Back to Journals » Drug Design, Development and Therapy » Volume 15
Network Pharmacology and Experimental Evidence Identify the Mechanism of Astragaloside IV in Oxaliplatin Neurotoxicity
Authors Xu J, Guan Z, Wang X, Sun D, Li Y, Pei B, Lu Y, Yuan L, Zhang X
Received 26 June 2020
Accepted for publication 22 December 2020
Published 12 January 2021 Volume 2021:15 Pages 99—110
DOI https://doi.org/10.2147/DDDT.S262818
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qiongyu Guo
Jingyu Xu,1,* Zhenbiao Guan,2,* Xiaowei Wang,1,* Dazhi Sun,1 Yongjin Li,1 Bei Pei,1 Ye Lu,1 Liangxi Yuan,3 Xuan Zhang1
1Department of Traditional Chinese Medicine, Changzheng Hospital, Naval Medical University, Shanghai 200003, People’s Republic of China; 2Department of Respiration, Changhai Hospital, Naval Medical University, Shanghai 200433, People’s Republic of China; 3Department of Vascular Surgery, Changhai Hospital, Naval Medical University, Shanghai 200433, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liangxi Yuan
Department of Vascular Surgery, Changhai Hospital, Naval Medical University, Shanghai 200433, People’s Republic of China
Email [email protected]
Xuan Zhang
Department of Traditional Chinese Medicine, Changzheng Hospital, Naval Medical University, Shanghai 200003, People’s Republic of China
Email [email protected]
Background and Objective: Neurotoxicity is a common side effect of oxaliplatin; the effect of current drugs such as methylcobalamin and gabapentine is not obvious. Astragaloside IV (AS-IV) is an important active ingredient of Astragali Radix, which can protect the nervous system and inhibit tumor growth to a certain extent. However, whether AS-IV can reduce oxaliplatin neurotoxicity and its molecular mechanism remain unclear.
Methods: The network pharmacology method was used to determine the collective targets of AS-IV and oxaliplatin neurotoxicity. The model of neurotoxicity was established by intraperitoneal injection of oxaliplatin in rats. Bodyweight, mechanical withdrawal threshold (MWT), cold allodynia, and nerve conduction velocity (NCV) were examined, pathological changes were observed by hematoxylin-eosin staining, number of Nissl bodies were assessed by Nissl staining, the key collective targets were measured by spectrophotometry and immunohistochemistry.
Results: Through network pharmacological analysis, 25 collective targets of AS-IV and oxaliplatin neurotoxicity were identified, mainly related to inflammation and oxidative stress. AS-IV could increase body weight, elevate MWT, and reduce cold allodynia of model rats, it also raised NCV. Neuropathology was improved and the number of Nissl bodies was increased by AS-IV administration. It reduced TNF-α, IL-6, and IL-1β in the spinal cord of model rats to inhibit inflammation; it also decreased MDA, raised SOD, CAT, and GSH-Px in the spinal cord of model rats to block oxidative stress.
Conclusion: AS-IV improves oxaliplatin neurotoxicity by regulating neuroinflammation and oxidative stress; the results can provide a new perspective for the potential treatment strategy of oxaliplatin neurotoxicity.
Keywords: network pharmacology, astragaloside IV, oxaliplatin neurotoxicity, neuroinflammation, oxidative stress
Introduction
Oxaliplatin is a third-generation platinum-based chemotherapy agent that is widely applied to treat advanced metastatic, ovarian, colorectal, breast, and lung cancers.1 However, neurotoxicity is a common adverse reaction that occurs with the clinical application of oxaliplatin. The main characteristics of neurotoxicity include hyperalgesia, mechanical, and cold allodynia without heat sensitivity. As far as we know, the progression of the disease is closely associated with oxaliplatin accumulation.2 Neurotoxicity seriously affects the quality of patients’ life and also interrupts effective treatment.3
Although many studies have examined the pathogenesis of oxaliplatin neurotoxicity, the available drugs can only relieve symptoms and have low efficacy or an unpredictable risk of side effects.4 Therefore, it is essential to develop new drugs to treat the disease.
