Back to Journals » Research and Reports in Neonatology » Volume 8
Neonatal periventricular leukomalacia: current perspectives
Authors Ahya KP, Suryawanshi P
Received 7 August 2017
Accepted for publication 17 October 2017
Published 10 January 2018 Volume 2018:8 Pages 1—8
DOI https://doi.org/10.2147/RRN.S125575
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Robert Schelonka
Kunal P Ahya,1 Pradeep Suryawanshi2
1Department of Neonatology, Maahi Newborn Care Centre, Rajkot, Gujarat, 2Department of Neonatology, BVDU Medical College, Pune, Maharashtra, India
Abstract: Significant advances in the neonatal ICU have improved the survival of extreme premature neonates; with this comes the importance of intact survival. Periventricular leukomalacia (PVL) is the commonest white matter brain injury in preterm infants. It has a typical distribution at the watershed areas adjacent to the lateral ventricles. PVL occurs because of ischemic injury to periventricular oligodendrocytes of the developing brain. It can be detected by cranial ultrasonography (CUS) as initial periventricular echodensities, followed later by cystic formation. Recent magnetic resonance imaging studies have shown that it helps in early visualization of PVL and also detection of non-cystic form of PVL, which is not picked up by CUS. It is the commonest cause of cerebral palsy, intellectual impairment or visual disturbances. Currently, no medical treatment is available for PVL; prevention and close developmental follow-up are the only options.
Keywords: periventricular leukomalacia, preterm brain injury, cranial ultrasonography
Introduction
The word “leukomalacia” is derived from “leukos” meaning white and “malacia” means softening.1 Periventricular leukomalacia (PVL) is the leading cause of nonhemorrhagic neuropathological abnormality in the cerebral white matter of a premature infant.2 PVL is more common in premature infants than in term infants, and the incidence increases with decreasing gestation age.3 This disease affects the immature white matter of the cerebral hemispheres and peaks at 24–32 gestational weeks and in those with a weight <1500 g.4 The incidence of PVL reported in some countries was 19.8%–34.1% for overall PVL and 2.5%–23% for cystic PVL. The evidence of PVL was found to be up to 75% in preterm infants and up to 20% in term infants in previous neuropathological studies.5,6 PVL is defined morphologically by two histopathologic components: 1) a “focal”, necrotic component in the periventricular region of the cerebral white matter and 2) a “diffuse” component characterized by reactive gliosis in the surrounding white matter.7 PVL is the leading cause of cerebral palsy (CP) and cognitive deficits in premature infants.8 Cranial ultrasonography (CUS) is the preferred bedside tool for diagnosis of PVL but is able to identify only cystic form of PVL. Magnetic resonance imaging (MRI), however, is more ideal as it can identify the lesion early and can also identify the non-cystic form of PVL.9
Periventricular white matter damage was observed in preterm babies over 100 years ago in 1867 by Virchow.10 Banker and Larroche11 initially described focal coagulation necrosis in periventricular white matter of autopsy brain specimens in preterm babies and used the term PVL. CUS grading of PVL was done by de Vries et al12 in 1992 and serves as a guide for assessing severity and prognosis.
