Back to Journals » Cancer Management and Research » Volume 11
Neutrophils infiltration and its correlation with human papillomavirus status in the oral squamous cell carcinoma
Authors Li C, Zhao L, Wang Q, Ma S, Sun J, Ma C, Liu J, Jing X, Ai D, Nan Z, Qu X
Received 22 January 2019
Accepted for publication 6 April 2019
Published 5 June 2019 Volume 2019:11 Pages 5171—5185
DOI https://doi.org/10.2147/CMAR.S202465
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Chen Li,1,2 Lei Zhao,1 Qingjie Wang,1 Shao Ma,3 Jintang Sun,1 Chao Ma,1 Jia Liu,1 Xuanxuan Jing,1 Dan Ai,1 Zhaodi Nan,1 Xun Qu1
1Institute of Basic Medical Sciences, QiLu Hospital of Shandong University, Jinan 250012, Shandong, People’s Republic of China; 2Department of Radiation Oncology, QiLu Hospital of Shandong University, Jinan 250012, Shandong, People’s Republic of China; 3Department of Breast Surgery, QiLu Hospital of Shandong University, Jinan 250012, Shandong, People’s Republic of China
Purpose: One of the cardinal etiological factors for oral squamous cell carcinoma (OSCC) is Human papillomavirus (HPV). Neutrophils were potential targets of immune therapy for patients with OSCC. The objective of this study was to determine if neutrophils density and HPV status can be used to define a high-risk category of patients in OSCC and to investigate the possible relationship between them.
Patients and methods: We performed immunohistochemistry to probe neutrophils infiltration and HPV (P16) expression in 81 patients with OSCC. Prognostic factors for cancer-related survival were evaluated by univariate and multivariate analyses. We used the detection of cytokines to investigate the possible molecular mechanisms between neutrophils infiltration and HPV status.
Results: There were significantly higher numbers of CD15+ neutrophils infiltration in OSCC tissues. Higher numbers of CD15+ neutrophils infiltration was related to stage Ⅲ,Ⅳ (p<0.001), poor grade (p<0.001), lymph node metastasis (p=0.014), and the higher preoperative neutrophil-lymphocyte ratio (NLR) (p<0.001). HPV-negative status was also associated with stage Ⅲ,Ⅳ (p=0.001), poor grade (p=0.002), lymph node metastasis (p=0.005), radiotherapy (p=0.038), and the higher NLR (p=0.002). The high density of neutrophils was associated with worse cancer-related survival time (p<0.001) and was an independent prognostic factor for OSCC, while the HPV-positive group was associated with better cancer-related survival time. Moreover, high density of neutrophils was correlated with HPV-negative status in OSCC (p<0.001). Detection of cytokines and chemokines revealed that one of the chemotactic factors of neutrophils, IL-8, was exhibited relatively low expression by HPV-positive OSCC cells, whereas HPV-negative OSCC cells were found to drive an IL-8 secretion profile.
Conclusion: Neutrophils infiltration and HPV status appear to be prognostic parameters for OSCC. Overexpression of HPV18 E7 on OSCC cells may participate in depressing neutrophils infiltration to some extent through downregulating expression of IL-8.
Keywords: human papillomavirus, neutrophils, oral squamous cell carcinoma, IL-8
Introduction
It is noticed that the incidence of head and neck squamous cell carcinomas (HNSCCs) is increasing compared with carcinomas of other origins, which cause more than 300,000 deaths worldwide every year and attract public attention. It can occur in multiple sites, and oral cancer, especially oral squamous cell carcinoma (OSCC) accounts for one-third of HNSCCs, so OSCC has become the most common type of HNSCCs.1,2 Over the past few decades, the survival of OSCC remains poor which could be attributed to distant metastasis and local recurrence,3 even though the technology of diagnosis and treatment is more advanced than before. It has been proved that chronic inflammation is one uniform characteristic of the tumor microenvironment by epidemiological and experimental evidence.4–6 As we all know, inflammatory cells include neutrophils, macrophages, plasmocytes, lymphocytes, eosinophilic granulocytes etc, among which neutrophils accounted for 50–70% in total circulating leukocytes.5–8 Neutrophils which are famous for their antimicrobial functions have been proved to be a critical factor in the development of tumor with the exception of macrophages. Nevertheless, tumor-associated neutrophils (TANs) have been demonstrated playing important role in the pathological processes of the malignant tumor and in the modulation of the antitumor immunity.9 Evidence shows that neutrophil infiltration within tumors may be associated with worse outcomes in metastatic and localized clear cell carcinomas,10 as well as inbronchoalveolar carcinomas.11 However, there is few systematical investigation of the relationship between TAN infiltration and clinical prognosis with tumors, especially with OSCC. In addition, it seems to be important to understand the regulation of inflammatory status in the tumor microenvironment.