In recent years, many studies have turned to traditional Chinese medicine as therapeutic options for many diseases. Astragali Radix is an important traditional Chinese herbal medicine that is widely used in disease treatment, food, and healthcare products in Asia.5 Many components of Astragali Radix have been examined. Astragaloside IV (AS-IV) is the major active component of Astragali Radix, with a molecular weight of 784 KD. This drug has been applied to prevent and treat cardiovascular, diabetes, hepatic, and renal diseases, and exerts a neuroprotective effect in nervous system disease via inhibition of neuronal apoptosis and oxidative damage.6 AS-IV has also been identified to have anti-inflammatory and antiviral effects.7 Moreover, AS-IV can kill a variety of tumor cells, inhibit the invasion and metastasis of tumor cells, and enhance the sensitivity of colon cancer cells to oxaliplatin.8–10 Therefore, AS-IV may be able to repair oxaliplatin neurotoxicity without promoting tumor growth. However, to date, there are no definitive reports about this process. Network pharmacology emphasizes multi-target prediction of drugs and selects the key targets.11 The study will clarify the effects and mechanisms of AS-IV treatment on oxaliplatin neurotoxicity based on network pharmacology and experimental evidence. The results will provide an experimental basis for further clinical application of AS-IV on oxaliplatin neurotoxicity.
Materials and Methods
Materials
Male Sprague-Dawley rats (6–8 weeks old) were purchased from the Animal Laboratory Center of the Chinese Academy of Sciences (Shanghai, SCXK 2012–0002). AS-IV (purity 98%) was purchased from Nanjing Spring & AutumnBiological Engineering Co. Ltd (Nanjing, China). Oxaliplatin was purchased from Hengrui Pharmaceutical Co. Ltd (Jiangsu, China). Superoxide dismutase (SOD) (No: A001-3-2), glutathione peroxidase (GSH-Px) (No: A005-1-2), malondialdehyde (MDA) (No: A003-1-2), and catalase (CAT) (No: A007-1-1) Assay Kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Anti-TNF-αtumor necrosis factor-α (TNF-α) (No: ab1793), anti-interleukin-1β (IL-1β) (No: ab2105), and anti- interleukin-6 (IL-6) (No: ab6672) antibodies were purchased from Abcam (Shanghai, China).
Identification of Candidate Targets of AS-IV
The molecular structure of AS-IV was obtained from PubChem Database (https://pubchem.ncbi.nlm.nih.gov/). Forty-three targets of AS-IV were selected by TTD Database (http://db.idrblab.net/ttd/), Drugbank Database (https://www.drugbank.ca) and CTD Database (http://ctdbase.org/).
Identification of Candidate Targets of Oxaliplatin Neurotoxicity
About 3107 targets of oxaliplatin were acquired from Genecards Database (https://www.genecards.org/) and CTD Database (http://ctdbase.org/). About 5774 targets of neurotoxicity were gained from Disgenet Database (http://www.disgenet.org) and Genecards Database (https://www.genecards.org/). The intersection of candidate targets of oxaliplatin and neurotoxicity were about 1217.
Protein–Protein Interaction Network
The collective targets of AS-IV and oxaliplatin neurotoxicity were used to establish the protein–protein interaction regulation network by String Database (https://string-db.org/) and cytoscape3.7.2 (http://www.cytoscape.org/).
Oxaliplatin Neurotoxicity Model Construction and Drug Administration
All male Sprague-Dawley rats were housed under specific pathogen-free conditions under a 12 h light/dark cycle. The room temperature was set at 25±2°C, the humidity was 50–60%, and they had free access to food and water. The rats were divided into six groups: control, model, Low AS-IV, Medium AS-IV, High AS-IV, control + High AS-IV (n=10).