Pathology
PVL consists of initial periventricular focal coagulation necrosis at 3–6 hours after the initial insult, followed by microglial activation at 6–8 hours and several days later karyorrhexis and astrocytic degeneration with macrophage infiltration. Subsequent formation of microcavities occurs between 8 and 12 days, and microcavities are formed by 2 weeks. These cavities are not in communication with the lateral ventricles.1,11 Topographically, the lesions are uniform, affecting the white matter in the zone within the subcallosal, superior fronto-occipital and superior longitudinal fasciculi, the external and internal border zones of the temporal and occipital horn of lateral ventricles and parts of corona radiata.6,13 Anatomical distribution of PVL correlates with the development of perforating medullary arteries and areas that represent arterial border or end zones that arise between ventriculopetal and ventriculofugal arteries within the deep white mater.14
Pathophysiology
The pathophysiology of PVL is a multifactorial and complex process (Figure 1).15 There are several evidences that imply the vulnerability of pre-oligodendrocytes, which are present between gestation of 24 and 32 weeks and are more sensitive to toxic effects than progenitor and mature oligodendrocytes.16,17 There is a marked increase in microglial activity in diffuse white matter as identified by CD68 markers.18 By 22 weeks of gestation, microglial cells are dispersed throughout the white matter and are capable of producing toxic inflammatory mediators, free radicals and reactive oxygen intermediates.19 Axonal and neuronal damages from the corticospinal tract, thalamocortical fibers, optic radiation, superior occipital fasciculus and the superior longitudinal fasciculus may be affected and result in motor, sensory, visual and higher cortical function deficits.20 The subplate neurons are selectively sensitive to hypoxia–ischemia, leading to increased apoptosis and reduction in cortical and thalamic volumes in infants with PVL.21–23
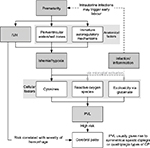  | Figure 1 Pathogenesis of PVL. Note: Reproduced with permission from Eric Wong and Sultan Chaudhry, McMaster Pathophysiology Review, www.pathophys.org.15 Abbreviations: CP, cerebral palsy; IVH, intraventricular hemorrhage; PVL, periventricular leukomalacia. |
Data indicate that the pathogenesis of PVL is the effect of hypoxia, ischemia and inflammation on the progenitor oligodendrocyte cells present during 23–32 weeks of gestation in the periventricular area.24 Risk in the preterm brain is due to arterial border and end zones within the white matter and impairment in the autoregulation of cerebral blood flow.1 The periventricular region is more vulnerable because it is perfused by long penetrators forming distal arterial fields that are sensitive to fall in cerebral perfusion.14,25 Poor perfusion in the shorter penetrators that anastomose with long penetrators leads to diffuse white matter injury. The short penetrators and their anastomoses increase in the third trimester, hence decreasing white matter vulnerability. Hypocarbia and patent ductus arteriosus (PDA) leading to cerebral steal are shown to have been linked to PVL.26,27 Failure of cerebral autoregulation in sick premature infant is demonstrated by continuous near-infrared spectrometry, which increases the vulnerability of white matter in babies with decreasing systemic blood pressure as a result of sepsis or other causes.28
Any inflammatory or infective process leads to systemic upregulation of proinflammatory cytokines and diffuse activation of microglia within the immature white matter, which includes intrauterine infections, neonatal or fetal infections and ischemia-induced inflammation.29 Combined elevation of serum CRP and interleukin 6 at birth are predictors for white matter injury in preterm infants with fetal inflammatory response.30
Excitotoxicity and free radical attack as mechanisms of cell injury are linked. After the initial insult either by ischemia or inflammation, injury to immature premyelinating oligodendrocytes (pre-OLs) occurs either by free radical or excitotoxicity.15 Excitotoxicity renders the pre-OL permeable to calcium influx, activation of cytotoxic enzymes, caspase activation and excitotoxic-induced apoptotic cell death.31 Pre-OL excitotoxicity is thought to result from interferon-γ, tumor necrosis factor-α and glutamate.1
Ischemia and reperfusion of the brain tissue produce huge amount of reactive oxygen and nitrogen species that overwhelm the low concentration of superoxide dismutase, catalase and glutathione peroxidase in pre-OLs.24 Active incorporation of iron occurs during normal pre-OL differentiation, which adds to the free radical burden.32 Nitrotyrosine and other nitrosative products are also found in injured pre-OLs, and inducible nitric oxide synthase is expressed in reactive microglia in diffuse PVL.33 Oxidative and nitrosative stresses lead to generation of harmful reactive oxygen and nitrogen species that damage membrane lipids and initiate cascade of events leading to apoptotic cell death of various cerebral white matter, including pre-OLs.