OSCC is a head and neck cancer with a marked increase in incidence. Infections with oncogenic high-risk (HR) human papillomaviruses (HPV) may lead to this development as they are increasingly found in OSCC. Based on the published paper, in addition to tobacco and alcohol, viruses such as HPV are also the risk factor for OSCC.12 According to the recently published series, HPV plays an important role in the occurrence and development of OSCC.13 P16 protein is a great substitute for carcinogenic HPV in the oropharyngeal tissues in immunohistochemistry.14,15 Research has suggested that tumor-infiltrating immunocytes between HPV-positive and HPV-negative tumors was quite different. There were more CD8+ T-cells infiltrating in HPV+ cancer, which could lead to better clinical outcomes.16 However, the effects of oncogenic HPV on immune cells are poorly studied. Here, the objective was to determine if neutrophils density and HPV status can be used to define a HR category of patients in OSCC and to investigate the possible relationship between them.
Material and methods
Patients and specimens
Institutional Medical Ethics Committee of the Qilu Hospital of Shandong University reviewed and approved this study, and each patient had signed informed consent. In this study, a series of 81 patients with OSCC who underwent primary and curative resection at Qilu Hospital of Shandong University, from January 2007 to December 2011, were included in the analysis. All the 81 patients underwent neck dissection, 30 of which had lymph node metastasis. All the diagnoses were made following the Pathology and Genetics of Head and Neck Tumors of the World Health Organization Classification of Tumors.17 Clinicopathological, tumor-specific, and adjuvant therapy data were obtained from the patients’ medical recording system of Qilu Hospital. Descriptive statistics were used to summarize the demographic and clinical characteristics of the patients. Conventional clinicopathologic parameters, including age, gender, smoking history, drinking history, clinical stage, histological grade, lymph node metastasis, tumor extension radiotherapy, and chemotherapy are recorded in Tables 1 and 2.
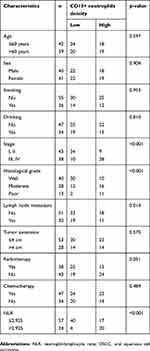 | Table 1 The relationship of CD15+ neutrophils count in OSCC and clinicopathologic parameters |
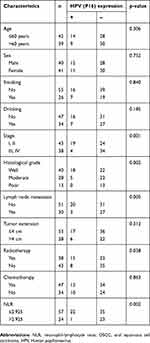 | Table 2 The relationship of HPV (P16) expression in OSCC and clinicopathologic parameters |
The study was approved by ethics committee of Qilu Hospital of Shandong University and consented by all participants (KYLL-2017(KS)-281), a parent or legal guardian signed informed consent for any patient under the age of 18 years, and that this was conducted in accordance with the Declaration of Helsinki.
Tissue microarray (TMA) and immunohistochemistry
We used the H&E staining to premarked the representative areas which were away from the hemorrhagic and necrotic materials in the paraffin-embedded wax blocks. TMA included 81 patients’ duplicates of 1-mm-diameter cylinders in the tumor center that designated as peritumoral tissues and tumor, respectively. Each block that contained 42 cylinders was sectioned at a thickness of 4-μm thick. We placed the blocks on the glass slides coated with 3-aminopropyl triethoxysilane. Thus, this is preparation for immunohistochemistry.
To investigate whether the density of neutrophils infiltration correlated with the HPV expression in OSCC, we detected the expression of CD15 (a marker of human neutrophils), P16 (a marker for HPV) in 81 patients with OSCC by immunochemical staining. Infiltration of neutrophils was detected with a rabbit anti-CD15 (FUT4) polyclonal antibody (19497-1-AP, Proteintech Group, Inc., USA), and P16 was detected with a mouse anti-P16INK4a monoclonal antibody (MA5-17093, Thermo Scientific, USA). Microwave-repaired antigens on the sections and then the tissue sections were immersed by 3% H2O2 to block endogenous oxidase. Antigen impurities were blocked by sera at room temperature for 20 or 30 mins and then washed three times with 0.01M PBS, the sections were incubated with primary antibody anti-P16 (dilution 1:200) or anti-CD15 (dilution 1:200) overnight at 4°C. The sections were washed with 0.01M PBS and then incubated with secondary antibody for 20 mins. The anti‐biotin‐labeled peroxidase solution was used to terminate the reaction. Finally, diaminobenzidine colorized the sections, counterstain with hematoxylin, dehydrate with absolute ethanol and fix with neutral glue, then observe the nuclei under 200× microscope.
CD15 and P16 evaluation
Two independent observers who did not know prognosis or clinicopathologic variables use the 2006 magnification of light microscopy to evaluate the TMAs. Then, another conclusive judgment evaluated the disagreements for the third time. We calculated the positive cells in each 1-mm- diameter cylinder and used them as a repeated mean (cell/core) to obtain the CD15+ neutrophils count. The median value cutoff was designed by cut-point survival analysis. For patients with positive P16, according to previous reports, the positive cell rate of tumor cells stained with P16 immunohistochemistry is 25% or more, that is, the surgical specimens are positive.18 An experienced pathologist determines whether P16 is positive.
