Back to Journals » Journal of Pain Research » Volume 12
Neuropathic Characteristics In Patients With Persistent Idiopathic Facial Pain
Authors Sukenaga N, Matsuki Y , Maeda L, Nagai T, Hashimoto K, Takao Y, Hirose M
Received 4 June 2019
Accepted for publication 17 September 2019
Published 27 September 2019 Volume 2019:12 Pages 2801—2805
DOI https://doi.org/10.2147/JPR.S218332
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Norihiko Sukenaga,1 Yuka Matsuki,2 Lynn Maeda,3 Takako Nagai,1 Kazuma Hashimoto,1 Yumiko Takao,1 Munetaka Hirose1
1Department of Anesthesiology and Pain Medicine, Hyogo College of Medicine, Hyogo, Japan; 2Department of Anesthesiology and Reanimatology, Faculty of Medicine Sciences, University of Fukui, Fukui, Japan; 3Department of Anesthesiology and Pain Management, Nishinomiya Municipal Central Hospital, Hyogo, Japan
Correspondence: Munetaka Hirose
Department of Anesthesiology and Pain Medicine, Hyogo College of Medicine, 1-1 Mukogawa-cho, Nishinomiya, Hyogo 663-8501, Japan
Tel +81-798-45-6392
Email [email protected]
Background: Persistent idiopathic facial pain (PIFP) is a subtype of painful cranial neuropathies and other facial pains. The involvement of neuropathic mechanisms in PIFP, however, remains controversial. Using the Douleur Neuropathique 4 (DN4) questionnaire, the present study examined neuropathic characteristics in patients with PIFP.
Methods: The multi-institutional retrospective study collected the following clinical data from 205 consecutive patients with adult chronic pain: gender, age, BMI, diseases causing chronic pain, disease duration, visual analogue scale score of pain strength, and DN4 score. To compare neuropathic characteristics between PIFP and postherpetic neuralgia (PHN), we selected patients with PIFP (n=19) and patients with PHN (n=33), and performing a case–control study in which each patient with PHN or PIFP was matched by age and gender (n=16 in each group).
Results: DN4 score was significantly lower in the PIFP group than in the PHN group before and after matching. The incidence when DN4 was ≥4 was 10.5% before matching and 12.5% after matching in the PIFP group, both of which were significantly lower than those in the PHN group before and after matching (66.7% and 75.0%).
Conclusion: Ten percent of the PIFP patients likely show neuropathic pain characteristics.
Keywords: DN4, neuropathic pain, orofacial pain
Introduction
The International Classification of Headache Disorders – third edition (beta version) differentiates headache and orofacial pain into three categories of the primary headaches, the secondary headaches, and painful cranial neuropathies, other facial pains and other headaches.1 Painful cranial neuropathies and other facial pains include trigeminal neuralgia, glossopharyngeal neuralgia, burning mouth syndrome, or persistent idiopathic facial pain.1
Neuropathic pain is caused by a lesion or disease of the somatosensory nervous system. Although whether neuropathic pain can or cannot describe some of the underlying mechanisms of painful cranial neuropathies and other facial pains is unresolved, recent reports, using the Douleur Neuropathique 4 (DN4) questionnaire for screening neuropathic pain,2,3 have shown that 69.2% of the patients with trigeminal neuralgia present with neuropathic pain4 and 31% of the patients with burning mouth syndrome present with neuropathic pain.5
On the other hand, PIFP, previously termed atypical facial pain, which shows characteristic presence of daily or near-daily persistent facial pain, deep pain, and poorly localized pain, is not associated with sensory loss or abnormalities in laboratory or imaging studies, nor is it associated with the distribution of peripheral nerves.6,7 The underlying pathophysiology remains unclear, but some investigators have suggested that PIFP is a form of neuropathic pain.7 Conversely, other investigators have denied neuropathic mechanisms, instead suggesting neurophysiological mechanisms in the brain.6,8 In the present study, we used the DN4 questionnaire to examine the incidence of neuropathic pain among patients with PIFP and to compare neuropathic characteristics between PIFP and postherpetic neuralgia (PHN) which is a complex neuropathic pain condition.9
Methods
This multi-institutional retrospective study was approved by the Ethics Committees of Hyogo College of Medicine, Nishinomiya Municipal Central Hospital, and University of Fukui. The present study being retrospective, the requirement for written informed consent was waived by the institutional ethics committee. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Patients
We retrospectively obtained clinical data of consecutive patients who visited the pain clinic at the Hyogo College of Medicine, Nishinomiya Municipal Central Hospital, or University of Fukui, between November 2016 and December 2017. Eligibility criteria for the present study were as follows: age between 18 and 90 years, and chronic non-cancer pain of >3 months’ duration, with a visual analogue scale (VAS) score for pain ≥20. We excluded patients if they had pain of presumably mixed origin (e.g., PHN and chronic low back pain).