Axon and astrocytes are more resistant to injury; all cellular components are injured in cystic PVL. In diffuse PVL, pre-OLs are the primary cell types undergoing apoptosis.34 Post 32 weeks of gestation, pre-OLs differentiate into mature myelin-producing oligodendrocytes that are more resistant to free radical injury.24
Clinical aspects
PVL is more common in preterm infants; however, it is not uncommon in term infants subjected to hypoxic ischemic insults.6 Clinical risk factors for PVL could be prenatal, perinatal or neonatal, which increases the chances of hypoxia, ischemia or inflammation (Table 1).24 The role of prenatal factors leading to development of PVL is well established.35 Clinical and epidemiological studies suggest a relation between maternal/fetal infection and inflammation and the occurrence of PVL, which can be due to fetal systemic inflammatory response.6
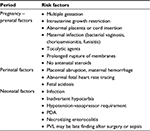  | Table 1 Clinical risk factors associated with the development of PVL Abbreviations: PDA, patent ductus arteriosus; PVL, periventricular leukomalacia. |
Infants developing PVL may not show any specific neurological symptoms and have a relatively benign clinical course. Initial symptoms may include decreased tone in lower extremities, increased tone in neck extensors, apnea, bradycardia, irritability and pseudobulbar palsy with poor feeding.36 Long-term correlates of PVL include spastic diplegia, motor deficits, cognitive deficits, visual deficits and behavioral/attentional deficits.6 Although clinical seizures are rare, may be seen in severe forms of PVL, electroencephalography (EEG) can show positive rolandic sharp waves in the central region or lower spectral frequency.37,38 However, the role of EEG as a screening tool is unclear.
Diagnosis
PVL can be diagnosed in neonates by neuroimaging using CUS, computed tomography (CT) or MRI.
CUS
CUS is the initial modality of choice for evaluation of PVL in premature infants.39 Sonographically, PVL initially appears as a highly echogenic area in the parietal lobe adjacent to the lateral ventricles or in the frontal lobes. Diagnosis relies on the echogenicity of the periventricular brain; if the parenchyma adjacent to the lateral ventricles appears more echogenic than the choroid plexus, PVL must be considered.40
Classification of PVL
PVL is classified as follows:12
- Grade I: transient periventricular echodensities persisting for >7 days;
- Grade II: transient periventricular echodensities evolving into small, localized frontoparietal cysts
- Grade III: periventricular echodensities evolving into extensive periventricular cystic lesions and
- Grade IV: densities extending into the deep white matter evolving into extensive cystic lesions.
There are two phases of the evolution of PVL:
- Early acute phase: the acute insult may occur in the late antenatal or early postnatal period. Hence, echogenicity may be seen in the end of first week up to 10 days.
- Late chronic changes: seen as a “Swiss-cheese” multicystic appearance. This evolves over a period of 4–6 weeks.
– Grade I PVL (Figure 2): this is very similar to normal anatomical variations, often referred to as venous congestion or peritrigonal blush. The choroid plexus can be used as a reference point for diagnosing leukomalacia. Echogenicity more than that of the choroid plexus may be significant. A “peritrigonal blush” is soft, symmetrical, radial echodensity, normally seen around frontal horns and the parieto-occipital junction of the lateral ventricles. Persistence of “flare” >7–14 days may be considered abnormal and indicative of white matter damage, which correlates with abnormalities on MRI. Grade I PVL may not progress to cyst formation but may lead only to discrete ventriculomegaly.
– Grade II PVL: limited (focal) cystic PVL (Figure 3): white matter cysts within the flare may be localized and few in number and usually noted in the frontoparietal white matter. If the cysts start coalescing, the neonate may develop spastic diplegia.
– Grade III extensive cystic PVL (Figure 4): cyst formation takes place during the second or third week after insult and represents total tissue necrosis. When they are widespread across the fronto–parieto–occipital region, they are referred to as Grade III PVL. The cystic zone is smaller than the hyperechoic zone. Smaller cysts may merge into larger cysts, leading to an area of irregular cavitation with intraluminal septae (Swiss-cheese pattern).
– Grade IV: subcortical leukomalacia (Figure 5): this rare condition is seen in more mature preterm neonates and post asphyxial term neonates where periventricular and cortical cysts develop. Prognosis is dismal, with inevitable microcephaly, spastic quadriplegia and severe learning disability.
