Back to Journals » Journal of Experimental Pharmacology » Volume 14
Neurobehavioral and Immunohistochemical Studies of the Cerebral Cortex Following Treatment with Ethyl Acetate Leaf Fraction of Tamarindus indica During Prenatal Aluminum Chloride Exposure in Wistar Rats
Authors Usman IM , Adebisi SS, Musa SA, Iliya IA, Ochieng JJ , Ivang AE, Peter AB, Okesina AA
Received 6 April 2022
Accepted for publication 12 October 2022
Published 20 October 2022 Volume 2022:14 Pages 275—289
DOI https://doi.org/10.2147/JEP.S369631
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Stephen Safe
Ibe Michael Usman,1,2 Samuel Sunday Adebisi,1 Sunday Abraham Musa,1 Ibrahim Abdullahi Iliya,3 Juma John Ochieng,2 Andrew Ekpeyong Ivang,4 Akwu Bala Peter,2 Akeem Ayodeji Okesina2,5
1Department of Human Anatomy, Ahmadu Bello University, Zaria, Kaduna State, Nigeria; 2Department of Human Anatomy, Kampala International University, Bushenyi, Uganda; 3Department of Human Anatomy, Federal University Dutse, Dutse, Jigawa State, Nigeria; 4Department of Clinical Biology, University of Rwanda, Kigali, Rwanda; 5Department of Clinical Medicine and Community Health, University of Rwanda, Kigali, Rwanda
Correspondence: Ibe Michael Usman, Tel +256706666798, Email [email protected]
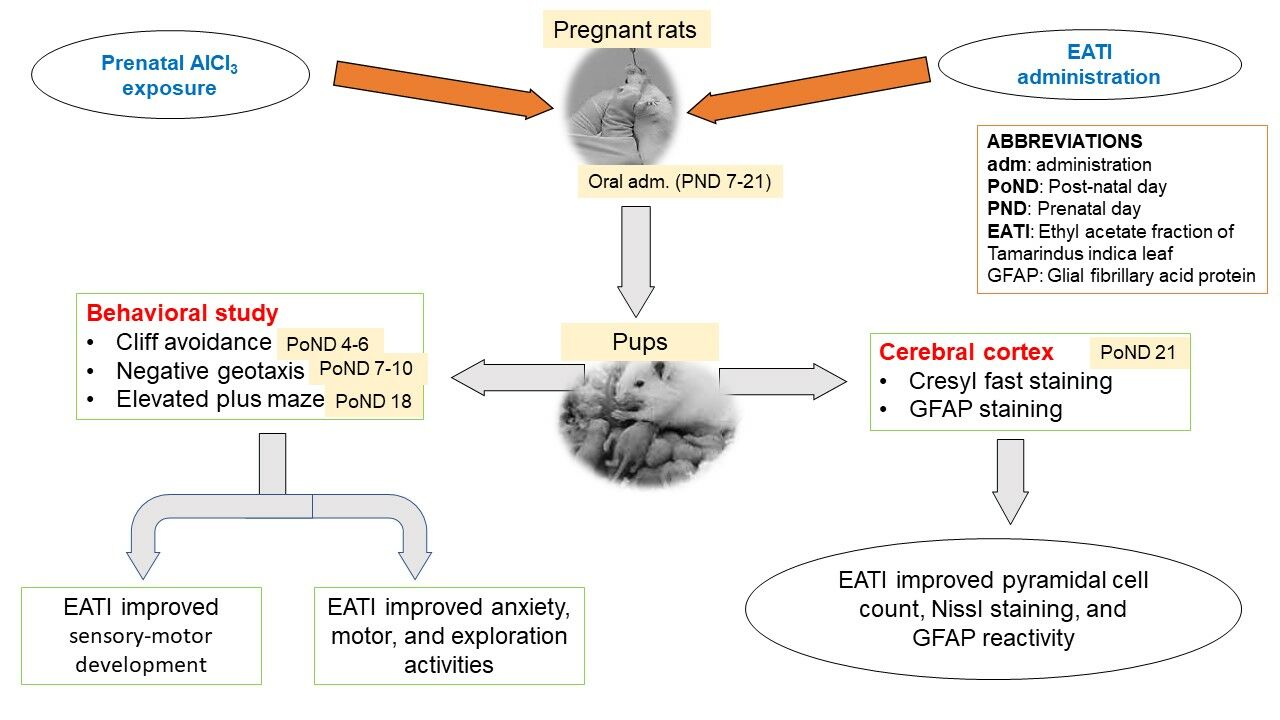
Purpose: The recent increase in aluminum exposure and its effect on the development of the brain call for serious attention. The study investigated the behavioral and immunohistochemical changes in the cerebral cortex of Wistar rats following prenatal co-administration of ethyl acetate leaf fraction of Tamarindus indica (EATI) and aluminum chloride (AlCl3).
Methods: Pregnant Wistar rats were divided into 5 groups (n=4). Group I (negative control), Group II–V were experimental groups treated with 200 mg/kg of AlCl3 s/c. Group III and IV received an additional 400 mg/kg and 800 mg/kg of EATI respectively, while Group V received an additional 300 mg/kg of Vitamin E for 14 days (prenatal days 7– 21) via the oral route. The pups were then exposed to cliff avoidance, negative geotaxis, and elevated plus maze (EPM) test on the post-natal day (PoND) 4– 6, 7– 10, and 18 respectively. On PoND 21 pups were sacrificed, and the skull dissected to remove the brain. The harvested brain tissues were processed for Cresyl fast (CF) and glial fibrillary acid protein (GFAP).
Results: The study showed that EATI administration during AlCl3 exposure was associated with significant improvement in sensory-motor development. The EPM, CF, and GFAP results revealed significant improvement in anxiety-like behavior, motor activities, GFAP expression, pyramidal cell count, and Nissl staining following prenatal EATI administration during AlCl3 exposure.
Conclusion: The present study concludes that EATI was associated with some protective potential during prenatal AlCl3 exposure in Wistar rats.