CXCL8 evaluation
To quantify the expression of CXCL8 expression level, a score was created based on two criteria: I. The intensity of CXCL8 staining was classified by the following scale: negative=0; weak=1; and strong=2. II. We calculated and classified the percentage of immunoreactive tumor cells on a 5-point scale (0=0%, 1=1–25%, 2=26–50%, 3=51–75%, 4=76–100%).19 For statistical analysis, a final score of 0–1 indicates low gene expression; a score of 2–4 indicates high expression of CXCL8
Cell culture
Human tongue squamous cell carcinoma cell line Cal-27 which were purchased from Culture Collection of Chinese Academy of Science (Shanghai, China) expanded with Dulbecco’s modified Eagle’s medium (DMEM, Hyclone, Thermo scientific, USA) with 10% fetal bovine serum (FBS, Gibco Life Technologies, Carlsbad, CA, USA) then cultured in an incubator with a humidified atmosphere containing 5% CO2 at 37°C for 48 hrs.
Plasmid DNA transient transfection
HPV18-E7-HA-Flag-targeting Plasmid DNA sequences inserted into the PC-DNA 3.1 vector were purchased from Shanghai Boshang biotechnology company. An empty vector PC-DNA 3.1 was used as a negative control. The cal-27 cells were plated on a six-well plate with a density of 60% before transfection. Then, cells were transfected with HPV18-E7-HA-Flag-DNA (2 μg plasmid) by 5 mL LipofectamineTM 2000 (Invitrogen, USA) according to the manufacturer’s instructions.
Quantitative RT-PCR analysis of HPV18-E7 expression in different transfection groups
According to the manufacturer’s protocol, we extracted the total RNA from three groups of cells at the time of 48 hrs after (HPV18-E7-HA-Flag-DNA, PC-DNA3.1 and control), using TRIZOL Reagent (Invitrogen, Waltham, MA, USA). And it used for detection of the relative expression of RNA. We used the First Strand cDNA Synthesis kit (TOYOBO, JAPAN) to reverse transcribe 2 μg of each RNA samples to cDNA, and used the SYBR Green qPCR kit (Takara, Japan) to carry out the real-time quantitative transcription polymerase chain reaction (qRT-PCR). Cycling conditions were as follows: 30 s at 95°C, followed by 40 cycles of 5 s at 95°C, 10 s at 55°C, 15 s at 72°C, and recorded by Melting Curve Analysis finally. According to previous studies, we calculated and normalized relative expression values to b-actin using the comparative CT method.20 Each experimental reaction was performed in triplicate. The primer sequences used in this study are listed in Table 6.
 | Table 3 The relationship of HPV(P16) expression and density of neutrophils |
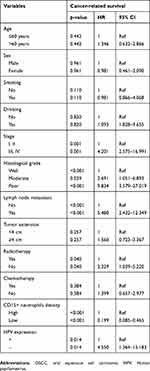 | Table 4 Univariate survival analysis in OSCC patients |
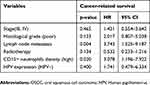 | Table 5 Multivariate survival analysis in OSCC patients |
 | Table 6 Primer sequences and size of PCR products |
Western blot analysis of E7-HA-Flag expression
Total cellular protein was extracted by using ice-cold RIPA buffer containing 1% phenylmethylsulfonyl fluoride for 60 mins. The protein concentration in the medium was measured by using a BCA Protein Assay Kit (Beyotime). The collected lysates were centrifuged at 15,000g for 10 mins at 4°C. Twenty-five micrograms of total protein was subjected to 8% SDS-polyacrylamide gels and transferred onto polyvinylidene difluoride membranes which were blocked with 5% non-fat milk blocking buffer for 1 hr. The blots were incubated with the primary monoclonal antibody against monoclonal rabbit anti-human HA-Tag (dilution 1:500, 3724S, CST; USA) and antibody against β-actin (dilution 1:1,000, Abcam, Cambridge, UK). Then, the membranes were washed with TBST and incubated with horseradish peroxidase-linked secondary antibody (dilution 1:5,000; Beyotime). The results were visualized through using enhanced chemiluminescence reagent (Thermo Fisher Scientific, USA). The density of the protein bands was analyzed by the software ImageJ (NIH, Bethesda, MD, USA).