A practice parameter on neuroimaging of the neonate by the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society made the following recommendations: routine ultrasound screening should be performed on all infants with a gestational age <30 weeks. Screening should be performed at 7–14 days of age and repeated at 36–40 weeks of postmenstrual age.41
CUS has limited sensitivity and specificity to detect PVL, especially if lesions are <0.5 cm.42 CUS is able to detect abnormalities in ~50% of infants who subsequently develop CP, indicating that a significant number of infants are missed.43 Serial ultrasound examination and MRI of the brain improve the detection of PVL.44 CUS also has a poor sensitivity for detection of non-cystic PVL, and MRI is the preferred modality for detecting non-cystic PVL.45
CT
CT scan is not the preferred modality of imaging in cases of PVL as it detects fewer lesions than CUS and MRI of the brain in preterm infants.46 The findings include ventriculomegaly involving the lateral ventricles with irregular margins and loss of deep white matter.36
MRI
MRI may not always be feasible in unstable premature infants but is often successful in visualizing PVL earlier than ultrasonography.47 Repeated studies and diffusion tensor imaging (DTI) can provide prognostic information by documenting gray matter atrophy and tract degenerations.8 PVL is seen as a hypointense periventricular lesion on T1-weighted MRI images and as a hyperintense periventricular lesion on T2-weighted images.48 MRI can detect all forms of cystic and non-cystic diffuse white matter injuries with higher sensitivity. Diffusion-weighted imaging (DWI) enables earlier detection of PVL.49 Infants having MRI suggestive of PVL are at a greater risk of developing cognitive and behavioral deficits. MRI clearly detects glial scars after resolution of cysts and changes in the internal capsule and thalamus.45,50 Signal changes in the posterior limb of internal capsule have been correlated with the development of CP.51 Diffuse excessive high signal intensity (DEHSI) is a T2-weighted MRI white matter abnormality seen in preterm infants.52 Water does not flow uniformly along the white matter tracts in injured brain; this alteration in water diffusion can be detected by DTI.16,52
Management
No specific treatment exists for PVL. Infants with PVL require close neurodevelopmental follow-up after discharge from the hospital. Follow-up should be done at high-risk clinics consisting of a team of pediatrician, pediatric neurologist, physiotherapist, occupational therapist, developmental specialist and ophthalmologist. Early intervention strategies carried out by occupational therapists or physical therapists may decrease symptoms and may increase the infant’s motor function.36 Neuroplasticity is the capacity of neurons and their network in the brain to change their connections and behavior in response to new information, sensory stimulation, development, damage or dysfunction. Exposure to enriched sensory environment and a nutritious diet are associated with improved cognitive outcomes and increased brain growth for infants with perinatal brain damage.53
Neurodevelopmental outcome
Cystic PVL damages deeper and more medial fiber tracts that control the lower extremity functions, leading to spastic diplegia. Upper extremity function can also be impaired by involvement of more lateral fibers.6 Initially, infants may have low tone and poor head control, increased tone in neck extensors, apnea, bradycardia, irritability and pseudobulbar palsy with poor feeding.36 After several weeks, characteristic features of spastic diplegia (ie, increased tone, brisk deep tendon reflex, scissoring of lower extremities, contractures and abnormal gross and fine motor incoordination) may be seen. Extensive white matter involvement may result in quadriplegia.24 Cerebral visual impairment is an important sequel of PVL and can be predicted with abnormalities within optic radiation on MRI.54 Ability to predict motor disability is better than predicting more subtle forms of neurodevelopmental impairment or neuropsychiatric disorders associated with diffuse PVL.
Prevention
Prevention of premature birth is the most important measure to prevent PVL.36 Tocolytic agents are used for the prevention of premature birth; however, this can have deleterious effects if used in cases of premature rupture of membranes with chorioamnionitis. Prevention of fall of Pco2 level to <35 mmHg substantially lowers the risk of CP, and careful monitoring of arterial blood gas values diminishes the severity of PVL.55,56 Diagnosis and treatment of chorioamnionitis may prevent PVL.57 Antenatal betamethasone to mothers at 24–31 weeks of gestation significantly reduced the risk of PVL, suggesting the possible effect of steroids on fetal inflammatory response.58,59 Avoiding maternal cocaine abuse and maternal fetal blood flow alterations can minimize the incidence of PVL. Postnatally, avoiding blood pressure fluctuations and hypotension also reduces the incidence of PVL.36 A few studies have shown that antenatal magnesium sulfate during preterm labor has reduced the incidence of moderate-to-severe CP, but it was not specific to reduction in PVL.60
Acknowledgment
The relatives of the patients provided written informed consent for the publication of the images.