Keywords: Tamarindus indica, aluminum toxicity, glial fibrillary acid protein, sensory-motor development, elevated plus maze, anxiety-like behavior
Graphical Abstract:
Introduction
Aluminum exists in a variety of forms and is widely distributed in the environment, thus posing a risk to humans especially when exposure is prolonged.1 Exposure to aluminum in humans and other mammals is through the ingestion of substances such as water or vegetables containing aluminum.1 Aluminum occurs naturally in the environment and is released through anthropogenic activities such as mining.2 Various compounds containing aluminum are produced and put into an array of uses, including paper making, fillers, food additives, colors, water treatments, and pharmaceuticals.1 The major route of exposure to aluminum for the general population is through ingestion of contaminated food.3 Foods with reported high aluminum concentrations include tea leaves, cocoa, herbs, and spices.3 Patients on dialysis or long-term treatment with total parenteral nutrition have been shown to accumulate aluminum in different organs including the cerebral cortex.4
The cerebral cortex forms a very important component of the brain, with its different parts having specific and well-determined functions. The developing cerebral cortex is particularly vulnerable to environmental neurotoxicants just like every other part of the brain.5 The major window of developmental vulnerability occurs during intrauterine life, infancy, and early childhood.6 Exposure of the developing cerebral cortex to aluminum is associated with an unprecedented accumulation, with consequent developmental toxicity manifesting later in life,7 as both structural and functional changes.8 Exposure of experimental mice to aluminum hydroxide for 105 days was associated with a 34.0% (1.41 µmol/g) increase in brain aluminum level compared to the control groups 0.32 µmol/g.9 Oral administration of 50 mg/kg (low) and 200 mg/kg (high) of AlCl3 for 8 weeks was associated with elevated brain aluminum levels.10 The brain aluminum levels of the control, the low and high dose of aluminum exposed groups were measured as 0.717±0.208 µg/g, 0.963±0.491 µg/g and 1.816±1.157 µg/g (wet weight), respectively.10 During prenatal aluminum exposure, the placenta plays an important role in the amount of aluminum reaching the fetus. 100% of aluminum reaching the placenta crosses to the fetus, ensuring fetal aluminum accumulation.11,12 The fetal brain receives a fair share of the fetal aluminum stores following prenatal exposure.11 The placenta serves the function of supplying nutrients, oxygen, hormone and supporting the general survival of the fetus.11 Prenatal aluminum exposure interferes with the normal function of the placenta, therefore, impairing nutrient, oxygen, and hormone supply which affects normal developmental processes.11
Our recent independent studies on the ethyl acetate leaf fraction of Tamarindus indica (EATI) indicated the presence of flavonoids and polyphenols, based on the preliminary phytochemical screening (unpublished observation). Major components detected, based on the GCMS screening of EATI, included oleic acid, n-Hexadecanoic acid, Phenol, 3,5-bis (1,1-dimethyl ethyl), and cis-9-Hexadecenal (unpublished observation). Other pharmacological investigations on Tamarindus indica extracts revealed the presence of antibacterial, and antifungal properties,13 cytotoxic,14 anti-inflammatory,15 gastrointestinal,16 antioxidant activities.17 However, there are no published articles on the effect of EATI on the cerebral cortex during prenatal AlCl3 exposure, therefore, the objective of the present study was to investigate the neurobehavioral and immunohistochemical changes in the cerebral cortex following the administration of EATI prenatal AlCl3 exposure in Wistar rats.
Materials and Methods
Materials
Ten adult male and 20 non-pregnant female Wistar rats, light microscope, absolute ethanol, test tubes, Gemser reagent, aluminum chloride, vitamin E, Tween 80, plant material (Tamarindus indica leaf), automatic vacuum tissue processor (model: KD-TS6B), citrate buffer, hydrogen peroxide, horse serum, mouse monoclonal anti-GFAP, biotinylated secondary antibody, avidin-biotin complex, diaminobenzidine, hematoxylin, Cresyl violet acetate, water bath, distilled water, pestle and mortar, MXBAOHENG digital weighing balance, dissecting set, elevated plus maze, and negative setup.
Chemical and Drug Purchase/Preparation
Anhydrous AlCl3 powder, 99.999% (CAS Number: 7446–70-0) was obtained from Sigma-Aldrich. AlCl3 stock solution was prepared by dissolving 1g of AlCl3 in 10 mL of distilled water.
Capsules of vitamin E (Gujarat liquid pharmacaps Pvt) were obtained from a reputable drug store in Nigeria. The obtained capsules were cut open and emptied into a neat container. The stock solution was prepared using Tween 80, ensuring 0.2 mL of suspension contained 60 mg of vitamin E. The stock solution containing vitamin E was then shielded from direct light to avoid photodegradation using aluminum foil.
Plant Material Acquisition and Extraction/Fractionation
Tamarindus indica leaves were collected from the Botanical Garden of Ahmadu Bello University, Zaria. The leaves were authenticated in the Herbarium unit of the Department of Botany, Faculty of Life Science, Ahmadu Bello University, Zaria. A verification number of 2417 was assigned by Namadi Sunusi the Botanist in charge. The extraction of the leaves was carried out by maceration, followed by subsequent fractionation as outlined by Ajiboye et al18 in the Department of Pharmacognosy and Drug Development, Faculty of Pharmaceutical Science, Ahmadu Bello University, Zaria. Shade dried Shade-driedindica leaves were pounded to obtain a fine powder. The fine powder (100 g) of Tamarindus indica leaves then was suspended in 500 mL ethanol for 4 days with continuous shaking. The mixture was then filtered using Whatman filter paper (No. 41). The filtrate was collected and condensed at 60 ℃ in a rotary evaporator and then dried under vacuum, with a percentage yield of 6.95 g. The different fractions (n-butanol, n-hexane, and ethyl acetate) of Tamarindus indica leaves were prepared by dissolving 6 g of the ethanol extract in 200 mL of warm water and successively partitioned with n-hexane (3 x 100 mL), ethyl acetate (3 x 100 mL), and n-butanol (3 x 100 mL). A Rotary evaporator was used to dry the different fractions at 40℃. The fractions were weighed separately, transferred into universal bottles for storage at low temperature (4℃), and subsequently analyzed. EATI was selected due to its rich oil-like composition, and its stock solution was prepared by dissolution in Tween 80 since EATI was not soluble in water due to the same reason.