Detection of cytokines and chemokines
Cell-free supernatants were collected from cultures of CAL-27 cells at the time of 48 hrs after the HPV18 E7 transfection process. All the steps were performed in a cell ultra clean platform. The desired solution which was used for the detection of cytokines was collected and stored in a −80°C refrigerator until analysis. As described in the previous study, cytokines and chemokines in the supernatant were quantified using the bio-plex protein array system (bio-rad Laboratories, Hercules, CA),21 such as IL-1β, IL-8, G-CSF, and GM-CSF.
qRT-PCR analysis of IL-8
cDNA was used for qRT-PCR analysis, and the mRNA expression of iL-8 in the three groups was detected after verifying the transfection efficiency of E7-HA-Flag. We listed the primers information for b-actin, IL-8 in Table 6.
Statistical analysis
Statistical analyses were performed by using Graph Prism Program, Version 7.0 (GraphPad Software, Inc., La Jolla, CA, USA) and the SPSS statistical software package (Version 20.0; IBM Corporation, Armonk, NY, USA). The correlation of clinicopathologic parameters and CD15+ neutrophils density infiltration as well as HPV18-E7 expression was examined by using the Chi-square test. Correlations of infiltration of neutrophils and HPV18-E7 expression were analyzed by Spearman’s rho coefficient test. Kaplan–Meier curves and log-rank were used for survival analysis to test significance. Statistical analysis included single univariate analysis and multifactor analysis. Survival time was calculated from the date of surgery to the event or the last follow-up. Univariate Cox regression analysis was performed using disease recurrence or death as a significant level of outcome p<0.05. Student t test was used to evaluate the results of qRT-PCR.
Tumor size was defined referring to the classification of TNM (T2= tumor size ≤4 cm): tumor size ≤4 cm vs tumor size >4 cm. The neutrophil-lymphocyte ratios (NLRs) were analyzed in two-categories according to the optimal cutoff values determined using the receiver operating characteristic curve analysis and You den’s index (sensitivity + specificity − 1): NLR ≤2.85 vs NLR >2.85 (Figure S1).
Results
Patients’ characters
Eight-one patients (100%) with OSCC were included in the analysis. Variables of pathological characteristics of patients are shown in Tables 1 and 2. The median age of 81 patients was 57.8 years (ranging from 17 to 79 years), and 49.4% of these patients were male. The 38 patients had T3/T4 tumor, and the 13 patients had the highest pathological grade. Some patients had positive lymph node metastasis, and some of them were in stage III/IV.
IHC analysis of neutrophils infiltration, HPV(P16) expression, and their correlation
Density of neutrophils in OSCC tissues, and its relationship with clinical pathological features
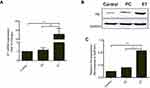
In order to analyze neutrophils infiltration in OSCC, tumor specimens from 81 patients diagnosed with OSCC were stained for CD15, a marker of human neutrophils. As shown in Figure 1A, there were many CD15+ neutrophils in OSCC (Figure 1A a). Outside of the blood vessels in each 1-mm-diameter tissue, the number of CD15+ cells ranged from 1 to 2290/core. The median density was 41.0/core. If the average number of repetitions in a condition was less than 41.0, it was classified as a low-density group; otherwise, it was classified as a high-density group. The percentage of high CD15+ neutrophil density was 45.7% in all tumor specimens. In OSCC tissues, the majority of CD15+ cells were closely adjacent to tumor cells, and they mainly penetrated into the tumor tissues (Figure 1A b, c) or tumor infiltration margins (Figure 1A d). Neutrophils surrounding the nests were distributed in the stroma (Figure 1A b) or in the nests (Figure 1A c) in the intratumoral region. The high-density CD15+ neutrophils had been analyzed to be associated with higher clinical stage (p<0.001), poor histological grade (p<0.001), lymph node metastasis (p=0.014), and the higher preoperative NLR (p<0.001) by using Chi-square test. There were no significant differences in age, gender, smoking history, drinking history, tumor size, radiotherapy, and chemotherapy (Table 1).
Immunostaining of P16 in OSCC tissues
To investigate the HPV expression in OSCC, we detected the expression of P16 (a marker of HPV) in 81 OSCC by immunochemical staining. As shown in Figure1B, P16 expression was upregulated in tumor tissues (Figure 1B). In OSCC tissues, P16 expression has obvious heterogeneity. Among the 81 cases, 58 were negative, 23 were positive. The HPV-negative status had been analyzed to be associated with higher clinical stage (p=0.001), poor histological grade (p=0.002), lymph node metastasis (p=0.005), radiotherapy (p=0.038), and higher NLR (p=0.002) as shown in Table 2.
Correlation of HPV expression on OSCC with neutrophils count
The density of CD15+ neutrophils was detected to be negatively correlated with HPV (P16) expression (Table 3) by using Spearman’s rho coefficient test analysis. In other words, high-density neutrophils were less infiltrated in the HPV (P16) positive expression group (21.74%, Figure 1C b), while high-density neutrophils were more infiltrated in the HPV (P16) negative group (55.17%, Figure 1C a).