Rats were acclimated for 7 days before experiments. Establishment of model rats, according to the method of Homles.12 Oxaliplatin was diluted in 5% glucose to form a final solution with a concentration of 2 mg/mL. The model rats were injected intraperitoneally with the solution twice a week (first and second day of the week) at a dose of 4 mg/kg. The Control group was intraperitoneally injected with 5% glucose solution; the Low, Medium and High AS-IV groups received a daily gavage of AS-IV 10, 20, or 40 mg/kg body weight, respectively, the control+High AS-IV group was received 5% glucose solution by intraperitoneally injection and 40 mg/kg body weight AS-IV by gavage in order to exclude the effect of AS-IV on control group. The treatment lasted for 4 weeks. All experimental and animal handling procedures were approved by the Ethics Committee for Animal Experiments of Naval Medical University (License No. NMMU-20200610D) and conformed to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Mechanical Withdrawal Threshold (MWT) Evaluation
To quantify the mechanical sensitivity of rats, the rats were placed in a plexiglass cage and keep at a resting state for 30 min so they could adapt to the environment. After the rats were quiet, 1–15 g of von Frey fiber filaments were used to stimulate the mid-plantar region of the hind paw. The paw was touched with a weight of 2.0 g from the beginning; a larger degree of stimulus was applied when 2.0 g did not cause a positive reaction such as foot lifting or foot licking. Conversely, if the positive reaction occurred with the 2.0 g stimulus, a smaller stimulus was given. Repeated measurements were conducted on each hind paw 5 times at intervals of 30 s. Three or more positive reactions were regarded as mechanical hyperalgesia. The maximum stimulus (when ≥15 g) was recorded as 15 g.13
Choi Test for Cold Allodynia
Cold sensitivity of the rat hindpaw to acetone was quantified by paw withdrawal frequency, as described previously. In brief, a 100 μL drop of acetone was added to the center of the plantar surface of the paw. Acetone was added to each paw a total of 5 times with a 5-min interval. The number of brisk paw withdrawals accompanied by nocifensive behaviors was recorded.
Detection of Sciatic Nerve Conduction Velocity
The rats were anesthetized by 10% chloral hydrate (350 mg/kg) and the left hind and midline were kept about 45°, then the rats were sterilized and fixed, furthermore, the skin was cut at the middle and lower 1/3 of the left thigh, the left sciatic nerve was bluntly separated and exposed. At last, the left sciatic nerve of each rat was cut open quickly and placed in an insulated box before treatment with a biphasic pulse at the distal end for duration of 0.1 s at intensity of 200 mV. Motor nerve conduction velocity (MNCV) was calculated by dividing the distance between the stimulating electrodes by the average latency difference among the peaks of the compound muscle action potentials triggered from the sciatic notch and ankle. Sense nerve conduction velocity (SNCV) was detected by dividing the distance between the stimulating and recording electrodes by the latency of the signal from the stimulation artifact to the onset of the peak signal. The sciatic nerve conduction velocity was measured by Medlab-U/4CS biosignal acquisition and analysis system.
Evaluation of Histological Changes
The observers were blinded to the experiment design. Hematoxylin-eosin (HE) staining and Nissl staining were used to evaluate histological changes. L4-L6 spinal cords were collected. Then, 1 mm segments (spanning a 3 mm length in the spinal cord centered at the epicenter) were harvested and immediately fixed in 4% paraformaldehyde for 24 h before paraffin embedding. The paraffin sections were then cut into 4 μm slides and submitted to HE staining. A light microscope (Olympus, Tokyo, Japan) was used to photograph the HE staining.
Prior to Nissl staining, the slides were submitted to the same procedure as that described above for HE staining. Then, they were blocked with 3% H2O2 (v/v) and incubated with sodium citrate buffer (10.2 mM) for 20 min at 95°C for antigen retrieval. Next, the slides were incubated with 1% cresyl violet for Nissl staining and recorded under a light microscope (Olympus). The number of Nissl bodies in three randomly selected fields was assessed using Image-Pro Plus software (version 6.0, Media Cybernetics, Rockville, MD, USA).