Ethics Approval
Expediated ethical approval from Ahmadu Bello University Committee on Animal Use and Care was acquired and registered as ABUCAUC/2019/001. The experimental rats received human care following the National Institute of Health guidelines for the care and use of laboratory animals.19,20
Experimental Animals
Twenty (20) adult nonpregnant females and 10 adult male Wistar rats were obtained from the animal house of the Human Anatomy Department, Faculty of Medicine, Ahmadu Bello University, Zaria, and were acclimatized for 2 weeks before the commencement of the experiment. The rats were allowed free access to feed and water before and during the experiment.
Determination of the Estrous Cycle
The estrous cycle was determined by the collection of vaginal smears from the 20 female rats using a small platinum wire loop that had been sterilized in flame and dipped in distilled water. The vaginal opening was cleaned with cotton wool dipped in methylated spirit followed by gentle insertion of the sterile platinum wire loop into the rat’s vagina, but not deep to avoid excessive stimulation of the rats, with the platinum wire loop still inside the vaginal lumen, the platinum wire loop was gently rubbed on the posterior wall of the vagina to remove some of its content. This was smeared on a grease-free glass slide and allowed to dry, after which the slide was stained for 5–10 minutes with 10% Geimser reagent diluted in 9 parts with buffer. The stained slide was rinsed in distilled water and then allowed to dry. The stained slides were examined under a light microscope at a magnification of x100.21,22
Mating of Rats and Confirmation of Pregnancy
Proestrus rats were caged overnight with sexually matured male rats for mating in the ratio of 2 females to 1 male. The presence of a vaginal plug the following morning confirms the animal mated. Distilled water with the aid of a plastic pipette was used to flush the vaginal lumen. The content of the vaginal lumen was gently aspirated and quickly transferred to a grease-free microscope slide and assessed under a light microscope at a magnification of x40. The presence of sperm cells in the vaginal smear confirmed copulation. Pregnancy was confirmed via vaginal smear examination 2 days after copulation. A persistent diestrus phase indicated an alteration in the normal cycle as a result of pregnancy.22
Dosage Determination
A dosage of 200 mg/kg was adopted for AlCl3 based on previous studies using the Wistar rat model.23,24 The authors conducted independent pilot studies to justify the selected dosage can induce the desired moderate developmental toxicity. The adopted dosage for EATI was 400 mg/kg (low dose) and 800 mg/kg (high dose). These doses were determined based on the result of the LD50 studies for EATI (above 3000 mg/kg bw using the up and down method). The up and down method was used because it ensured the use of a reduced number of animals.25 A dosage of 300 mg/kg was adopted for vitamin E, based on a previous study.26
Experimental Design and Sample Size Determination
Twenty pregnant timed Wistar rats were assigned randomly into 5 groups (n=4) to minimize selection bias on prenatal day 7. The sample size was determined using the power resource equation; Minimum n=10/k+1, Maximum n=20/k+1
where n is the number of animals per group, k is the number of groups.27
Therefore, the minimum number of animals needed per group for the studies = (10/5) +1 = 3.
While the maximum number of animals needed per group for the studies = (20/5) +1 = 5.
The choice of four rats per group was therefore within the required limit for the present studies.
Group 1: 2mL/kg of distilled water.
Group 2: 200 mg/kg of AlCl3.
Group 3: 200 mg/kg of AlCl3 and 400 mg/kg EATI
Group 4: 200 mg/kg of AlCl3 and 800 mg/kg EATI
Group 5: 200 mg/kg of AlCl3 and 300 mg/kg of Vitamin E.
The total duration of the experiment was 8 weeks; period of acclimatization of the rats (2 weeks before mating of rats), the period from mating till littering (3 weeks, prenatal day 1 to 21), and the period from littering to sacrifice of pups (3 weeks, post-natal day 1 to 21). All administrations in the study were done orally by gastric intubation for fourteen days from prenatal day 7 to 21, followed by the subsequent humane sacrifice of pups on postnatal day 21 (Figure 1).
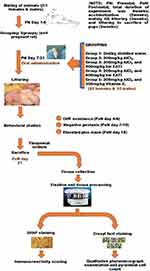 |
Figure 1 Flow chart. |
Neurobehavioral Study
Six male pups were randomly selected and curled for the different behavioral studies; cliff avoidance and negative geotaxis for sensory-motor development,28,29 and elevated plus-maze to assess anxiety, motor, and exploratory activities.30
Cliff Avoidance
For the cliff avoidance test, the selected pup was placed on a table, with the forepaws and nose at the edge of the table The time taken to completely turn away from the table edge was recorded for each pup on the post-natal day (PoND) 4 to 6 (n=6) and computed as a measure for sensory-motor development. Each test pup underwent two trials, with each trial lasting for 30 seconds as outlined by Zhang et al.28
Negative Geotaxis
From the post-natal day 8 to 10, the pups from the different groups were subjected to the negative geotaxis test (n=6). The time taken for a complete 180° turn when placed in a head-down position on a plane inclined at 25° was recorded. Each test pup underwent two trials, which lasted for 30 seconds as outlined by Ellenbroek et al.29
Elevated Plus-Maze Test
The elevated plus-maze test was performed as outlined by Shrestha and Singh,30 on the post-natal day 18. The test was used to assess anxiety, motor and exploratory activities in rodents.30 The setup consisted of two open arms (50 cm × 10 cm) and two closed arms (50 cm × 10 cm × 40 cm) which are connected via the central platform (10 cm × 10 cm). The arms are arranged in a cross shape with the two open arms facing each other and two closed arms facing each other. The maze was kept 50 cm above the floor. The pup for the test was placed at the center of the maze with its face directed towards one of the closed arms and observed for 5 minutes. The number of entries into the open arm and closed arm as well as the duration of time spent in each of the arms were recorded. Before the next trial, the floor and the walls of the open and closed arms were cleaned with 70% alcohol.
Animal Sacrifice
On post-natal day 21, the pups were weighed using MXBAOHENG digital weighing balance (2000g; 0.01g, Error ≤2d), followed by the humane sacrifice of the pups using thiopental sodium (5mg/kg intravenously) as the anesthetic agent as this is ethically acceptable in experimental animals.31,32 The skull was dissected to expose the whole brain which was weighed before fixation in 10% neutral buffered formalin.
Tissue Processing
The fixed brain tissues were trimmed and processed with the aid of an automated vacuum tissue processor (TP-1020). The processed tissue was cut using a rotary microtome at a thickness of 6µ. The cut tissue sections were stained for expression of Nissl substance and glial reactivity using Cresyl fast and Glial fibrillary acidic protein respectively.