Analysis of the effects of neutrophils infiltration and HPV expression on OSCC patients’ survival
Eighty-one patients were followed up to assess the effect of neutrophil infiltration and HPV expression on OSCC survival. The CD15+ neutrophil density, HPV (P16) expression, lymph node metastasis, and high clinical staging were detected to be associated with cancer-related survival time by using Kaplan–Meier survival analysis. The median cancer-related survival of this cohort was 35 months (range 6–80 months). Kaplan–Meier curves of cancer-related survival based on Neutrophils Infiltration and HPV Expression are shown in Figure 2. Survival analysis showed a significant difference in cancer-related survival among different neutrophils Infiltration (p<0.001) and HPV Expression categories (p=0.007) in these entire data. Furthermore, we first performed univariate analysis with COX proportional hazard model, and then selected variables related to cancer-related survival time to be included in multivariate survival analysis, so as to test whether the above parameters were independent prognostic factors of OSCC patients (Tables 4 and 5). As shown in Table 5, high-density CD15+ neutrophils and lymph node metastasis were independent prognostic factors of OSCC patients.
HPV may participate in depressing neutrophils infiltration to some extent through downregulating expression of IL-8
It was because of the low level of HPV expression in the cal-27 cell line that we constructed HPV18-E7-HA-Flag-targeting Plasmid DNA vectors and transfect them into cal-27 to simulate the in vivo environment. By qRT-PCR (Figure 3A) and western blot analysis (Figure 3B and C), the expression of HPV18 E7 in the HPV18 E7 transfection group was significantly higher than that in the empty vector transfection group and the control group. It was precise because the IHC results showed a negative correlation between HPV (P16) expression and CD15+ neutrophil density that we speculated that there might be some relationship between the two. The possible effect of overexpression of HPV in OSCC on neutrophil infiltration was investigated. The overexpression of HPV18 E7 was investigated to possibly downregulate the expression of IL-8 (p<0.05), but not IL-1β, G-CSF and GM-CSF in Cal-27 cell line (Figure 4) by detecting cytokines and chemokines. Moreover, HPV18 E7 overexpression was found possibly downregulate the mRNA expression of IL-8 (Figure 4) by using qRT-PCR. We confirmed that the results of gene level and protein secretion level were consistent. Previous studies have found that IL-8 is the important chemokine for neutrophils.22–24
In order to analyze the expression of IL-8 in OSCC, tumor specimens from 81 patients diagnosed with OSCC were stained for CXCL8. As shown in Figure 5, CXCL8 expression was upregulated in tumor tissues (Figure 5). It was found that the density of CXCL8 expression was detected to be positively correlated with CD15+ neutrophils (Table 7) and negatively correlated with HPV (P16) expression (Table 7) by using Spearman’s rho coefficient test analysis.
 | Table 7 The relationship of IL-8 expression with the density of neutrophils and the relationship of IL-8 expression with HPV (P16) |
The results revealed that HPV may participate in depressing neutrophils infiltration to some extent through downregulating expression of IL-8.
In addition, we validated the results based on mRNA expression data from the Gene Expression Omnibus (GEO) database and The Cancer Genome Atlas (TCGA) database. In (GEO) database (GSE3292 and GSE6791 datasets), the expression of IL-8 (CXCL8) was significantly different. The expression of CXCL8 in HPV-group was significantly up-regulated (Figures S2 and S3). In the TCGA database (
Discussion
Increasing studies have shown that inflammation is related to the development of tumors.5,25 Many of the leukocytes including macrophages and neutrophils involved in the tumor microenvironment may play a dual role in the development and progression of the tumor. TANs have been considered to be very insignificant in tumor-associated immune responses due to their short lifespan and adequate phenotypic differentiation. However, recent studies have pointed out that neutrophils play a very important role in the occurrence and development of tumors, which is another type of tumor-infiltrating myeloid cells in addition to the well-behaved TAM.9 There have been many reports that neutrophil infiltration is associated with patients’ clinical prognosis, that more neutrophil infiltration is associated with poorer prognosis and shorter overall survival.26–29 Abundant of tumor-associated macrophage and neutrophil infiltration predicted poor prognosis in HPV-negative head and neck cancers.30–32 However, in OSCC tissues, there was little research on neutrophil infiltration, and the regulatory mechanism was not yet clear.
In recent years, HPV has been linked to squamous cell carcinoma of the head and neck in addition to alcohol consumption and smoking.33–37 HPV+ HNSCC, which has specific clinical and molecular characteristics, is a stand-alone subtype, which has the characteristics of younger patients, no obvious history of smoking and drinking, more sensitive to radiotherapy and chemotherapy, and better prognosis.38 In this study, the expression of HPV and the density of neutrophils in OSCC tissues were explored. The relationship between them and the possible effect of HPV on neutrophils in OSCC tissues and the possible mechanism were also investigated.