Immunohistochemistry (IHC) and Evaluation
The paraffin-embedded spinal cords were cut into 4 μm glass slides and dewaxed, then dehydrated with xylene and gradient concentrations of alcohol. Antigen retrieval was operated by autoclaving at 120°C for 15 min in citrate buffer, and then sections were incubated with 3% hydrogen peroxide for 10 min to inactivate endogenous catalase. After that, sections were blocked with 10% goat serum and primer antibodies TNF-α (1:100 dilution), IL-6 (1:500 dilution), IL-1β (1:100 dilution) were added all night at 4°C. In addition, the sections were incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG secondary antibody (1:1000 dilution) for 1 h at room temperature and added with the streptavid in HRP in the end. The expression of TNF-α, IL-6, and IL-1β of spinal cords were observed under light microscope (Olympus, Tokyo, Japan) and analyzed by Image Pro Plus 6.0 software.
Evaluation of the Oxidative Stress
After the last behavioral test, the rats were sacrificed. L4-6 spinal cord was taken out and weighed, and then it was added Radio Immunoprecipitation Assay (RIPA) Lysis Buffer. Afterwards, the spinal cord was homogenized on ice with a glass homogenizer and centrifuged (4°C, 10,000g) to obtain the supernatant. BCA method was used to determine protein concentration. The activity of SOD, GSH-PX, CAT, and MDA content of the spinal cord was measured according to the kit instructions.
Statistical Analysis
Statistical analyses were carried out using GraphPad Prism (version 6.0) software. The results are presented as mean ± standard deviation (SD) and were analyzed using one-way analysis of variance (ANOVA) followed by posthoc tests, and two-way repeated analysis of ANOVA by R (https://www.r-project.org/), p<0.05 was considered statistically significant.
Results
The Targets of AS-IV Were Used to Establish the Protein–Protein Regulation Network
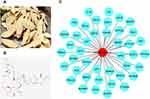
AS-IV is isolated from Astragali Radix (Figure 1A), and its two-dimensional structure is shown in Figure 1B. Forty-three target of AS-IV were identified using TTD Database, Drugbank Database and CTD Database, and these genes were further used to construct a protein regulatory network composed of about 44 link nodes, these nodes represent the possible targets of AS-IV (Figure 1C).
The Collective Targets of AS-IV and Oxaliplatin Neurotoxicity Were Used to Construct the Protein–Protein Regulation Network
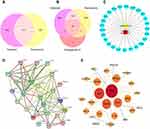
Genecards Database and CTD Database were used to identify 3107 targets of oxaliplatin, Disgenet Database and Genecards Database were utilized to identify 5774 targets of neurotoxicity. The intersection of the 1217 targets was defined as the candidate targets of oxaliplatin neurotoxicity (Figure 2A). Twenty-five collective targets of AS-IV and oxaliplatin neurotoxicity were further obtained (Figure 2B), the targets were used to construct a protein regulatory network composed of 27 link nodes (Figure 2C). The protein–protein interaction network of 25 targets was drawn by string database (Figure 2D) and analyzed by cytoscape 3.7.2 software to obtain the most important indicators related to inflammation and oxidative stress, the larger area and the darker color mean the indicators are more important (Figure 2E).
Effects of AS-IV Administration on Body Weight and MWT and Cold Allodynia of Model Rats
Body weights were recorded to evaluate the general health status of the rats. Oxaliplatin-induced rats exhibited an obvious decrease in body weight, and AS-IV improved this (Figure 3A). The MWT of rats in the model group was significantly decreased after 7, 14, 21, and 28 days of administration of oxaliplatin compared with the control group (p<0.05). AS-IV administration improved the MWT induced by oxaliplatin in a dose-dependent manner (10 mg/kg AS-IV vs model group, day 21 and 28, p<0.05; 20 mg/kg AS-IV vs model group, day 14, 21, and 28, p<0.05; 40 mg/kg AS-IV vs model group, day 7, 14, 21, and 28, p<0.05) (Figure 3B). Compared with the control group, cold withdrawal times were significantly increased in model rats following 7, 14, 21, and 28 days of oxaliplatin treatment (p<0.05). These effects were significantly neutralized by AS-IV administration in a dose-dependent manner (10 mg/kg AS-IV vs model group, day 21 and 28, p<0.05; 20 mg/kg AS-IV vs model group, day 14, 21, and 28, p<0.05; 40 mg/kg AS-IV vs model group, day 7, 14, 21 and 28, p<0.05) (Figure 3C). There was no significant difference between control + 40 mg/kg group and control group (p>0.05). The results indicate that AS-IV administration might normalize the behavior of model rats.