Cresyl Fast Staining
The staining was done as outlined by Fitzpatrick.33 The tissue sections were deparaffinized and rehydrated in distilled water, then placed in Cresyl violet acetate solution for about 5 minutes. The stained sections were rinsed in 3 changes of distilled water, then dehydrated in graded alcohol, and cleared in 3 changes of xylene. The cleared slides were subsequently mounted with synthetic resin for light microscopy.
Pyramidal Cell Count
Photomicrographs of the stained slides were taken with the aid of a CMOS C-Mount microscope camera at a magnification of ×400 for the pyramidal cell count. At least 5 images per slide were taken by scanning through 5 different fields of the slides. The photomicrograph was then imported to the digimizer image analyzer, then divided into segments by gridline to ensure efficient manual counting of cells within the different grids. The number of normal and vacuolated pyramidal cells were recorded as normal pyramidal cell/field and vacuolated pyramidal cells/field by the pathologist, who was blinded to the experimental grouping; the blinding was to minimize bias.
Glial Fibrillary Acidic Protein (GFAP) Slide Preparation
Staining for glial reactivity was evaluated using glial fibrillary acidic protein (GFAP) (DAKO, USA). GFAP is the hallmark intermediate filament protein in astrocytes and becomes reactive in central nervous associated trauma from radiation or chemical, ischemia, and neurodegenerative conditions.34 Sections were treated with 0.01 M citrate buffer (pH 6.0) for 10 minutes to unmask the antigen. The sections were then incubated in 0.3% H2O2 for 30 min to get rid of tissue endogenous peroxidase activity followed by blocking with 5% horse serum for at least one to two hours. The sections were then incubated with the primary antibody (1:500 mouse monoclonal anti-GFAP) for 18 to 20 hours at a temperature of 4℃. The sections were washed, then incubated with biotinylated secondary antibodies (ABC kit, 1:200), and then with avidin-biotin complex. The sections were finally developed with 0.05% diaminobenzidine, followed by counterstaining with hematoxylin before mounting.35
Immunoreactivity Scoring
Klein’s semi-qualitative immune-reactive scoring approach was adopted as outlined by Fedchenko and Reifenrath36 and Archibong et al37 and slightly modified for the sake of this study. In brief, the scoring was established by scoring each sample twice by two independent pathologists who were both blinded to the experimental grouping to reduce possible bias. Five photomicrographs were snapped at random per section from individual rats for examination by the independent pathologist. For the percentage labeling; 0 (Absence of astrocyte labeling), 1 (<30% astrocyte labeling), 2 (30–60% astrocyte labeling), and 3 (>60% astrocyte labeling). For the intensity of the immunostaining; 0 (no staining), 1 (weak) 2 (mild), and 3 (strong staining). The percentage labeling was multiplied by the intensity of the immunostaining, which gave a range of scores between 0 and 9. The mean of scores from the different examiners for the different groups was then computed and presented as the final scores.
Statistical Analysis
The obtained data were entered into excel, then imported to the statistical package for social science (SPSS) version 20 for onward analysis. For the establishment of significant differences, the data were analyzed using a one-way analysis of variance (ANOVA). Differences were considered significant at p< 0.05, where applicable a post hoc test was applied. Data files used can be accessed at https://figshare.com/s/3c06f742a64ed611b2f1
Results
Morphometrics Studies
The result of the morphometric studies revealed that prenatal administration of 200 mg/kg of AlCl3 did not significantly impact the mean brain weight gain (Figure 2A). On the other hand, prenatal administration of 200 mg/kg of AlCl3 negatively affected the mean body weight gain. However, the administration of 800 mg/kg EATI was observed to be associated with improvement in the mean body weight gain in the experimental animals (Figure 2B).
Cliff Avoidance
The cliff avoidance test on post-natal days 4, 5, and 6 revealed that prenatal administration of 200 mg/kg of AlCl3 negatively impacted the mean turning latency, which was suggestive of delay in sensory-motor maturation. The administration of 800 mg/kg EATI and 300 mg/kg of vitamin E were both observed to have improved the recorded mean turning latency, suggestive of an improvement in sensory-motor maturation (Table 1).
 |
Table 1 Cliff Avoidance Test Result |
Negative Geotaxis
The negative geotaxis task revealed that prenatal administration of 200 mg/kg of AlCl3 negatively impacted mean turning latency during the task, suggestive of possible persistence of delay in sensory-motor maturation when compared to the normal control administered distilled water. However, the administration of 800 mg/kg EATI was observed to improve mean turning latency in the experimental animals on post-natal day eleven (Table 2).
 |
Table 2 Negative Geotaxis Result |
Elevated Plus-Maze
The elevated plus-maze test revealed that prenatal administration of 200 mg/kg of AlCl3 negatively impacted anxiety-like behavior, motor, and exploratory activities in the experimental rats, evidenced by the decrease in the mean number of entry into the open arm, duration of time spent in the open arm, and the number of entry into the closed arm (Figure 3A, B and D respectively), with an increase in the duration of time spent in the closed arm (Figure 3C). The administration of 800 mg/kg EATI was associated with an improvement in anxiety-like behavior, motor, and exploratory activities, evident by the recorded increase in the mean duration of time spent in the open arm and the number of entries into the open arm, and a decrease in the mean duration of time spent in the closed arm (Figure 3B–D). The administration of 300 mg/kg of vitamin E was also observed to be associated with improvement of anxiety-like behavior, motor, and exploratory activities, evident by the recorded increase in the mean number of entries into the open arm and the number of entries into the open arm (Figure 3A and D respectively).
Nissl Staining
The qualitative examination of sections from the cerebral cortex using Cresyl fast on post-natal day 21 revealed that the group treated with 200 mg/kg of AlCl3 (negative control group) and 400 mg/kg EATI were characterized by the presence of vacuolated pyramidal cell and pale/reduced Nissl staining (Figure 4B and C) when compared to the distilled water treated group (normal control group) (Figure 4A). On the other hand, relatively normal pyramidal cells, with improved Nissl staining were observed in the group treated with 800 mg/kg EATI and 300 mg/kg of vitamin E (Figure 4D and E) when compared to the distilled water treated group.