First of all, we explored that the majority of CD15+ cells were closely adjacent to tumor cells, and they mainly penetrated into the tumor tissues or tumor infiltration margins (Figure 1A). The lymph node metastasis group’s infiltrated CD15+ neutrophils density was significantly higher than the no lymph node metastasis group, which was the same as the clinical stage III/IV and poor histological grade group (Table 1). This finding gave backing to the strong association between CD15+ neutrophil infiltration and poor clinical outcomes. Furthermore, we found by survival analysis that high CD15+ neutrophil infiltration was an independent prognostic factor in OSCC patients undergoing primary and therapeutic resection, which seems to be consistent with the observation of other groups in other tumors.26–28 Previously published articles have shown that neutrophils are involved in tumorigenesis by releasing nitric oxide derivatives and reactive oxygen species (ROS).39,40 It was noted that ROS of neutrophil origin, for example, MPO-mediated HOCl formation, had a relationship with point mutation and DNA damage.39 Furthermore, HOCl can also activate multiple proteolytic enzymes, such as MMP-2, to enhance the activity of MMP-9 by inactivating the tissue inhibitor metalloprotease 1, thus facilitating the invasion and metastasis of cancer cells.41 Neutrophils also inhibit the effector function and proliferation of T-cells by secreting stored arginase 1. The degradation of extracellular arginine by arginase 1 is a necessary factor for the normal activity of T-cells.42,43 All of these studies revealed that neutrophils can promote the development of tumor through a series of mechanisms, as well as the invasion and metastasis of cancer cells. However, the causes and mechanisms of large numbers of neutrophil infiltration in tumors are still poorly studied.
Next, the expression of HPV in OSCC tissues and its possible role in cancer tissues were investigated in this study. The expression of P16 was detected by immunohistochemistry to study the expression of HPV in OSCC. Our study showed that HPV was highly expressed in OSCC tissues (Figure 1B). We also performed statistical analysis, and the results showed that the expression of P16 was associated with lymph node metastasis (p=0.000), and the clinical stage was relatively high (p=0.001) (Table 2). Survival analysis suggested that the overexpression of HPV in OSCC was associated with better clinical outcomes. Consistent with previous studies on tonsillar carcinomas44 and other head and neck squamous cancer,45 this result indicated that the overexpression of HPV in OSCC was correlated with better clinical outcomes by survival analysis. The clinical manifestations of HPV-related oropharyngeal squamous cell carcinoma are significantly different from those of non-HPV-related oropharyngeal squamous cell carcinoma, and due to the high sensitivity to radiotherapy, it has been reported that the clinical efficacy of HPV-related OSCC is significantly better than that of non-HPV-related oropharyngeal squamous cell carcinoma.34,35,46 The reason for the better survival outcome of HPV+ OSCC patients was not completely clear. Based on the abundance of head and neck lymph nodes and blood vessels, the activation of local immunity may play a role in limiting the spread and metastasis of HNSCC and/or enhancing the response of patients to treatment, which may be the reason for this phenomenon16 Therefore, the study on the characterization of neutrophil infiltration in tumor microenvironment may be helpful to further understand the reasons for the improved prognosis of OSCC.
Furthermore, the results of our study explored that neutrophils were less infiltrated in the HPV (P16) expression positive group (Figure 1C a), while neutrophils were more infiltrated in the HPV (P16) expression negative group (Figure 1C b). HPV expression was found to be negatively correlated with CD15+ neutrophil density in OSCC tissues with Spearman’s rho coefficient detection (Table 3). Based on this result, HPV-negative expression in OSCC affected neutrophil infiltration and location. We detected cytokines and chemokines in different HPV18 E7 transfection groups in order to explore how HPV18 E7 affects neutrophils in OSCC (Figure 4). Data from the chemokine protein array demonstrated that the conditioned medium from HPV-negative OSCC increased the expression of IL-8, which was a member of the chemokine family. In order to further explore the possible mechanism of HPV blocking CD15+ neutrophil infiltration, we performed immunohistochemical staining on TMA of 81 patients. We found that some of them had high expression of IL-8. As shown in Figure 5, CXCL8 expression was upregulated in tumor tissues (Figure 5). In addition, the density of CXCL8 expression was detected to be positively correlated with CD15+ neutrophils (p<0.001, Table 7) and negatively correlated with HPV(P16) expression (p<0.001, Table 7) by using Spearman’s rho coefficient test analysis. The results of this in vivo experiment were highly consistent with those of our in vitro experiment. Therefore, combined with in vitro experiments, we could conclude that HPV may participate in inhibiting neutrophils infiltration to some extent through downregulating expression of neutrophil chemokine, IL-8. Previous reports have shown that squamous cell carcinoma cells of the head and neck are capable of expressing chemokines.47–49 However, the secretion of these and other chemokines increased substantially from HPV-negative cells rather than HPV-positive cells in the conditioned medium. Indeed, previous studies have reported the expression of IL-8 in HPV-negative oropharyngeal cancer, and our study is consistent with this conclusion, while in OSCC, the data on how the status of HPV affect the role of neutrophils through the expression of neutrophil chemokines is very limited.50 Our results suggest that HPV status plays a role in the recruitment of leukocyte subsets in OSCC tumor microenvironment and affects inflammatory activities. Given that neutrophils can also be involved in tumor development and metastasis in a number of ways,51–54 the association between HPV-negative status and neutrophil infiltration may be another reason for poor clinical outcomes in cancer with HPV-negative status. To sum up, this phenomenon was first detected in OSCC patients, which was the first time that both HPV-negative expression and neutrophil abundance revealed poor clinical outcomes. Neutrophil infiltration in tumor cells was correlated with HPV-negative status in tumor cells. The HPV-negative OSCC cells may attract more neutrophils by releasing the significant neutrophil chemokine IL-8, whereas in HPV-positive OSCC cells this molecular mechanism is not obvious. The overexpressed HPV may participate in inhibiting the accumulation of neutrophils in the tumor site by downregulating the expression of IL-8 in OSCC.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81772879) and National Key Research and Development Program of China (2018YFC1002803).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Brauswetter D, Dános K, Gurbi B, et al. Copy number gain of PIK3CA and MET is associated with poor prognosis in head and neck squamous cell carcinoma. Virchows Archiv. 2016;468(5):579–587. doi:10.1007/s00428-016-1905-1
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386.