Effects of AS-IV Administration on Sciatic Nerve Conduction Velocity in Model Rats
As damage to spinal cord neurons can lead to neurotoxicity, we explored the effects of AS-IV administration on sciatic nerve conduction velocity in oxaliplatin neurotoxicity rats. Oxaliplatin administration significantly decreased the MCV and SCV of rats, whereas 10 mg/kg, 20 mg/kg, and 40 mg/kg AS-IV rescued these decreases (10 mg/kg and 20 mg/kg AS-IV vs model group, p<0.05; 40 mg/kg AS-IV vs model group, p<0.01) (Figure 4). There was no significant difference between control + 40 mg/kg group and control group (p>0.05). This suggests that AS-IV administration exerts a protective role against oxaliplatin neurotoxicity in rats.
Effects of AS-IV Administration on Histological Changes in the Spinal Cords of Model Rats
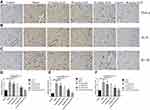
The structure of the spinal cord, morphology, and Nissl bodies of neurons were normal in the control group. In the model group, the nucleus and nucleoli of neurons were pyknotic, some neurons had shrunk, and the cytoplasm was condensed and had separated from the surrounding cells. Different concentrations of AS-IV improved these histological changes (Figure 5A). In addition, the number of Nissl bodies was significantly decreased in model rats, and this was reversed by AS-IV administration (vs model group, p<0.01) (Figure 5B and C). There was no significant difference between control + 40 mg/kg group and control group (p>0.05). These results further suggest that AS-IV administration might repair damaged nerves in the spinal cords of model rats.
Effects of AS-IV Administration on Inflammation of Spinal Cord in Model Rats
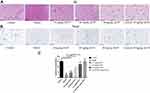
As inflammation is one of the hallmarks which leads to neuropathic pain[6], we explored the effects of AS-IV administration on the inflammation of spinal cord in model rats using IHC. Compared with the control group, the expression levels of TNF-α, IL-6, and IL-1β were significantly elevated in the model group, and AS-IV administration obviously rescued the increased expressions of TNF-α, IL-6, and IL-1β induced by oxaliplatin (TNF-α 10 mg/kg AS-IV vs model group, p<0.05, 20 mg/kg and 40 mg/kg AS-IV vs model group, p<0.01; IL-6 and IL-1β all AS-IV vs model group, p<0.01) (Figure 6). There was no significant difference between control + 40 mg/kg group and control group (p>0.05). These results suggest that AS-IV administration can repress the inflammatory responses in rat spinal cord induced by oxaliplatin.
Effects of AS-IV Administration on Oxidative Stress in the Spinal Cords of Model Rats
Compared with the control group, the relative absorbance of MDA was significantly increased (10 mg/kg AS-IV vs model group, p<0.05; 20 mg/kg and 40 mg/kg AS-IV vs model group, p<0.01), while the levels of SOD, CAT, and GSH-Px were decreased in the model group (SOD and GSH-Px, all AS-IV vs model group, p<0.01; CAT, 10 mg/kg AS-IV vs model group, p<0.05; 20 mg/kg and 40 mg/kg AS-IV vs model group, p<0.01). AS-IV administration reversed these changes in a dose-dependent manner (Figure 7). There was no significant difference between control + 40 mg/kg group and control group (p>0.05). These results suggest that AS-IV administration can suppress oxidative stress induced by oxaliplatin.
Discussion
Neurotoxicity is a common and dose-limiting side effect of oxaliplatin.14 Oxaliplatin neurotoxicity is characterized by a specific somatosensory profile including cold and mechanical allodynia.15 There is no specific drug to treat it; thus, it is critical to find new treatment options.