Pyramidal Cell Count
The result of the pyramidal cell count revealed prenatal administration of 200 mg/kg of AlCl3 negatively impacted the number of normal and vacuolated pyramidal cells, evidenced by a marked decrease in the number of relatively normal pyramidal cells and a significant increase in the number of vacuolated pyramidal cells (Figure 5A and B respectively). The administration of EATI showed a dose-dependent increase in the number of relatively normal pyramidal cells and a decrease in the number of vacuolated pyramidal cells per field when compared to the negative control (Figure 5A and B respectively). The administration of 300 mg/kg of vitamin E during prenatal administration of 200 mg/kg of AlCl3 was also associated with improvement in the number of relatively normal pyramidal cells.
GFAP Expression
The result of the cerebral cortical GFAP expression on post-natal day 21 from the different treatment groups revealed a marked increase in GFAP expression in the negative control group, the 400 mg/kg EATI and 300 mg/kg of vitamin E administered groups (Figure 6B, C, E and F) when compared to the normal control administered distilled water (Figure 6A). On the other hand, the administration of 800 mg/kg EATI was restorative, as shown by the observed decrease in GFAP expression (Figure 6D).
Discussion
The present study revealed that prenatal AlCl3 exposure was associated with lower mean body weight gain, a phenomenon possibly linked with altered food and water intake, and other likely metabolic disturbances associated with prenatal aluminum exposure.38 On the other hand, the administration of EATI and vitamin E were both associated with improvement in mean body weight gain, an observation that may be linked with possible improvement in food intake.
Delayed sensory-motor development, increased anxiety-like behavior, decreased exploration, motor activities, presence of degenerating pyramidal (evidenced by significantly higher vacuolated pyramidal cell count and reduced Nissl staining), and interference in GFAP expression. On the other hand, the administration of EATI and vitamin E were both associated with improved sensory-motor development, the number of normal pyramidal cell counts, Nissl staining, and GFAP expression. The administration of EATI and vitamin E were also associated with improved anxiety-like behavior, motor activities, and exploratory activity.
The reported effects of prenatal AlCl3 administration in the present study may be linked with the disruptive attribute of AlCl3, especially during the development of the cerebral cortex. Neuronal cell loss with the consequent decrease in the overall neuronal cell number is the major outcome of prenatal and post-natal aluminum exposure.39 Neuronal insults from heavy metals are most time associated with both functional and structural interference with astrocytes in the central nervous system.40 Reduction in the staining intensity of Nissl substance is usually an indication of reduced cellular metabolic activity, as it relates to protein synthesis.41 This is common in heavy metal exposure-related tissue damage, and therefore explains the observed alteration in astrocyte reactivity and neurobehavioral changes. The observed phenomenon is linked with the associated tissue injury and cell death. Astrocytes form an important component of the nervous system as they play a critical role in the normal function and general survival of neuronal cells, they also stand out in the normal development of the nervous system.42,43 The observed increase in immunoreactivity may be linked to the impacts of aluminum exposure on neuronal cells. The impact of neurotoxicants on the neuronal cell generates a cascade of events associated with astrocyte activity, to preserve neuronal cells.44 Neurotoxicants could interfere directly with the astrocyte pool of the nervous,44 as such attacks on the nervous tissue manifest as an increase in GFAP expression. Our observation is in line with the findings of Bernuzzi et al,45 who reported that prenatal administration of AlCl3 at a dosage of 160mg/kg and 200 mg/kg were both associated with delay in sensory-motor development in Wistar rats. Zhang et al28 reported that prenatal administration of alumina particles was associated with delayed neurodevelopmental behaviors and an increase in anxiety-like behavior in the treatment group. Abu-Taweel et al46 reported a dose-dependent suppression of sensory-motor maturation, decreased exploration and motor activities, and an increase in anxiety-like behavior in Wistar rats following prenatal exposure to aluminum at a dose of 300 mg/kg and 600 mg/kg of body weight. Thenmozhi et al47 reported that aluminum exposure was associated with significantly decreased locomotive activity, rearing, and grooming in the open field test, indicating an increase in anxiety-like behavior.
AlCl3 in the gastrointestinal tract of humans and experimental animals is absorbed mainly through the duodenum.48 The absorbed aluminum ends up in the bloodstream and is expected to be eliminated by the kidney and partly through the bile duct.49 Aluminum accumulation has been reported in various tissues of the body.50 Aluminum enters the brain, extracellular fluid, and cerebrospinal fluid more rapidly than it enters the blood.51 The uptake of aluminum by the different body tissues is very slow and assumed to be through endocytosis and intracellular transfer of the aluminum bound to transferrin.52 Accumulation of aluminum in the various tissues of the body is linked with various pathologic conditions both during fetal life and in adulthood.48 The impact of ingested aluminum is often more in fetal tissues than in adults considering the rudimentary nature of the different systems, especially the urinary system involved with the excretion of aluminum; therefore, a lower level of aluminum is required to elicit toxicity in fetal tissue.53,54 Aluminum can cross the fetal placental barrier and the blood-brain barrier, giving it unrestricted access to the supposed protected fragile fetal and brain tissue, with resultant cytotoxicity.55–57 The mechanism of aluminum-induced toxicity is dependent on its ability to accelerate the generation of free radicals, disruption of the antioxidant defense system, induction of DNA damage, iron dyshomeostasis, proinflammatory effects, amyloidogenesis, and provoking dysfunction of glial cells,48,58 with consequent cellular damage and altered developmental process. Structural consequence following exposure to aluminum both during development and in adulthood is often linked to various behavioral manifestations, in the form of delayed sensory-motor development, impaired motor activities, and increased anxiety-like behavior.28,45–47
The observed improvement in sensory-motor development, anxiety-like behavior, exploration, and motor activities, Nissl staining, reduction in degenerating cells, and GFAP expression following the treatment with EATI is not unconnected with the protective potentials accrued to Tamarindus indica.17,26,59 EATI from our previous report contains flavonoid, polyphenols, and cis-9-Hexadecenal; a phytochemical that possibly anchor its major combat against oxidative stress-related tissue disturbance in heavy metal exposure.60 Flavonoids are known to be effective metal chelators and can form stable products with metals such as aluminum, zinc, and iron.61 Polyphenols target various signaling molecules consequently influencing cellular activity.62 Polyphenol attenuates oxidative stress elicited cell viability loss, mitochondrial dysfunction, and DNA damage by reducing the level of aluminum exposure induced elevated reactive oxygen concentration.63 Therefore, augmenting the function of the endogenous antioxidant defense system and minimizing disruption in the normal developmental process possibly caused by AlCl3 exposure. Associated metal chelation and oxidative stress attenuative potential of phytochemicals within EATI may be the possible explanation for the associated or observed dose-dependent improvement during prenatal AlCl3 exposure in the present study. Improvements in cellular survival rate, mitochondrial function, and alleviated/reduced DNA damage have both structural and functional implications.64 Therefore, manifests as improved sensory-motor development, anxiety-like behaviors, motor activity, the viability of pyramidal cells, and GFAP expression in the present studies.
