et al as they reported that all Acinetobacter isolates were resistant to third and fourth generation cephalosporins and monobactams.30,45 Our findings proved that all (42/42) Acinetobacter isolates that harbored the blaNDM-1 gene exhibited 100% resistance to carbapenems and all other tested antibiotics except for colistin. Similar to our findings, Tran et al and Mulla et al reported a significantly higher level of carbapenem resistance in Acinetobacter isolates with coexistent blaNDM-1 when compared with other blaNDM-1-negative isolates.33,45 In agreement with our results, Abd El-Glil and El-Sayed-Ahmed et al also reported that most of the blaNDM-1-producing Acinetobacter spp. remained susceptible only to colistin and that the co-occurrence of blaNDM-1 with blaOXA-23-like genes in Acinetobacter isolates caused overexpression of those genes, increased carbapenem resistance, and resistance to different antibiotics as well.35,36 The resistance to antibiotics reflects on the policy of antimicrobial usage and drug-resistant clones in hospitals and ICUs.30
Regarding the resistance of Acinetobacter to colistin, only 6/64 (9.3%) isolates displayed colistin resistance, which was in accordance, to some extent, with previous studies in India and Egypt1,30 in which Acinetobacter spp. showed low colistin resistance (9.5% and 3%).This might be due to the fact that colistin is a reserve drug for MDR pathogens.46 However, several studies reported that PDR Acinetobacter has raised in clinical relevance, perhaps due to the increase in the use of colistin; hence, resistance to it is also emerging.33
The hallmark of Acinetobacter isolates is their resistance genes such as blaNDM-1 gene, with MDR Acinetobacter spp. affecting an estimated 10%–30% of ventilated patients worldwide with a high mortality rate reaching up to 45.6%–60.9%.46 The prognostic impact of Acinetobacter VAP infection remains controversial and conflicting. Thus, this study aimed to identify factors predictive of a poor outcome in patients with late-onset VAP caused by Acinetobacter isolates with coexistent blaNDM-1 gene when compared with other blaNDM-1-negative isolates.
As far as risk factors are concerned, our finding suggested that prolonged time on mechanical ventilation and prolonged ICU stay days were significantly recognized risk factors associated with infection due to blaNDM-1-positive Acinetobacter isolates in VAP patients. These observations were in parallel with previous studies, which reported MDR blaNDM-1-positive Acinetobacter as a late-onset pathogen.4,45 In agreement with our finding, previous Egyptian studies by Amr and Abdel Razek and Hamid et al concluded that prolonged ICU stay and mechanical ventilation days with prior use of antibiotics were the recognized factors increasing the risk of VAP in >70% of patients due to multidrug and imipenem-resistant Acinetobacter infection.27,47
In this study, there was a significant relationship between prior use of carbapenem treatment (76.1% vs 40.9%; P=0.0052) and the acquisition of infections caused by blaNDM-1-positive Acinetobacter isolates. Prior use of antibiotics is a recognized factor increasing the risk of VAP due to MDR Acinetobacter infection.47 Moreover, Nhu et al and Aneta et al suggested that there is a risk that prior carbapenem exposure predisposes patients to subsequent colonization and infection with resistant pathogens.48,49 On the other hand, there were no statistically significant differences regarding age or sex and VAP infection as previously reported.27