Astragali Radix is a traditional Chinese medicine and has been reported to reduce blood pressure and cholesterol levels, improve liver and heart function,16 and regulate immune responses.17 Based on current knowledge, Astragali Radix does not affect the therapeutic effects of anticancer drugs. On the other hand, accumulating evidences suggest that Astragali Radix can be used as an adjunct drug for cancer treatment.18 It can enhance chemotherapy sensitivity and significantly reduce therapy-induced toxicity. In addition, Di Cesare Mannelli reported protective properties of an aqueous and two hydroalcoholic extracts of Astragali Radix in oxaliplatin-treated nervous cells, with no obvious influence on oxaliplatin-mediated cancer cell lethality in human colon cancer HT-29 cells.19 As AS-IV is a primary constituent of Astragali Radix, we hypothesized that AS-IV would also play a protective role in oxaliplatin neurotoxicity.
Network pharmacology is a new discipline aiming to find special drug targets based on the theory of system biology.20 Compared with experimental pharmacology, it emphasizes multi-pathway regulation of targets and is particularly suitable for explaining the mechanism of Traditional Chinese Medicine with multiple chemical components and numerous targets.21,22 This new method can increase the therapeutic effect of drugs, reduce toxic and side effects, enhance the success rate of clinical trials, and save development costs.23
“AS-IV-targets-Oxaliplatin Neurotoxicity” regulation network was established based on the principles of network pharmacology and multiple databases. It was found that targets of AS-IV on Oxaliplatin Neurotoxicity are closely related with inflammation and oxidative stress. Especially some targets such as TNF-α, IL-6, SOD, and CAT were worthy of further testing.
Then, we measured the behavior of rats of oxaliplatin neurotoxicity and evaluated the effect of AS-IV. The results of this study indicated that administration of oxaliplatin to rats significantly decreased bodyweight, MWT, MCV, SCV, and the number of Nissl bodies, and significantly increased the cold allodynia. This indicates that the neurotoxicity model was successfully induced in the rats. Further, the results indicated that changes in spinal cord structure and function caused by oxaliplatin may lead to the occurrence and maintenance of abnormal tactile and pain perception in rats.
Based on the network pharmacology and the efficacy evaluation, we further studied the mechanism of AS-IV on Oxaliplatin Neurotoxicity. Inflammation and oxidative stress play an important role in nerve damage.
Nerve injury can activate the glial cells in spinal cord to release inflammatory mediators such as TNF-α, IL-6, and IL-1β. These mediators lead to the enhancement and maintenance of neuropathic pain by regulating the excitability and sensitivity to injury stimuli. Neuropathic pain shows increased sensitivity to hyperalgesia and abnormal pain, which is also one of the common symptoms of oxaliplatin neurotoxicity. Gopalsamy found that chronic constriction injury model rats reached the peak of IL-1β content after 24 h of nerve injury.24 In the rat model of sciatic nerve cryolysis, IL-6 was observed in the dorsal and ventral horns of the spinal cord. Another study verified that IL-1β and IL-6 caused nerve damage and induced the occurrence of joint neuropathic pain.25 Early rapid intervention on inflammation could relieve osteoarthritis. The application of TNF-α and IL-1β antagonists around or within the painful area can effectively suppress the pain.26 Our experiments showed that AS-IV can down-regulate the expression of TNF-α, IL-6, and IL-1β, thereby alleviating the neurotoxicity of oxaliplatin.
Oxidative stress plays an important role in oxaliplatin neurotoxicity.27 All kinds of harmful stimuli, including oxaliplatin, can block the imbalance between the oxidation system and the antioxidant system, resulting in tissue damage. Oxidative stress is involved in the occurrence and development of neurotoxicity.28 A study found that early application of antioxidant enzymes can reduce neuron dysfunction.29 Since there are many types of GPX (GSH-Px) in the network of 25 targets, GSH-Px can also be used as one of the indicators in oxaliplatin neuropathy. SOD and GSH-Px are the main enzymes for scavenging oxygen-free radicals, which can protect the structural integrity of the body’s cell membrane. MDA is the oxidation product of oxygen-free radicals and cell membrane lipids. A higher concentration of MDA means that there is more serious oxidative damage, and thus, this can be used as an indicator to evaluate the level of oxidative stress.30 To this end, we administered different concentrations of AS-IV (10, 20, and 40 mg/kg) to oxaliplatin-treated rats or normal rats. We observed that all concentrations of AS-IV significantly increased the content of SOD and GSH-Px, reduced the content of MDA, and inhibited oxidative stress induced by oxaliplatin.