Disclosure
The authors report no conflicts of interest in this work.
References
Dyet LE, Rennie JM. Preterm brain injury. In: Rennie JM, editor. Text Book of Neonatology. 5th ed. London: Elsevier; 2012:1156–1181. | ||
Volpe JJ. Confusions in nomenclature: “periventricular leukomalacia” and “white matter injury” – identical, distinct or overlapping? Pediatr Neurol. 2017;73:3–6. | ||
Romero-Guzman GJ, Lopez-Munoz F. Prevalence and risk factors for periventricular leukomalacia in preterm infants. A systematic review. Rev Neurol. 2017;62(2):57–62. | ||
Kinney HC. The near-term (late preterm) human brain and risk of periventricular leukomalacia: a review. Semin Perinatol. 2006;30(2):81–88. | ||
Chen HJ, Wei KL, Zhou YJ, et al. Incidence of brain injuries in premature infants with gestational age ≤34 weeks in ten urban hospitals in China. World J Pediatr. 2013;9(1):17–24. | ||
Volpe JJ. Neurology of the Newborn. 5th ed. Philadelphia, PA: WB Saunders; 2008. | ||
Kinney HC, Haynes RL, Folkerth RD. White matter lesions in the perinatal period. In: Golden JA, Harding B, editors. Pathology and Genetics: Acquired and Inherited Diseases of the Developing Nervous System. Basel: ISN Neuropathology Press; 2004:156–170. | ||
Deng W, Pleasure J, Pleasure D. Progress in periventricular leukomalacia. Arch Neurol. 2008;65(10):1291–1295. | ||
Bonifacio SL, Gonzalez F, Ferriero DM. Central nervous system injury and neroprotection. In: Gleason CA, Devaskar SU, editors. Diseases of the Newborn. 9th ed. Elsevier; Philadelphia: Elsevier; 2012:869–891. | ||
Virchow R. Zur pathologischen Anatomie des Gehirns I Congenitale Encephalitis und Myelitis. Virchows Arch Pathol Anat. 1867;38:129–142. | ||
Banker JC, Larroche JC. Periventricular leukomalacia of infancy. A form of neonatal anoxic encephalopathy. Arch Neurol. 1962;7(5):386–410. | ||
de Vries LS, Eken P, Dubowitz L. The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res. 1992;49(1):1–6. | ||
Gilles FH, Murphy SF. Perinatal telencephalic leucoencephalopathy. J Neurol Neurosurg Psychiatry. 1969;32:404–413. | ||
Inage YW, Itoh M, Takashima S. Correlation between cerebrovascular maturity and periventricular leukomalacia. Pediatr Neurol. 2000;22(3):204–208. | ||
Rogers L, Wong E. In: Caturay A, Chaudhry S, editors. Cerebral Palsy. Available from: http://www.pathophys.org/cerebralpalsy/. Accessed September 17, 2017. | ||
Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21(4):1302–1312. | ||
Back SA, Han BH, Luo NL, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia–ischemia. J Neurosci. 2002;22(2):455–463. | ||
Haynes RL, Folkerth RD, Keefe RJ, et al. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol. 2003;62(5):441–450. | ||
Rezaie P, Male D. Colonisation of the developing human brain and spinal cord by microglia: a review. Microsc Res Tech. 1999;45(6):359–382. | ||
Iai M, Takashima S. Thalamocortical development of parvalbumin neurons in normal and periventricular leukomalacia brains. Neuropediatrics. 1999;30(1):14–18. | ||
McQuillen PS, Sheldon RA, Shatz CJ, Ferriero DM. Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J Neurosci. 2003;23(8):3308–3315. | ||
Robinson S, Li Q, DeChant A, Cohen ML. Neonatal loss of gamma-aminobutyric acid pathway expression after human perinatal brain injury. J Neurosurg. 2006;104(6):396–408. | ||
Nagasunder AC, Kinney HC, Bluml S, et al. Abnormal microstructure of the atrophic thalamus in preterm survivors with periventricular leukomalacia. AJNR Am J Neuroradiol. 2011;32(1):185–191. | ||
Bass WT. Periventricular leukomalacia. NeoReviews. 2011;12(2):76–83. | ||
Rorke LB. Anatomical features of the developing brain implicated in pathogenesis of hypoxic-ischemic injury. Brain Pathol. 1992;2(3):211–221. | ||