Inappropriate empirical therapy of VAP patients is an important predictor of mortality in ICUs.39 Our study aimed at identifying the optimal treatment choice in patients with blaNDM-1-positive Acinetobacter VAP and different outcomes associated with treatment strategies. The use of colistin has recently gained popularity, as other treatment options are limited. It is noteworthy that the antibiotics used for patients in the present study were either colistin alone or colistin plus imipenem, which was selected as an independent predictor of better survival.10 In the present work, complete clinical response was achieved in 40/64 (62.5%) VAP patients and it was better among VAP patients who received combined carbapenem plus colistin therapy (24/28; 85.7%) compared with those who received colistin alone (16/36; 44.4%). Colistin combined therapy was previously reported to be synergic and prevent the emergence of colistin-resistant mutants.10 There was a discrepancy between studies comparing colistin monotherapy and combined therapies, providing inconsistent results, with insufficient evidence supporting the superiority of combined therapies.23 Some literatures reported that no additional benefit was achieved by combination therapy,50 while others were consistent with our study and reported superiority for combined therapy to provide a synergistic effect for the salvage therapy because of the debate about low penetration of colistin into the lungs.10 Drug choice can be customized according to drug access, drug interactions, side effects, and costs.23
In the present study, the mortality and relapse rates were higher in patients infected with blaNDM-1-positive Acinetobacter isolates, particularly among those patients who received colistin monotherapy compared to those who received combined therapy. However, a meta-analytic study demonstrated no difference in mortality between monotherapy and combined therapy for carbapenem-resistant Acinetobacter VAP infections.50 Previously, there has been confusion between the concepts of “recurrence”, which usually indicates prevention failure, and “persistence”, which usually indicates treatment failure.3 Low relapse rate 8/64 (12.5%) was detected in this study. Supporting this finding, El-Saed et al reported that Acinetobacter spp. were the only pathogens significantly associated with recurrent VAP but with a low rate (16.8%),3 though a higher rate of VAP relapse was associated with infection by Acinetobacter spp. (26.8%).51
The overall mortality of VAP patients was found to be high (30/64; 46.8%); of these, 27/42 (64.3%) patients were infected with blaNDM-1-positive Acinetobacter isolates, and only 3 patients (13.6%) were infected with blaNDM-1-negative isolates with a statistically significant difference (P=0.0001). Similar high mortality rates in VAP patients were previously recorded with Acinetobacter infections.4,47 Available data in the literature suggested that mortality related to Acinetobacter VAP varies from 26% to 68%.47 This high mortality, despite appropriate therapy, can be explained by low colistin pulmonary penetration.51
Conclusion and recommendations
Acinetobacter isolates harboring the blaNDM-1 gene conferring carbapenem resistance are among the major causes of late-onset VAP circulating in Egyptian hospitals. Awareness of Acinetobacter resistance patterns is considered the most important information to guide optimal antimicrobial therapy as its management still continues to be a challenge for clinicians. This study highlights the risk factors associated with poor outcomes and mortality in VAP patients. The most significant prognostic factors were infection with blaNDM-1-positive Acinetobacter isolates, longer duration of mechanical ventilation >14 days, longer hospital and ICU stays for >7 days, prior exposure to antimicrobial agents, and inappropriate empirical therapy. Timely detection, implementation of infection control measures, formulation of antibiotic policy, and preventive strategies to control the dissemination of such strains are urgently required to reduce incidence and mortality among hospitalized patients suffering from VAP. Clinical response was superior with colistin plus carbapenem combination over colistin alone, but this did not represent a statistically significant difference.
Acknowledgments
We acknowledge the assistance of our colleagues in the Chest Department and different ICUs of both Menoufia and Kasr Al Ainy Hospitals for helping us collect the needed specimens, and providing us with essential clinical information and therapeutic data of the study population throughout the course of the study.
Disclosure
The authors report no conflicts of interest in this work.
