Back to Journals » International Medical Case Reports Journal » Volume 7
Never neglect the atmospheric pressure effect on a brain with a skull defect
Received 18 December 2013
Accepted for publication 24 January 2014
Published 26 March 2014 Volume 2014:7 Pages 67—69
DOI https://doi.org/10.2147/IMCRJ.S59410
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Hsiao-Yue Wee,1 Jinn-Rung Kuo1,2
1Department of Neurosurgery, Chi-Mei Medical Center, 2Department of Biotechnology, Southern Taiwan University of Science and Technology, Tainan, Taiwan
Abstract: Herein, we report an unusual case of a patient who presented with a severe, sinking skin flap after a decompressive craniectomy and ventriculoperitoneal shunt surgery due to a traumatic brain injury. After cranioplasty, the patient's neurological deficiency improved and was confirmed by transcranial Doppler sonography. In addition to discussing the pathogenesis of the sinking skin flap, we emphasize the importance of cranioplasty for neurological improvement and remind the surgeon to “never neglect the atmospheric pressure effect on a brain with a skull defect”.
Keywords: traumatic brain injury, decompressive craniectomy, hydrocephalus, cranioplasty
Introduction
Decompressive craniectomy is a common practice and considered to be a life-saving procedure in patients with severe traumatic brain injury.1 Post-traumatic hydrocephalus is a complication needing a ventriculoperitoneal shunt to divert cerebrospinal fluid.2 A sinking scalp flap occasionally develops after decompressive craniectomy and could be aggravated by a ventriculoperitoneal shunt.3–5 In such cases, the purpose of cranioplasty at the craniectomy site is to eliminate atmospheric pressure, restore the cerebrospinal fluid, and improve neurological status.6–10 Here we present an unusual case of a severe sinking scalp flap after decompressive craniectomy and ventriculoperitoneal shunt.
Case report
A 65-year-old male patient was transferred to our hospital with a large, right-sided skull defect and severe scalp depression. He had suffered a traumatic brain injury 5 months earlier and undergone decompressive craniectomy and removal of an intracranial hemorrhage at that time. Hydrocephalus developed one month after the operation and he received a ventriculoperitoneal shunt. However, his level of consciousness progressively deteriorated thereafter. On neurological examination, he was drowsy with a Glasgow Coma Scale score of E1V1M4 and profound left hemiplegia. A computed tomography (CT) brain scan revealed marked concavity of the brain at the craniectomy site associated with midline shift to the left (Figure 1). Transcranial Doppler sonography (Multi-Dop® X2, DWL; Elektronische Systeme GmbH, Schlotheim, Germany) was unable to detect the wave pattern of the blood flow in the middle cerebral artery of the concave brain. The impression was of sinking skin flap syndrome, so cranioplasty with bone cement was performed. Postoperatively, the patient was treated with hydration and bed rest for 3 days. Four days after his cranioplasty, follow-up CT images showed reversal of the midline shift with no significant complications in the underlying brain (Figure 2). Follow-up transcranial Doppler revealed a mean velocity of 30 cm per second in the right middle cerebral artery. After the surgery, the patient’s mentality gradually improved to an alert state and he was discharged with a Glasgow Coma Scale score of E34V1M5–6 one month after cranioplasty.
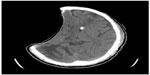  | Figure 1 Computed tomography scan of the axial brain showing sinking at the craniectomy site with extensive midline shift and the proximal tube of the ventricular peritoneal shunt. |
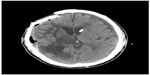  | Figure 2 Computed tomography scan showing reversal of the sunken brain contour and the midline shift after an autologous cranioplasty repair of the skull defect. |
Discussion
Decompressive craniectomy is widely used as a life-saving measure to treat intracranial hypertension following traumatic brain injury.1 However, it has been identified as a risk factor for changes in cerebrospinal fluid and development of post-traumatic hydrocephalus, for which a ventriculoperitoneal shunt may be necessary for diversion of cerebrospinal fluid.2
Mean atmospheric pressure is 1,033 cm H2O of water and equivalent to 14.7 psi.2 A ventriculoperitoneal shunt for hydrocephalus in patients with a skull defect may lead to excessive sinking at the craniectomy site as a result of the atmospheric pressure gradient, which could be aggravated by diversion of cerebrospinal fluid and change in position, especially when there is post-traumatic brain parenchymal atrophy or loss, as in our case.3,4 Thus, the goal of treatment in a patient with a sinking skin flap is to eliminate atmospheric pressure at the craniectomy site via cranioplasty.