Data Collection
Information on gender, age, BMI, diseases causing chronic pain, disease duration, visual analogue scale (VAS) score for pain, pain medications, and DN4 questionnaire score. The VAS score for pain was determined using a 100-mm line from “no pain” to “the worst imaginable pain”. The DN4 questionnaire evaluates 10 items: characteristics of pain (burning (1), painful cold (2), electric shocks (3)), symptoms in the region of pain (tingling (4), pins and needles (5), numbness (6), itching (7)), localized pain (hypoesthesia to touch (8), hypoesthesia to pricking (9)), and pain caused or increased by brushing in the painful area (10). Items from #1 to #7 of the DN4 questionnaire are answered by interviewing patients, and items from #8 to #10 require examination of patients. DN4 score is the total of these 10 items existing in each patient, and the cut-off value for a diagnosis of neuropathic pain is a score of 4/10.2,3 The reliability, validity, and specificity of the Japanese version of the DN4 diagnostic questionnaire were confirmed in our previous study.3
Study Design
Figure 1 shows how we selected patients in the present study. To evaluate pain states of PIFP, we selected consecutive patients with PIFP or PHN and compared neuropathic characteristics of PIFP with PHN, which is the most common neuropathic pain.9 We then performed a case–control study in which each patient with PHN (control) or PIFP was matched by age and gender. Diagnosis of PIFP and PHN was performed through medical interviewing and physical examinations. Additional image diagnostics were not required. PIFP was diagnosed as persistent facial and/or oral pain, which is poorly localized, recurring daily for more than 2 hrs per day over more than 3 months in the absence of clinical neurological deficit.1 PHN was diagnosed as pain persisting more than 3 months following zoster diagnosis.9
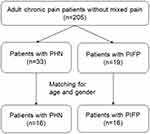 |
Figure 1 Diagram showing patient inclusion in this study. Abbreviations: PHN, postherpetic neuralgia; PIFP, persistent idiopathic facial pain. |
Statistics
All statistical testing was performed using IBM SPSS Statistics 24 software (IBM Corp., Chicago, IL). The Mann–Whitney U-test or chi-square test was used to compare appropriate variables between the two groups. Multiple regression analysis was also performed to examine associations between DN4 scores and patient characteristics. A value of P < 0.05 was considered statistically significant.
Results
Comparison Of DN4 Questionnaire Items Between PIFP And PHN
Before Matching For Age And Gender
In a total of 205 adult patients with chronic pain, 19 patients were diagnosed with PIFP, and 33 patients were diagnosed with PHN (Figure 1, Table 1). Patients with PIFP were significantly younger than those with PHN (P < 0.001). Disease duration was significantly longer in patients with PIFP than in patients with PHN (P = 0.003). DN4 score was significantly lower in patients with PIFP than in patients with PHN (P < 0.001). Incidence when DN4 score was ≥4 in patients with PHN was 66.7%, significantly higher than that in patients with PIFP (10.5%: P < 0.001). There was no significant difference in VAS score between patients with PIFP and PHN. The number of patients with PIFP who were prescribed with anticonvulsants (26.3%) was significantly lower than that with PHN (69.7%: P =0.004) (Table 1).