References
Rynga D, Shariff M, Deb M. Phenotypic and molecular characterization of clinical isolates of Acinetobacter baumannii isolated from Delhi, India. Ann Clin Microbiol Antimicrob. 2015;14:40. | ||
Muthusamy D, Boope A. Phenotypic methods for the detection of various beta-lactamases in carbapenem-resistant isolates of Acinetobacter baumannii at a tertiary care hospital in South India. J Clin Diagn Res. 2012;6(6):970–973. | ||
El-Saed A, Balkhy HH, Al-Dorzi HM, Khan R, Rishu AH, Arabi YM. Acinetobacter is the most common pathogen associated with late-onset and recurrent ventilator-associated pneumonia in an adult intensive care unit in Saudi Arabia. Int J Infect Dis. 2013;17(9):e696–e701. | ||
Gupta V, Singla N, Gombar S, Palta S, Chander J. Prevalence of multidrug-resistant pathogens and their antibiotic susceptibility pattern from late-onset ventilator-associated pneumonia patients from a Tertiary-Care Hospital in North India. J Assoc Chest Physicians. 2018; 6(1):4–8. | ||
Kabbaj H, Seffar M, Belefquih B, et al. Prevalence of metallo-B-lactamases producing Acinetobacter baumannii in a Moroccan hospital. ISRN Infect Dis. 2013;2013(1):1–3. | ||
Amudhan MS, Sekar U, Kamalanathan A, Balaraman S. bla(IMP) and bla(VIM) mediated carbapenem resistance in Pseudomonas and Acinetobacter species in India. J Infect Dev Ctries. 2012;6(11):757–762. | ||
Mobashshera T, Aruna K. Phenotypic and molecular characterization of MBL genes among uropathogens isolated in Mumbai city. Br Microbiol Res J. 2015;5(4):368–383. | ||
Bush K. Alarming β-lactamase-mediated resistance in multidrug-resistant Enterobacteriaceae. Curr Opin Microbiol. 2010;13(5):558–564. | ||
Levy Hara G, Gould I, Endimiani A, et al. Detection, treatment, and prevention of carbapenemase-producing Enterobacteriaceae: recommendations from an International Working Group. J Chemother. 2013;25(3):44–51. | ||
Yilmaz GR, Guven T, Guner R, et al. Colistin alone or combined with sulbactam or carbapenem against A. baumannii in ventilator-associated pneumonia. J Infect Dev Ctries. 2015;9(5):476–485. | ||
Kollef MH, Silver P, Murphy DM, Trovillion E. The effect of late-onset ventilator-associated pneumonia in determining patient mortality. Chest. 1995;108(6):1655–1662. | ||
Zilberberg MD, Shorr AF. Ventilator-associated pneumonia: the clinical pulmonary infection score as a surrogate for diagnostics and outcome. Clin Infect Dis. 2010;51 Suppl 1:S131–S135. | ||
UK Standards for Microbiology Investigations (SMIs) are a comprehensive referenced collection of recommended algorithms and procedures for clinical microbiology; 2014. | ||
Perkins GD, Chatterjee S, Giles S, et al. Safety and tolerability of nonbronchoscopic lavage in ARDS. Chest. 2005;127(4):1358–1363. | ||
Koneman E, WinnWJ, Allen SD, et al. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology. 6th ed. London: Lippincott, Williams and Wilkins; 2006; Chapter 2:211–226. | ||
Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 20th informational supplement. CLSI document M100-S26. Wayne, PA: CLSI; 2017. | ||
European Society of Clinical Microbiology and Infectious Diseases. Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution; European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Clin Microbiol Infect. 2000;6(9):509–515. | ||
Brown SD, Traczewski MM. Comparative in vitro antimicrobial activity of tigecycline, a new glycylcycline compound, in freshly prepared medium and quality control. J Clin Microbiol. 2007;45(7):2173–2179. | ||
Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 20th informational supplement. CLSI document M100-S22. Wayne, PA: CLSI; 2012. | ||
Pitout JD, Gregson DB, Poirel L, McClure JA, Le P, Church DL. Detection of Pseudomonas aeruginosa producing metallo-beta-lactamases in a large centralized laboratory. J Clin Microbiol. 2005;43(7):3129–3135. | ||
Manchanda V, Rai S, Gupta S, et al. Development of TaqMan real-time polymerase chain reaction for the detection of the newly emerging form of carbapenem resistance gene in clinical isolates of Escherichia coli, Klebsiella pneumoniae, and Acinetobacter baumannii. Indian J Med Microbiol. 2011;29(3):249–253. | ||
Amin M, Rashad A, Fouad A, Abdel Azeem A. Re-emerging of colistin for treatment of nosocomial pneumonia due to Gram negative multi-drug resistant pathogens in critically ill patients. Egypt J Chest Dis Tuberc. 2013;62(3):447–451. | ||
Kara Iskender, Yildirim F, Bilaloglu B. Comparison of the efficacy of colistin monotherapy and colistin combination therapies in the treatment of nosocomial pneumonia and ventilator-associated pneumonia caused by Acinetobacter baumannii. S Afr J Crit Care. 2015;31(2):51–58. | ||
Gurjar M, Saigal S, Baronia AK, et al. Carbapenem-resistant Acinetobacter ventilator-associated pneumonia: clinical characteristics and outcome. Indian J Crit Care Med. 2013;17(3):129–134. | ||
Dube M, Goswami S, Singh A, Raju BM, Dube P, Bhatia GC. Pattern and incidence of ventilator associated pneumonia among mechanically ventilated patients. Int J Adv Med. 2018;5(2):442–445. | ||
Hasanin A, Mukhtar A, El-Adawy A, et al. Ventilator associated pneumonia caused by extensive-drug resistant Acinetobacter species: colistin is the remaining choice. Egypt J Anaesth. 2016;32(3):409–413. | ||
Amr GE, Abdel Razek GM. Characterization of carbapenem resistant Acinetobacter baumannii causing ventilator associated pneumonia in ICUs of Zagazig University Hospitals, Egypt. Int J Curr Microbiol Appl Sci. 2016;5(12):660–671. | ||
Chung DR, Song JH, Kim SH, et al. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med. 2011;184(12):1409–1417. | ||
Inchai J, Pothirat C, Bumroongkit C, Limsukon A, Khositsakulchai W, Liwsrisakun C. Prognostic factors associated with mortality of drug-resistant Acinetobacter baumannii ventilator-associated pneumonia. J Intensive Care. 2015;3:9–17. | ||
Al-Agamy MH, Khalaf NG, Tawfick MM, Shibl AM, El Kholy A. Molecular characterization of carbapenem-insensitive Acinetobacter baumannii in Egypt. Int J Infect Dis. 2014;22(22):49–54. | ||
Al-Hassan L, El Mehallawy H, Amyes SG. Diversity in Acinetobacter baumannii isolates from paediatric cancer patients in Egypt. Clin Microbiol Infect. 2013;19(11):1082–1088. | ||
Cicek AC, Saral A, Iraz M, et al. OXA- and GES-type β-lactamases predominate in extensively drug-resistant Acinetobacter baumannii isolates from a Turkish University Hospital. Clin Microbiol Infect. 2014;20(5):410–415. | ||
Tran DN, Tran HH, Matsui M, et al. Emergence of New Delhi metallo-beta-lactamase 1 and other carbapenemase-producing Acinetobacter calcoaceticus-baumannii complex among patients in hospitals in Ha Noi, Viet Nam. Eur J Clin Microbiol Infect Dis. 2017;36(2):219–225. | ||
Hrabák J, Stolbová M, Studentová V, Fridrichová M, Chudáčková E, Zemlickova H. NDM-1 producing Acinetobacter baumannii isolated from a patient repatriated to the Czech Republic from Egypt, July 2011. Euro Surveill. 2012;17(7):80–85. | ||
Abd El-Glil RR. New Delhi Metallo-β-lactamase 1 (NDM-1) producing Acinetobacter baumannii in Egyptian hospitals. Int J Adv Res. 2015;3(4):470–478. | ||