The observed improvement in the present study following treatment with EATI is in line with the finding of Usman et al26 who reported improvement in oxidative parameter, sialic concentration, Nissl staining, and general histo-architecture of the cerebral cortex following treatment with ethanol extract of Tamarindus indica during prenatal ethanol exposure. Muhammad et al59 reported improvements in oxidative stress parameters, level of proinflammatory cytokines, and memory impairment following treatment with Tamarindus indica seed extract following AlCl3 exposure. An independent study we conducted linked EATI administration during prenatal AlCl3 exposure with improvement in brain metal concentration, oxidative stress parameter, motor coordination, memory, and learning (unpublished observation).
Another important observation worth noting is that the high dose of EATI was as protective as vitamin E. Vitamin E is a known antioxidant.65 The observed improvement in the vitamin E treated group following prenatal AlCl3 exposure in the present study is possibly linked with the neuroprotective potential of vitamin E. Previous studies have linked vitamin E with neuroprotective potential in heavy metal exposure.66
The findings from this study, though basic, sheds light on the developmental effect of EATI during prenatal AlCl3 exposure, as there is no information on this, therefore, adding to the pool of known pharmacological and therapeutic benefits associated with the use of Tamarindus indica in complementary medicine. However, the present study failed to investigate the finer cellular events associated with prenatal Tamarindus indica and AlCl3 exposure following financial and infrastructure challenges. The authors also failed to statistically quantify the histological images, and estimate total cell count and volume using current stereological tools due to the above mention of resource and time constraints, thus creating room for further studies on EATI. Given the mentioned limitations, Cellular mechanisms interrupted and gene expression studied can be looked into for adequate interpretation of our findings.
Conclusion
The present study concluded that administration of EATI during prenatal AlCl3 exposure was of protective value, with the observed improvement in sensory-motor development, anxiety-like behavior, motor activity, Nissl staining intensity, and GFAP expression. Therefore, the authors suggest investigation of the finer cellular events, such as gene expression, and possible visualization of subcellular changes using ultramicroscopy to better understand the protective potential of Tamarindus indica, especially in prenatal aluminum exposure is recommended.
Acknowledgments
The authors acknowledge the support of the Human Anatomy Department, Ahmadu Bello University, Zaria, Nigeria for providing a conducive environment for the conduct of the present studies. The authors also acknowledge the support from Prof Susan Christina Welburn at the University of Edinburgh on the language editing of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Klotz K, Weistenhöfer W, Neff F, Hartwig A, Van Thriel C, Drexler H. The health effects of aluminum exposure. Dtsch Arztebl Int. 2017;114(39):653–659. doi:10.3238/arztebl.2017.0653
2. Exley C. Human exposure to aluminium. Environ Sci Process Impacts. 2013;15(10):1807–1816. doi:10.1039/c3em00374d
3. Aguilar F, Autrup H, Barlow S, et al. Safety of aluminium from dietary intake 1 scientific opinion of the panel on food additives, flavourings, processing aids and food contact materials (AFC). EFSA J. 2008;754:1–34.
4. Yokel RA, McNamara PJ. Aluminium toxicokinetics: an updated minireview. Pharmacol Toxicol. 2001;88:159–167. doi:10.1111/j.1600-0773.2001.880401.x
5. Bondy SC, Campbell A. Developmental neurotoxicology. J Neurosci Res. 2005;81(5):605–612. doi:10.1002/jnr.20589
6. Rice D, Barone S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108:511. doi:10.2307/3454543
7. Saunders NR, Liddelow SA, Dziegielewska KM. Barrier mechanisms in the developing brain. Front Pharmacol. 2012;3:1–18. doi:10.3389/fphar.2012.00046
8. Choi AL, Sun G, Zhang Y, Grandjean P. Developmental fluoride neurotoxicity: a systematic review and meta-analysis. Environ Health Perspect. 2012;120(10):1362–1368. doi:10.1289/ehp.1104912
9. Şahin G, Varol I, Temizer A, Benli K, Demirdamar R, Duru S. Determination of aluminum levels in the kidney, liver, and brain of mice treated with aluminum hydroxide. Biol Trace Elem Res. 1994;41(1–2):129–135. doi:10.1007/BF02917223
10. Baydar T, Papp A, Aydin A, et al. Accumulation of aluminum in rat brain: does it lead to behavioral and electrophysiological changes? Biol Trace Elem Res. 2003;92(3):231–244. doi:10.1385/BTER:92:3:
11. Sharma P, Mishra KP. Aluminum-induced maternal and developmental toxicity and oxidative stress in rat brain: response to combined administration of Tiron and glutathione. Reprod Toxicol. 2006;21(3):313–321. doi:10.1016/j.reprotox.2005.06.004
12. Yumoto S, Nagai H, Matsuzaki H, et al. Transplacental passage of 26Al from pregnant rats to fetuses 26Al transfer through maternal milk to suckling rats. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater Atoms. 2000;172(1–4):925–929. doi:10.1016/S0168-583X(00)00096-3
13. Mahomoodally MF. Traditional medicines in Africa: an appraisal of ten potent African medicinal plants. Evidence-Based Complement Altern Med. 2013;2013:1–14. doi:10.1155/2013/617459
14. Kobayashi A, Kajiyama SI, Adenan MI, Kanzaki H, Kawazu K, Cytotoxic A. Principle of Tamarindus indica, di-n-butyl malate and the structure-activity relationship of its analogues. Zeitschrift fur Naturforsch Sect C J Biosci. 1996;51(3):233–242. doi:10.1515/znc-1996-3-415
15. Rimbau V, Cerdan C, Vila R, Iglesias J. Antiinflammatory activity of some extracts from plants used in the traditional medicine of North-African countries (II). Phyther Res. 1999;13(2):128–132. doi:10.1002/(SICI)1099-1573(199903)13:2<128::AID-PTR399>3.0.CO;2-7
16. Coutino-Rodriguez R, Cruz Hernandez P, Giles-Rı́os H. Lectins in fruits having gastrointestinal activity: their participation in the hemagglutinating property of Escherichia coli 0157: H7. Arch Med Res. 2001;32(4):251–257. doi:10.1016/S0188-4409(01)00287-9
17. Usman IM, Buraimoh A, Ibegbu A. Effect of prenatal ethanol exposure and extract of Tamarindus indica pulp on the cerebral cortex of Wistar rats. Afr J Cell Pathol. 2016;7(1):1–5.