 |
Table 1 Patient Demographic And Pain Characteristics In Patients With PHN And PIFP Before Matching |
Figure 2 shows the incidence of the DN4 questionnaire items. Incidences of items including #8, # 9, and #10, which requires examination of patients, were lower in patients with PIFP (0.0%, 10.5%, 5.3%) than in patients with PHN (60.0%, P < 0.001; 50.0%, P = 0.006; 60.0%, P < 0.001). Incidences of tingling (#4) and itching (#7) were significantly lower in patients with PIFP (52.6%, 5.3%) than in patients with PHN (96.7%, P < 0.001; 56.7%, P < 0.001).
After Matching For Age And Gender
The matching procedure selected 16 patients in each group of patients with PIFP and PHN (Figure 1, Table 2). After matching, no significant differences in age and gender were seen between the two groups (P = 0.287 and P = 0.716). Disease duration was significantly longer in the PIFP group than in the PHN group (P < 0.001). VAS score for pain was higher in the PIFP group than in the PHN group (P = 0.026). DN4 score was significantly lower in the PIFP group than in the PHN group (P = 0.004), and also the incidence of DN4 ≥4 was significantly lower in the PIFP group than in the PHN group (P = 0.001). The number of patients with PIFP who were prescribed with anticonvulsants (12.5%) or opioids (12.5%) was significantly lower than that with PHN (81.3%: P < 0.001 or 56.3%: P = 0.023) (Table 2).
 |
Table 2 Patient Demographic And Pain Characteristics In Patients With PHN And PIFP After Matching |
Figure 3 shows the incidences of DN4 questionnaire items in the PHN and PIFP groups. Incidences of itching in addition to 3 items requiring examination were significantly lower for patients in the PIFP group (6.3%, 0.0%, 12.5%, 6.3%, respectively) compared to the PHN group (78.6%, P < 0.001; 71.4%, P < 0.001; 64.3%, P = 0.007; 64.3%, P = 0.002, respectively).
Multiple Regression Analysis
We performed the multiple regression analysis using a stepwise method to reveal the associations of the DN4 scores with age, gender, BMI, disease duration, diagnosis of pain state (PHN or PIFP), and VAS score before and after matching. Only the diagnosis of PIFP was selected to be negatively associated with the DN4 scores before and after matching.
Discussion
The present study showed that 10% of the patients with PIFP may involve neuropathic mechanisms. Pain states of hypoesthesia to touch, hypoesthesia to prick, and pain caused or increased by brushing (allodynia) suggest somatosensory nerve involvement. The incidences of these pain states were also significantly lower in patients with PIFP than in patients with PHN. The number of patients receiving a prescription of anticonvulsants, which are recommended as the first-line drugs for neuropathic pain pharmacotherapy,10 was significantly lower in the PIFP group than that in the PHN group. The decrease in DN4 scores was significantly associated with diagnosis of PIFP. Our results were consistent with the previous study using quantitative sensory testing, which found that PIFP is maintained by mechanisms that do not involve somatosensory processing of stimuli from the pain area.8
The incidence of itching in patients with PIFP was 5–6%, significantly lower than the 57–79% in patients with PHN in the present study. This incidence of itching in PHN patients in the present study corresponds to a previous report in which 65% of the patients with PHN had itching.11 Itch associated with PHN is neuropathic itch caused by damage to the sensory nerve.12,13 The low incidence of itching in PIFP patients may thus also be explained by the underlying mechanisms without somatosensory processing in PIFP.
Pain in patients with PIFP has typical characteristics of dull and aching.1 In the DN4 questionnaire items, the incidence of numbness in patients with PIFP was around 53%, which was comparable to that in patients with PHN, was relatively high compared to the other items in the present study. The previous study also reported that 50% of the PIFP patients had numbness.14 Numbness might be one of the common symptoms in patients with PIFP.
Following nociceptive and neuropathic pain, nociplastic pain was added as a third mechanistic descriptor of chronic pain.15,16 Nociplastic pain is defined as pain that arises from altered nociception. Although nociplastic pain may describe some of the mechanisms underlying PIFP,17 precise investigations are needed to clarify the mechanisms of PIFP.