El-Sayed-Ahmed MA, Amin MA, Tawakol WM, Loucif L, Bakour S, Rolain JM. High prevalence of bla(NDM-1) carbapenemase-encoding gene and 16S rRNA armA methyltransferase gene among Acinetobacter baumannii clinical isolates in Egypt. Antimicrob Agents Chemother. 2015;59(6):3602–3605. | ||
Joshi PR, Acharya M, Kakshapati T, Leungtongkam U, Thummeepak R, Sitthisak S. Co-existence of blaOXA-23 and blaNDM-1 genes of Acinetobacter baumannii isolated from Nepal: antimicrobial resistance and clinical significance. Antimicrob Resist Infect Control. 2017;6:21. | ||
Shahida A, Shamsuzzaman SM. Distribution of New Delhi metallo-beta-lactamase producing Acinetobacter baumannii in patients with ventilator associated respiratory tract infection. IMC J Med Sci. 2018;12(1):37–41. | ||
Bialvaei AZ, Kafil HS, Asgharzadeh M, Yousef Memar M, Yousefi M. Current methods for the identification of carbapenemases. J Chemother. 2016;28(1):1–19. | ||
Sun K, Xu X, Yan J, Zhang L. Evaluation of six phenotypic methods for the detection of carbapenemases in Gram-negative bacteria with characterized resistance mechanisms. Ann Lab Med. 2017;37(4):305–312. | ||
Lifshitz Z, Adler A, Carmeli Y. Comparative study of a novel biochemical assay, the Rapidec Carba NP test, for detecting carbapenemase-producing Enterobacteriaceae. J Clin Microbiol. 2016;54(2):453–456. | ||
Pandya NP, Prajapati SB, Mehta SJ, Kikani KM, Joshi PJ. Evaluation of various methods for detection of metallo-β-lactamase (MBL) production in Gram negative bacilli. Int J Biol Med Res. 2011;2(3):775–777. | ||
Omair M, Usman J, Kaleem F, Hassan A, Khalid A, Fahim Q. Evaluation of combined disc method for the detection of metallo-β-lactamase producing Gram negative bacilli. Malays J Microbiol. 2012;8(1):21–25. | ||
Muneeza A, Ejaz H, Zafar A, Hamid H. Phenotypic detection of metallo-beta-lactamases in carbapenem resistant Acinetobacter baumannii isolated from pediatric patients in Pakistan. J Pathog. 2016;2016:8603964. | ||
Mulla S, Charan J, Rajdev S, Jaykaran C, Sangita R. Antibiotic sensitivity pattern in blaNDM-1-positive and carbapenemase-producing Enterobacteriaceae. Int J Appl Basic Med Res. 2016;6(1):14–17. | ||
Chaari A, Mnif B, Bahloul M, et al. Acinetobacter baumannii ventilator-associated pneumonia: epidemiology, clinical characteristics, and prognosis factors. Int J Infect Dis. 2013;17(12):e1225–e1228. | ||
Hamid RMA, Hassan SS, El-Mahallawy HA, Saber M. Molecular characterization of carbapenem resistant Acinetobacter baumannii in cancer patients. J Curr Microbiol Appl Sci. 2016;5(11):637–647. | ||
Nhu NTK, Lan NPH, Campbell JI, et al. Emergence of carbapenem-resistant Acinetobacter baumannii as the major cause of ventilator-associated pneumonia in intensive care unit patients at an infectious disease hospital in southern Vietnam. J Med Microbiol. 2014;63(Pt 10):1386–1394. | ||
Aneta G, Maria KM, Oleksandr O, Natalia K. Ventilator-associated pneumonia (VAP) caused by Acinetobacter baumannii in view of the microbial properties of the ESKAPE group in neighbouring countries–Poland and Ukraine. J Pre Clin Clin Res. 2017;11:111–115. | ||
Inchai J, Pothirat C, Bumroongkit C, Limsukon A, Khositsakulchai W, Liwsrisakun C. Prognostic factors associated with mortality of drug-resistant Acinetobacter baumannii ventilator-associated pneumonia. J Intensive Care. 2015;3:9. | ||
Siempos II, Athanassa Z, Falagas ME. Frequency and predictors of ventilator-associated pneumonia recurrence: a meta-analysis. Shock. 2008;30(5):487–495. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.