3. Sasahira T, Kurihara M, Uk B, et al. Downregulation of miR-126 induces angiogenesis and lymphangiogenesis by activation of VEGF-A in oral cancer. Br J Cancer. 2012;107(4):700–706.
4. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet (London, England). 2001;357(9255):539–545.
5. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420(6917):860–867.
6. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674.
7. Carina S, Kerstin L, Bernd N, Zaenker KS, Frank E. Neutrophil granulocytes promote the migratory activity of MDA-MB-468 human breast carcinoma cells via ICAM-1. Exp Cell Res. 2010;316(1):138–148.
8. Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65(19):8896–8904.
9. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11(8):519–531.
10. Donskov F, von der Maase H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J Clin Oncol. 2006;24(13):1997–2005. doi:10.1200/JCO.2005.03.9594
11. Wislez M, Rabbe N, Marchal J, et al. Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: role in tumor progression and death. Cancer Res. 2003;63(6):1405–1412.
12. Petersen PE. Strengthening the prevention of oral cancer: the WHO perspective. Community Dent Oral Epidemiol. 2005;33(6):397–399. doi:10.1111/j.1600-0528.2005.00251.x
13. Vidal L, Gillison ML. Human papillomavirus in HNSCC: recognition of a distinct disease type. Hematol Oncol Clin North Am. 2008;22(6):1125–42, vii. doi:10.1016/j.hoc.2008.08.006
14. Doescher J, Veit JA, Hoffmann TK. [The 8th edition of the AJCC Cancer Staging Manual: updates in otorhinolaryngology, head and neck surgery]. Hno. 2017;65(12):956–961. doi:10.1007/s00106-017-0391-3
15. Mizumachi T, Homma A, Sakashita T, Kano S, Hatakeyama H, Fukuda S. Confirmation of the eighth edition of the AJCC/UICC TNM staging system for HPV-mediated oropharyngeal cancer in Japan. Int J Clin Oncol. 2017;22(4):682–689. doi:10.1007/s10147-017-1107-0
16. Oguejiofor K, Hall J, Slater C, et al. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV-positive oropharyngeal squamous carcinoma. Br J Cancer. 2015;113(6):886–893.
17. Barnes L, Eveson J, Reichart P, Sidransky D. World Health Organization Classification of Tumours. Pathology and Genetics of Head and Neck Tumours. Lyon: IARC Press; 2005.
18. Klaes R, Friedrich T, Spitkovsky D, et al. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92(2):276–284.
19. Gotanda Y, Akagi Y, Kawahara A, et al. Expression of monocarboxylate transporter (MCT)-4 in colorectal cancer and its role: MCT4 contributes to the growth of colorectal cancer with vascular endothelial growth factor. Anticancer Res. 2013;33(7):2941–2947.
20. Zhu L, Guo J, Zhu J, Zhou C. Enhanced expression of EsWAX1 improves drought tolerance with increased accumulation of cuticular wax and ascorbic acid in transgenic Arabidopsis. Plant Physiol Biochem. 2014;75:24–35.
21. Meixiang Y, Chunhong M, Shuxun L, et al. Hypoxia skews dendritic cells to a T helper type 2-stimulating phenotype and promotes tumour cell migration by dendritic cell-derived osteopontin. Immunology. 2010;128(1pt2):e237–e249.
22. Kobayashi Y. Neutrophil infiltration and chemokines. Crit Rev Immunol. 2006;26(4):307–316.
23. Himmel ME, Crome SQ, Ivison S, Piccirillo C, Steiner TS, Levings MK. Human CD4+ FOXP3+ regulatory T cells produce CXCL8 and recruit neutrophils. Eur J Immunol. 2011;41(2):306–312.