Conclusion
In conclusion, we have developed a comprehensive network of pharmacology to determine the 25 collective targets of AS-IV and oxaliplatin neurotoxicity, experiments confirmed that AS-IV could regulate the neuroinflammation and oxidative stress to improve oxaliplatin neurotoxicity (Figure 8), providing new insights into potential treatment strategies for this disease, the results also give data support for the clinical application of AS-IV on oxaliplatin neurotoxicity.
Acknowledgment
This study was supported by funding from the National Natural Science Foundation of China (Grant No.81202678, 81302933) and a Science and Technology Commission Guidance Project of Shanghai (Grant No.14401931300, 17401934300).
These authors are regarded as co-first authors: Jingyu Xu, Zhenbiao Guan, and Xiaowei Wang.
Disclosure
Xuan Zhang reports grants from National Natural Science Foundation of China and Science and Technology Commission Guidance Project of Shanghai, during the conduct of the study. The authors declare that they have no other potential conflicts of interest for this work.
References
1. Kim CA, Spratlin JL, Armstrong DE, et al. Efficacy and safety of single agent or combination adjuvant chemotherapy in elderly patients with colon cancer: a Canadian cancer institute experience. Clin Colorectal Cancer. 2014;13(3):
2. Xiao WH, Zheng H, Bennett GJ. Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience. 2012;203:
3. Han CH, Kilfoyle DH, Hill AG, et al. Preventing oxaliplatin-induced neurotoxicity: rationale and design of phase Ib randomized, double-blind, placebo-controlled, cross-over trials for early clinical evaluation of investigational therapeutics. Expert Opin Drug Metab Toxicol. 2016;12(12):
4. Hu S, Huang KM, Adams EJ, et al. Recent developments of novel pharmacologic therapeutics for prevention of chemotherapy-induced peripheral neuropathy. Clin Cancer Res. 2019;25(21):
5. Auyeung KK, Han QB, Ko JK. Astragalus membranaceus: a review of its protection against inflammation and gastrointestinal cancers. Am J Chin Med. 2016;44(1):
6. Kim S, Kang IH, Nam JB, et al. Ameliorating the effect of astragaloside IV on learning and memory deficit after chronic cerebral hypoperfusion in rats. Molecules. 2015;20(2):
7. Yang P, Zhou Y, Xia Q, et al. Astragaloside IV regulates the PI3K/Akt/HO-1 signaling pathway and inhibits H9c2 cardiomyocyte injury induced by hypoxia-reoxygenation. Biol Pharm Bull. 2019;42(5):
8. Ye Q, Su L, Chen D, et al. Astragaloside IV induced miR-134 expression reduces EMT and increases chemotherapeutic sensitivity by suppressing CREB1 signaling in colorectal cancer cell line SW-480. Cell Physiol Biochem. 2017;43(4):
9. Wang S, Mou J, Cui L, et al. Astragaloside IV inhibits cell proliferation of colorectal cancer cell lines through down-regulation of B7-H3. Biomed Pharmacother. 2018;102:
10. Zhu J, Wen K. Astragaloside IV inhibits TGF-β1-induced epithelial-mesenchymal transition through inhibition of the PI3K/Akt/NF-κB pathway in gastric cancer cells. Phytother Res. 2018;32(7):
11. Zhu B, Zhang W, Lu Y, et al. Network pharmacology-based identification of protective mechanism of panax notoginseng saponins on aspirin induced gastrointestinal injury. Biomed Pharmacother. 2018;105:
12. Holmes J, Stanko J, Varchenko M, et al. Comparative neurotoxicity of oxaliplatin, cisplatin, and ormaplatin in a wistar rat model. Toxicol Sci. 1998;46(2):