Shankaran S, Langer JC, Kazzi SN, Laptook AR, Walsh M; National Institute of Child Health and Human Development Neonatal Research Network. Cumulative index of exposure to hypocarbia and hyperoxia as risk factors for periventricular leukomalacia in low birth weight infants. Pediatrics. 2006;118(4):1654–1659. | ||
Shortland DB, Gibson NA, Levene MI, Archer LN, Evans DH, Shaw DE. Patent ductus arteriosus and cerebral circulation in preterm infants. Dev Med Child Neurol. 1990;32(5):386–393. | ||
Soul JS, Hammer PE, Tsuji M, et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res. 2007;61(4):467–473. | ||
Folkerth RD. Neuropathologic substrate of cerebral palsy. J Child Neurol. 2005;20(12):940–949. | ||
Inomata K, Mizobuchi M, Tanaka S, et al. Patterns of increases in interleukin-6 and C-reactive protein as predictors for white matter injury in preterm infants. Pediatr Int. 2014;56(6):851–855. | ||
Follett PL, Deng W, Dai W, et al. Glutamate receptor-mediated oligodendrocyte toxicity in periventricular leukomalacia: a protective role for topiramate. J Neurosci. 2004;24(18):4412–4420. | ||
Baud O, Greene AE, Li J, Wang H, Volpe JJ, Rosenberg PA. Glutathione peroxidase-catalase cooperativity is required for resistance to hydrogen peroxide by mature rat oligodendrocytes. J Neurosci. 2004;24(7):1531–1540. | ||
Haynes RL, Folkerth RD, Trachtenberg FL, Volpe JJ, Kinney HC. Nitrosative stress and inducible nitric oxide synthase expression in periventricular leukomalacia. Acta Neuropathol. 2009;118(3):391–399. | ||
Back SA. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev. 2006;12(2):129–140. | ||
Murphy DJ, Seller S, MacKenzie IZ, Yudkin PL, Johnson AM. Case-control study of antenatal and intrapartum risk factors for cerebral palsy in very preterm singleton babies. Lancet. 1995;346(8988):1449–1454. | ||
Zach T, Kaftan HA, Windle ML, Pramanik AK. In: Rosenkrantz T, MacGilvray SS, Brown JC, editors. Pediatric Periventricular Leukomalacia. 2015. Available from: https://emedicine.medscape.com/article/975728-overview. Accessed May 31, 2017. | ||
Marret S, Parain D, Samson-Dolfus D, Jeannot E, Fessard C. Positive rolandic waves and periventricular leukomalacia in the newborn. Neuropediatrics. 1986;17(4):199–202. | ||
Inder TE, Buckland L, Williams CE, et al. Lowered electroencephalographic spectral edge frequency predicts the presence of cerebral white matter injury in premature infants. Pediatrics. 2003;111(1):27–33. | ||
Sarkar S, Shankaran S, Laptook AR, et al. Screening cranial imaging at multiple time points improves cystic periventricular leukomalacia detection. Am J Perinatol. 2015;32(10):973–979. | ||
Suryawanshi P. Intraventricular hemorrhage and periventricular leukomalacia screening and classification. In: Thakre R, Murki S, editors. Protocols in Neonatology. 1st ed. New Delhi: Jaypee Brother; 2016:277–284. | ||
Ment LR, Bada HS, Barnes P, et al. Practice parameter: neuroimaging of the neonate: report of the quality standards subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;58(12):1726–1738. | ||
Hope PL, Gould SJ, Howard S, Hamilton PA, Costello AM, Reynolds EO. Precision of ultrasound diagnosis of pathologically verified lesions in the brains of very preterm infants. Dev Med Child Neurol. 1988;30(4):457–471. | ||
Mass YG, Mirmiran M, Hart AA, Koppe JG, Ariagno RL, Spekreijse H. Predictive value of neonatal neurological tests for developmental outcome of preterm infants. J Pediatr. 2000;137(1):100–106. | ||
Whyte HE, Blaser S. Limitations of routine neuroimaging in predicting outcomes of preterm infants. Neuroradiology. 2013;55(suppl 2):3–11. | ||