24. Gijsbers K, Gouwy M, Struyf S, et al. GCP-2/CXCL6 synergizes with other endothelial cell-derived chemokines in neutrophil mobilization and is associated with angiogenesis in gastrointestinal tumors. Exp Cell Res. 2005;303(2):331–342.
25. Cancer: MA. Inflaming metastasis. Nature. 2009;457(7225):36–37.
26. Zhao JJ, Pan K, Wang W, et al. The prognostic value of tumor-infiltrating neutrophils in gastric adenocarcinoma after resection. PLoS One. 2012;7(3):e33655.
27. Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol. 2009;27(28):4709–4717.
28. Li YW, Qiu SJ, Fan J, et al. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol. 2011;54(3):497–505.
29. Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137(2):425–428.
30. He KF, Zhang L, Huang CF, et al. CD163+ tumor-associated macrophages correlated with poor prognosis and cancer stem cells in oral squamous cell carcinoma. Biomed Res Int. 2014;2014:838632.
31. Trellakis S, Bruderek K, Dumitru CA, et al. Polymorphonuclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int J Cancer. 2011;129(9):2183–2193.
32. Huang SH, Waldron JN, Milosevic M, et al. Prognostic value of pretreatment circulating neutrophils, monocytes, and lymphocytes in oropharyngeal cancer stratified by human papillomavirus status. Cancer. 2015;121(4):545–555.
33. Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–720.
34. Fischer CA, Zlobec I, Green E, et al. Is the improved prognosis of p16 positive oropharyngeal squamous cell carcinoma dependent of the treatment modality? Int J Cancer. 2010;126(5):1256–1262.
35. Pernille L, Eriksen JG, Annelise K, et al. The influence of HPV-associated p16-expression on accelerated fractionated radiotherapy in head and neck cancer: evaluation of the randomised DAHANCA 6&7 trial. Radiother Oncol. 2011;100(1):49–55.
36. Marur S, D‘Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11(8):781–789.
37. Berman TA, Schiller JT. Human papillomavirus in cervical cancer and oropharyngeal cancer: one cause, two diseases. Cancer. 2017;123(12):2219–2229.
38. Elrefaey S, Massaro MA, Chiocca S, Chiesa F, Ansarin M. HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol Ital. 2014;34(5):299–309.
39. Gungor N, Knaapen AM, Munnia A, et al. Genotoxic effects of neutrophils and hypochlorous acid. Mutagenesis. 2010;25(2):149–154.
40. Sandhu JK, Privora HF, Wenckebach G, Birnboim HC. Neutrophils, nitric oxide synthase, and mutations in the mutatect murine tumor model. Am J Pathol. 2000;156(2):509–518.
41. De Larco JE, Wuertz BR, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res. 2004;10(15):4895–4900.
42. Rita R, Gaia B, Luca M, et al. IL-8 induces exocytosis of arginase 1 by neutrophil polymorphonuclears in nonsmall cell lung cancer. Int J Cancer. 2010;125(4):887–893.
43. Rodriguez PC, Quiceno DG, Zabaleta J, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64(16):5839–5849.
44. Klussmann JP, Gultekin E, Weissenborn SJ, et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol. 2003;162(3):747–753.
45. Chen X, Yan B, Lou H, et al. Immunological network analysis in HPV associated head and neck squamous cancer and implications for disease prognosis. Mol Immunol. 2018;96:28–36.
46. Okami K. Clinical features and treatment strategy for HPV-related oropharyngeal cancer. Int J Clin Oncol. 2016;21(5):827–835.
47. Marcus B, Arenberg D, Lee J, et al. Prognostic factors in oral cavity and oropharyngeal squamous cell carcinoma. Cancer. 2004;101(12):2779–2787.
48. St John MA, Li Y, Zhou X, et al. Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130(8):929–935.
49. Partlova S, Boucek J, Kloudova K, et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology. 2015;4(1):e965570.
50. Al-Sahaf S, Hunter KD, Bolt R, Ottewell PD, Murdoch C. The IL-1/IL-1R axis induces greater fibroblast-derived chemokine release in human papillomavirus-negative compared to positive oropharyngeal cancer. Int J Cancer. 2019;144(2):334–344.
51. Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103(33):12493–12498.
52. Tazzyman S, Lewis CE, Murdoch C. Neutrophils: key mediators of tumour angiogenesis. Int J Exp Pathol. 2009;90(3):222–231.
53. Kuang DM, Zhao Q, Wu Y, et al. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011;54(5):948–955.
54. Shojaei F, Singh M, Thompson JD, Ferrara N. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc Natl Acad Sci U S A. 2008;105(7):2640–2645.
Supplementary materials
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.