13. Kawashiri T, Egashira N, Watanabe H, et al. Prevention of oxaliplatin-induced mechanical allodynia and neurodegeneration by neurotropin in the rat model. Eur J Pain. 2011;15(4):
14. Argyriou AA, Polychronopoulos P, Iconomou G, et al. A review on oxaliplatin-induced peripheral nerve damage. Cancer Treat Rev. 2008;34(4):
15. Balayssac D, Ferrier J, Pereira B, et al. Prevention of oxaliplatin-induced peripheral neuropathy by a polyamine-reduced diet-NEUROXAPOL: protocol of a prospective, randomised, controlled, single-blind and monocentric trial. BMJ Open. 2015;5(4):e007479. doi:10.1136/bmjopen-2014-007479
16. Fu J, Wang Z, Huang L, et al. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother Res. 2014;28(9):
17. Zhang A, Zheng Y, Que Z, et al. Astragaloside IV inhibits progression of lung cancer by mediating immune function of tregs and CTLs by interfering with IDO. J Cancer Res Clin Oncol. 2014;140(11):
18. Chen M, May BH, Zhou IW, et al. FOLFOX 4 combined with herbal medicine for advanced colorectal cancer: a systematic review. Phytother Res. 2014;28(7):
19. Di Cesare Mannelli L, Zanardelli M, Bartolucci G, et al. In vitro evidence for the use of astragali radix extracts as adjuvant against oxaliplatin-induced neurotoxicity. Planta Med. 2015;81(12–13):
20. Li S, Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin J Nat Med. 2013;11(2):
21. Yuan H, Ma Q, Cui H, et al. How can synergism of traditional medicines benefit from network pharmacology? Molecules. 2017;22(7):1135. doi:10.3390/molecules22071135
22. Hao da C, Xiao PG. Network pharmacology: a rosetta stone for traditional Chinese medicine. Drug Dev Res. 2014;75(5):
23. Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6(1):13. doi:10.1186/1758-2946-6-13
24. Gopalsamy B, Farouk AAO, Tengku Mohamad TAS, Sulaiman MR, Perimal EK. Antiallodynic and antihyperalgesic activities of zerumbone via the suppression of IL-1β, IL-6, and TNF-α in a mouse model of neuropathic pain. J Pain Res. 2017;10:
25. Muley MM, Krustev E, Reid AR, McDougall JJ. Prophylactic inhibition of neutrophil elastase prevents the development of chronic neuropathic pain in osteoarthritic mice. J Neuroinflammation. 2017;14(1):168. doi:10.1186/s12974-017-0944-0
26. Ogawa N, Kawai H, Terashima T, et al. Gene therapy for neuropathic pain by silencing of TNF-α expression with lentiviral vectors targeting the dorsal root ganglion in mice. PLoS One. 2014;9(3):e92073. doi:10.1371/journal.pone.0092073
27. Pacurari M, Waugh S, Krajnak K. Acute vibration induces peripheral nerve sensitization in a rat tail model: possible role of oxidative stress and inflammation. Neuroscience. 2019;398:
28. Flatters SJL, Dougherty PM, Colvin LA. Clinical and preclinical perspectives on Chemotherapy-Induced Peripheral Neuropathy (CIPN): a narrative review. Br J Anaesth. 2017;119(4):
29. Karlsson JOG, Andersson RG, Jynge P. Mangafodipir a selective cytoprotectant - with special reference to oxaliplatin and its association to Chemotherapy-Induced Peripheral Neuropathy (CIPN). Transl Oncol. 2017;10(4):
30. Zhang X, Guan Z, Wang X, et al. Curcumin alleviates oxaliplatin-induced peripheral neuropathic pain through inhibiting oxidative stress-mediated activation of NF-κB and mitigating inflammation. Biol Pharm Bull. 2020;43(2):
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.