However, performing a cranioplasty on a patient with a severe concave scalp flap at the craniectomy site does increase the risks of accumulation of fluid and formation of hematoma.5 To relieve the pressure gradient between the atmosphere and the intracranial space and to reverse the scalp depression and midline shift, temporary occlusion of the shunt device5 or reprogramming of the ventriculoperitoneal shunt to a higher valve pressure before cranioplasty6 have been recommended. However, our patient underwent cranioplasty without temporary occlusion or shunt reprogramming, but rather hydration and postoperative bedrest for 3 days. Follow-up CT of the brain showed good brain expansion with no formation of hematoma. Nevertheless, we recommend early combined cranioplasty and programmable shunts for patients with skull bone defects to avoid sunken skin in accordance with the suggestion of Carvi et al.7
In our case, the possible reason for decreasing cerebral blood flow in a brain with a skull defect can be explained as impairment of brain autoregulation (due to insult of previous traumatic brain injury) and the local compression effect of atmospheric pressure. Several important principles have been described with regard to intracranial dynamics in relation to the conventional physiological regulatory mechanism of cerebral blood flow, including pressure autoregulation, regulation of CO2, and regulation of metabolism.8 In an experiment by Schaller et al, cats that underwent hemicraniectomy showed decreased cerebral blood flow in normal brain tissue that lasted for at least one day.9 We thought it might be related to a local compression effect by atmospheric pressure; however, the normal brain without traumatic injury is able to restore cerebral blood flow in a few days. Therefore, cranioplasty is able to improve neurological status in patients with a skull bone defect.10 It reduces the local compression effect of atmospheric pressure, thereby improving brain autoregulation and enhancing cerebral hemodynamic status.9–10 This result is consistent with our previous study about neurological improvement after cranioplasty, increasing velocity of cerebral blood flow can be seen on transcranial Doppler.11
We report here an unusual case of a patient who presented with a severe sinking skin flap after decompressive craniectomy and ventriculoperitoneal shunt due to traumatic brain injury. This case underscores the importance of cranioplasty and reminds surgeons to “never neglect the atmospheric pressure effect on a brain with a skull defect”.
Disclosure
The authors report no conflicts of interest in this work.
References
Coplin WM, Cullen NK, Policherla PN, et al. Safety and feasibility of craniectomy with duraplasty as the initial surgical intervention for severe traumatic injury. J Trauma. 2001;50(6):1050–1059. | |
Mazzini L, Campini R, Angelino E, Rognone F, Pastore I, Oliveri G. Posttraumatic hydrocephalus: a clinical, neuroradiologic, and neuropsychologic assessment of long-term outcome. Arch Phys Med Rehabil. 2003;84(11):1637–1641. | |
Yamamura A, Sato M, Meguro K, Nakamura T, Uemura K, Makino H. [Cranioplasty following decompressive craniectomy. Analysis of 300 cases]. No Shinkei Geka. 1997;5(4):345–353. Japanese. | |
Kelley GR, Johnson PL. Sinking brain syndrome: craniotomy can precipitate brainstem herniation in CSF hypovolemia. Neurology. 2004;62(1):157. | |
Liao CC, Kao MC. Cranioplasty for patients with severe depressed skull bone defect after cerebrospinal fluid shunting. J Clin Neurosci. 2002;9:553–555. | |
Han PY, Kim JH, Kang HI, Kim JS. Syndrome of the sinking skin-flap secondary to the ventriculoperitoneal shunt after craniectomy. J Korean Neurosurg Soc. 2008;43(1):51–53. | |
Carvi Y, Nievas MN, Hollerhage HG. Early combined cranioplasty and programmable shunt in patients with skull bone defects and CSF-circulation disorders. Neurol Res. 2006;28(2):139–144. | |
Schaller B, Graf R. Different compartments of intracranial pressure and its relationship to cerebral blood flow. J Trauma. 2005;59(6):1521–1531. | |
Schaller B, Graf R, Sanada Y, Rosner G, Wienhard K, Heiss WD. Hemodynamic and metabolic effects of decompressive hemicraniectomy in normal brain. An experimental PET-study in cats. Brain Res. 2003;982(1):31–37. | |
Segal DH, Oppenheim JS, Murovic JA. Neurosurgical recovery after cranioplasty. Neurosurgery. 1994;34(4):729–731. | |
Kuo JR, Wang CC, Chio CC, Cheng TJ. Neurological improvement after cranioplasty – analysis by transcranial Foppler ultrasonography. J Clin Neurosci. 2004;11(5):486–489. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