Inder TE, Anderson NJ, Spencer C, Wells S, Volpe JJ. White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. AJNR Am J Neuroradiol. 2003;24(5):805–809. | ||
Keeney SE, Adcock EW, McArdle CB. Prospective observations of 100 high-risk neonates by high-field (1.5 Tesla) magnetic resonance imaging of the central nervous system. II. Lesions associated with hypoxic-ischemic encephalopathy. Pediatrics. 1991;87(4):431–438. | ||
Sie LT, Van der knaap MS, van Wezel-Meijler G, Taets van Amerongen AH, Lafeber HN, Valk J. Early MR features of hypoxic-ischemic brain injury in neonates with periventricular densities on sonograms. AJNR Am J Neuroradiol. 2000;21(5):852–861. | ||
Counsell S, Rutherford M, Cowan F, Edwards A. Magnetic resonance imaging of preterm brain injury. Arch Dis Child Fetal Neonatal Ed. 2003;88(4):269–274. | ||
Inder T, Huppi PS, Zientara GP, et al. Early detection of periventricular leukomalacia by diffusion-weighted magnetic resonance imaging techniques. J Pediatr. 1999;134(5):631–634. | ||
Lin Y, Qkumura A, Hayakawa F, Kato T, Kuno K, Watanabe K. Quantitative evaluation of thalami and basal ganglia in infants with periventricular leukomalacia. Dev Med Child Neurol. 2001;43(7):481–485. | ||
Roelants-van AM, Groenendaal F, Eken P, de Vries LS. Parenchymal brain injury in preterm infant: comparison of cranial ultrasound, MRI and neurodevelopmental outcome. Neuropediatrics. 2001;32(2):80–89. | ||
Dyet LE, Kennea N, Counsell SJ, et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics. 2006;118(2):536–548. | ||
Aisen ML, Kerkovich D, Mast J, et al. Cerebral palsy: clinical care and neurological rehabilitation. Lancet Neurol. 2011;10(9):844–852. | ||
Ricci D, Anker S, Cowan F, et al. Thalamic atrophy in infants with PVL and cerebral visual impairment. Early Hum Dev. 2006;82(9):591–595. | ||
Collins MP, Lorenz JM, Jetton JR, Paneth N. Hypocapnia and other ventilation-related risk factors for cerebral palsy in low birth weight infants. Pediatr Res. 2001;50(6):712–719. | ||
Wiswell TE, Graziani LJ, Kornhauser MS, et al. Effects of hypocarbia on the development of cystic periventricular leukomalacia in premature infants treated with high-frequency jet ventilation. Pediatrics. 1996;98(5):918–923. | ||
Chau V, McFadden DE, Poskitt KJ, Miller SP. Chorioamnionitis in the pathogenesis of brain injury in preterm infants. Clin Perinatol. 2014;41(1):83–103. | ||
Baud O, Foix-L’Helias L, Kaminski M, et al. Antenatal glucocorticoid treatment and cystic periventricular leukomalacia in very premature infants. N Engl J Med. 1999;341(16):1190–1196. | ||
Canterino JC, Verma U, Visintainer PF, Elimian A, Klein SA, Tejani N. Antenatal steroids and neonatal periventricular leukomalacia. Obstet Gynecol. 2001;91(1):135–139. | ||
Crowther CA, Hiller JE, Doyle LW, Haslam RR; Australasian Collaborative Trial of Magnesium Sulphate (ACTOMg SO4) Collaborative Group. Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA. 2003;290(20):2669–2676. |
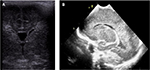  | Figure 2 (A, B) Grade I: transient periventricular echodensities persisting for >7 days. |
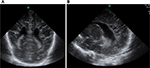  | Figure 3 Grade II periventricular leukomalacia. (A) Sagittal section and (B) coronal section, depicting small frontoparietal cysts. |
Figure 4 Grade III periventricular leukomalacia. (A) Sagittal section and (B) coronal section, showing extensive periventricular cysts.
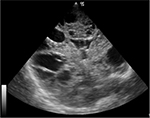  | Figure 5 Grade IV periventricular leukomalacia: densities extending into the deep white matter evolving into extensive cystic lesions. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