18. Ajiboye BO, Ojo OA, Okesola MA, Oyinloye BE, Kappo AP. Ethyl acetate leaf fraction of Cnidoscolus aconitifolius (Mill.) I. M. Johnst: antioxidant potential, inhibitory activities of key enzymes on carbohydrate metabolism, cholinergic, monoaminergic, purinergic, and chemical fingerprinting. Int J Food Prop. 2018;21(1):1697–1715. doi:10.1080/10942912.2018.1504787
19. Clark D, Baldwin RL, Bayne KA, Brown MJ. “National research council” guide for the care and use of laboratory animals; 1996.
20. Council NR. Guide for the care and use of laboratory animals: eighth edition; 2010.
21. Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Brazilian J Biol. 2002;62(4A):609–614. doi:10.1590/S1519-69842002000400008
22. Adebisi S. Determination of commencement of gestation from the vaginal cytology in the Wistar rats. J Exp Clin Anat. 2009;8(1). doi:10.4314/jeca.v8i1.48034
23. Elizabeth MA, Samson P, Itohan OR. Histomorphological evaluations on the frontal cortex extrapyramidal cell layer following administration of N-Acetyl cysteine in aluminum induced neurodegeneration rat model. Metab Brain Dis. 2020;35(5):829–839. doi:10.1007/s11011-020-00556-9
24. Chinoy N, Sorathia H, Jhala ahmedabad D. Fluoride+aluminium induced toxicity in mice testis with giant cells and its reversal by vitamin c. Fluoride. 2005;38(2):109–114.
25. Zhang YY, Huang YF, Liang J, Zhou H. Improved up-and-down procedure for acute toxicity measurement with reliable LD50 verified by typical toxic alkaloids and modified Karber method. BMC Pharmacol Toxicol. 2022;23(1):1–11. doi:10.1186/S40360-021-00541-7/TABLES/15
26. Usman I, Buraimoh A, Ibegbu A. Histological and biochemical studies of Tamarindus indica pulp extract on the cerebral cortex in prenatal ethanol exposure in Wistar rats. J Exp Clin Anat. 2016;15(2):96. doi:10.4103/1596-2393.200919
27. Wan NA, Wan MZ. Sample size calculation in animal studies using resource equation approach. Malaysian J Med Sci. 2017;24(5):101–105. doi:10.21315/mjms2017.24.5.11
28. Zhang Q, Ding Y, He K, et al. Exposure to alumina nanoparticles in female mice during pregnancy induces neurodevelopmental toxicity in the offspring. Front Pharmacol. 2018;9. doi:10.3389/fphar.2018.00253
29. Ellenbroek BA, Derks N, Park HJ. Early maternal deprivation retards neurodevelopment in Wistar rats. Stress. 2005;8(4):247–257. doi:10.1080/10253890500404634
30. Shrestha U, Singh M. Effect of folic acid in prenatal alcohol induced behavioral impairment in Swiss albino mice. Ann Neurosci. 2013;20(4):134–138. doi:10.5214/ans.0972.7531.200403
31. Kasozi KI, Namubiru S, Safiriyu AA, et al. Grain amaranth is associated with improved hepatic and renal calcium metabolism in type 2 diabetes mellitus of male Wistar rats. Evidence-Based Complement Altern Med. 2018;2018:1–10. doi:10.1155/2018/4098942
32. Usman IM, Iliya IA, Ivang AE, Ssempijja F, Ojewale AO, Yusuf HR. Microanatomical and biochemical changes of the cerebellum following ethanol gavage in adult Wistar rats. Anat J Africa. 2019;8(2):1662–1669. doi:10.4314/aja.v8i2.189708
33. Fitzpatrick M. Cresyl Violet Staining (Nissl Staining). The Open Lab Book v1.0; 2014.
34. Hol EM, Pekny M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol. 2015;32:121–130. doi:10.1016/j.ceb.2015.02.004
35. Gomes-Leal W, Corkill DJ, Freire MA, Picanço-Diniz CW, Perry VH. Astrocytosis, microglia activation, oligodendrocyte degeneration, and pyknosis following acute spinal cord injury. Exp Neurol. 2004;190(2):456–467. doi:10.1016/j.expneurol.2004.06.028
36. Fedchenko N, Reifenrath J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue - a review. Diagn Pathol. 2014;9:221. doi:10.1186/s13000-014-0221-9
37. Archibong VB, Ekanem TB, Igiri AO, et al. Immunohistochemical studies of codeine medication on the prefrontal cortex and cerebellum of adult Wistar rats. Cogent Med. 2020;7(1). doi:10.1080/2331205X.2020.1824390
38. Gonda Z, Lehotzky K. Effect of prenatal aluminium lactate exposure on conditioned taste aversion and passive avoidance task in the rat. J Appl Toxicol. 1996;16(6):529–532. doi:10.1002/(SICI)1099-1263(199611)16:6<529::AID-JAT392>3.0.CO;2-S
39. Hao Y, Li M, Zhang J, et al. Aluminum-induced “mixed” cell death in mice cerebral tissue and potential intervention. Neurotox Res. 2020;37(4):835–846. doi:10.1007/s12640-019-00123-w
40. Suárez-Fernández MB, Soldado AB, Sanz-Medel A, Vega JA, Novelli A, Fernández-Sánchez MT. Aluminum-induced degeneration of astrocytes occurs via apoptosis and results in neuronal death. Brain Res. 1999;835(2):125–136. doi:10.1016/S0006-8993(99)01536-X