Limitations to the present study were that we did not perform either neurological or histological examinations to confirm neuropathic pain in all patients for DN4 score ≥4. Further investigation is needed to confirm the incidence of neuropathic pain among patients with PIFP using precise examination.
Conclusion
Ten percent of the PIFP patients likely show neuropathic pain characteristics.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research KAKENHI (18K08875).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629–808. doi:10.1177/0333102413485658
2. Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114:29–36. doi:10.1016/j.pain.2004.12.010
3. Matsuki Y, Sukenaga N, Miyagi K, et al. Reliability and validity of the Japanese translation of the DN4 diagnostic questionnaire in patients with neuropathic pain. J Anesth. 2018;32:403–408. doi:10.1007/s00540-018-2495-7
4. VanDenKerkhof EG, Stitt L, Clark AJ, et al. Sensitivity of the DN4 in screening for neuropathic pain syndromes. Clin J Pain. 2018;34:30–36. doi:10.1097/AJP.0000000000000512
5. Sevrain M, Brenaut E, Le Toux G, Misery L. Primary burning mouth syndrome: a questionnaire study of neuropathic and psychological components. Am J Clin Dermatol. 2016;17:171–178. doi:10.1007/s40257-015-0170-4
6. Weiss AL, Ehrhardt KP, Tolba R. Atypical facial pain: a comprehensive, evidence-based review. Curr Pain Headache Rep. 2017;21:8. doi:10.1007/s11916-017-0609-9
7. Benoliel R, Gaul C. Persistent idiopathic facial pain. Cephalalgia. 2017;37:680–691. doi:10.1177/0333102417706349
8. Lang E, Kaltenhäuser M, Seidler S, Mattenklodt P, Neundörfer B. Persistent idiopathic facial pain exists independent of somatosensory input from the painful region: findings from quantitative sensory functions and somatotopy of the primary somatosensory cortex. Pain. 2005;118:80–81. doi:10.1016/j.pain.2005.07.014
9. Johnson RW, Rice ASC. Postherpetic neuralgia. New Engl J Med. 2014;371:1526–1533. doi:10.1056/NEJMoa1410490
10. The Committee for the Guidelines for the Pharmacologic Management of Neuropathic Pain (Revised) of JSPC, editors. Guidelines for the Pharmacologic Management of Neuropathic Pain.
11. Lee HJ, Kim GW, Kim WJ, et al. Clinical characteristics of postherpetic pruritus: assessment using a questionnaire, von Frey filaments and neurometer. Br J Dermatol. 2015;172:1672–1673. doi:10.1111/bjd.13569
12. Liu T, Ji RR. New insights into the mechanisms of itch: are pain and itch controlled by distinct mechanisms? Pflugers Arch. 2013;465:1671–1685. doi:10.1007/s00424-013-1284-2
13. Steinhoff M, Schmelz M, Szabó IL, Oaklander AL. Clinical presentation, management, and pathophysiology of neuropathic itch. Lancet Neurol. 2018;17:709–720. doi:10.1016/S1474-4422(18)30217-5
14. Siqueira SR, Siviero M, Alvarez FK, Teixeira MJ, Siqueira JT. Quantitative sensory testing in trigeminal traumatic neuropathic pain and persistent idiopathic facial pain. Arq Neuropsiquiatr. 2013;71:174–179. doi:10.1590/s0004-282x2013000300009
15. Kosek E, Cohen M, Baron R, et al. Do we need a third mechanistic descriptor for chronic pain states? Pain. 2016;157:1382–1386. doi:10.1097/j.pain.0000000000000507
16. Aydede M, Shriver A. Recently introduced definition of “nociplastic pain” by the International Association for the study of pain needs better formulation. Pain. 2018;159:1176–1177. doi:10.1097/j.pain.0000000000001184
17. Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the International Classification of Diseases (ICD-11). Pain. 2019;160:19–27. doi:10.1097/j.pain.0000000000001384
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