41. Angevine JB. Nervous system, organization of. In: Encyclopedia of the Human Brain. Elsevier; 2002:313–371.
42. De Keyser J, Mostert JP, Koch MW. Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J Neurol Sci. 2008;267(1–2):3–16. doi:10.1016/j.jns.2007.08.044
43. Chen Y, Qin C, Huang J, et al. The role of astrocytes in oxidative stress of central nervous system: a mixed blessing. Cell Prolif. 2020;53(3):e12781. doi:10.1111/cpr.12781
44. Zhou Y, Shao A, Yao Y, Tu S, Deng Y, Zhang J. Dual roles of astrocytes in plasticity and reconstruction after traumatic brain injury. Cell Commun Signal. 2020;18(1):1–16. doi:10.1186/s12964-020-00549-2
45. Bernuzzi V, Desor D, Lehr PR. Developmental alterations in offspring of female rats orally intoxicated by aluminum chloride or lactate during gestation. Teratology. 1989;40(1):21–27. doi:10.1002/tera.1420400104
46. Abu-Taweel GM, Ajarem JS, Ahmad M. Neurobehavioral toxic effects of perinatal oral exposure to aluminum on the developmental motor reflexes, learning, memory and brain neurotransmitters of mice offspring. Pharmacol Biochem Behav. 2011;101:49–56. doi:10.1016/j.pbb.2011.11.003
47. Thenmozhi AJ, Raja TRW, Janakiraman U, Manivasagam T. Neuroprotective effect of hesperidin on aluminium chloride induced alzheimer’s disease in Wistar rats. Neurochem Res. 2015;40:767–776. doi:10.1007/s11064-015-1525-1
48. Igbokwe IO, Igwenagu E, Igbokwe NA. Aluminium toxicosis: a review of toxic actions and effects. Interdiscip Toxicol. 2020;12(2):45–70. doi:10.2478/intox-2019-0007
49. Shirley DG, Lote CJ. Renal handling of aluminium. Nephron Physiol. 2005;101(4):p99–p103. doi:10.1159/000088331
50. Klein GL. Aluminum toxicity to bone: a multisystem effect? Osteoporos Sarcopenia. 2019;5(1):2–5. doi:10.1016/j.afos.2019.01.001
51. Al-Hazmi MA, Rawi SM, Hamza RZ. Biochemical, histological, and neuro-physiological effects of long-term aluminum chloride exposure in rats. Metab Brain Dis. 2021;36(3):429–436. doi:10.1007/s11011-020-00664-6
52. Hémadi M, Miquel G, Kahn PH, El Hage Chahine JM. Aluminum exchange between citrate and human serum transferrin and interaction with transferrin receptor 1. Biochemistry. 2003;42(10):3120–3130. doi:10.1021/bi020627p
53. Röllin HB, Nogueira C, Olutola B, Channa K, Odland J. Prenatal exposure to aluminum and status of selected essential trace elements in rural South African women at delivery. Int J Environ Res Public Health. 2018;15(7):1494. doi:10.3390/ijerph15071494
54. Clayton RM, Sedowofia SKA, Rankin JM, Manning A. Long-term effects of aluminium on the fetal mouse brain. Life Sci. 1992;51(25):1921–1928. doi:10.1016/0024-3205(92)90108-2
55. Song Y, Xue Y, Liu X, Wang P, Liu L. Effects of acute exposure to aluminum on blood-brain barrier and the protection of zinc. Neurosci Lett. 2008;445(1):42–46. doi:10.1016/j.neulet.2008.08.081
56. Banks WA, Kastin AJ. Aluminum-Induced neurotoxicity: alterations in membrane function at the blood-brain barrier. Neurosci Biobehav Rev. 1989;13(1):47–53. doi:10.1016/S0149-7634(89)80051-X
57. Yokel RA. Aluminum and the blood-brain barrier. In: Aluminium and Alzheimer’s Disease. Elsevier; 2001:233–260.
58. Morris G, Puri BK, Frye RE. The putative role of environmental aluminium in the development of chronic neuropathology in adults and children. How strong is the evidence and what could be the mechanisms involved? Metab Brain Dis. 2017;32(5):1335–1355. doi:10.1007/s11011-017-0077-2
59. Muhammad IN, Rahayu M, Soeharto S, Nurdiana Widodo MA, Widodo MA. Neuroprotective potency of Tamarindus indica seed extract for preventing memory impairment in rat model of alzheimer’s disease. Res J Pharm Technol. 2020;13(9):4041. doi:10.5958/0974-360x.2020.00714.3
60. Devi KV. GC-MS analysis of ethanol extract of entada pursaetha DC seed. Biosci Discov. 2012;3.:30–33.
61. Pavun LA, DimitrićMarković JM, Durdević PT, et al. Development and validation of a fluorometric method for the determination of hesperidin in human plasma and pharmaceutical forms. J Serbian Chem Soc. 2012;77(11):1625–1640. doi:10.2298/JSC111005060P
62. Tagde P, Tagde P, Islam F, et al. The multifaceted role of curcumin in advanced nanocurcumin form in the treatment and management of chronic disorders. Molecules. 2021;26(23):7109. doi:10.3390/MOLECULES26237109
63. Qi G, Mi Y, Wang Y, et al. Neuroprotective action of tea polyphenols on oxidative stress-induced apoptosis through the activation of the TrkB/CREB/BDNF pathway and Keap1/Nrf2 signaling pathway in SH-SY5Y cells and mice brain. Food Funct. 2017;8(12):4421–4432. doi:10.1039/C7FO00991G
64. Fricker M, Tolkovsky AM, Borutaite V, Coleman M, Brown GC. Neuronal cell death. Physiol Rev. 2018;98(2):813–880. doi:10.1152/physrev.00011.2017
65. Ulfanov O, Cil N, Adiguzel E. Protective effects of vitamin E on aluminium sulphate-induced testicular damage. Toxicol Ind Health. 2020;36(4):215–227. doi:10.1177/0748233720919663
66. Wang M, Ruan DY, Chen JT, Xu YZ. Lack of effects of vitamin E on aluminium-induced deficit of synaptic plasticity in rat dentate gyrus in vivo. Food Chem Toxicol. 2002;40(4):471–478. doi:10.1016/S0278-6915(01)00094-1
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.