Back to Journals » Infection and Drug Resistance » Volume 14
Naturally Occurring Resistance Associated Substitutions in Non-Cirrhotic, Treatment Naive HCV–HIV Co-Infected Patients Does Not Affect the Treatment Response for Anti-HCV Antiviral Therapy
Authors Gupta E , Agarwal R, Rastogi A, Rani N, Jindal A
Received 8 January 2021
Accepted for publication 13 March 2021
Published 12 April 2021 Volume 2021:14 Pages 1381—1387
DOI https://doi.org/10.2147/IDR.S301032
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Ekta Gupta,1 Reshu Agarwal,1 Aayushi Rastogi,2 Nitiksha Rani,1 Ankur Jindal3
1Department of Clinical Virology, Institute of Liver and Biliary Sciences, New Delhi, India; 2Department of Epidemiology, Institute of Liver and Biliary Sciences, New Delhi, India; 3Department of Hepatology, Institute of Liver and Biliary Sciences, New Delhi, India
Correspondence: Ekta Gupta
Department of Clinical Virology, Institute of Liver and Biliary Sciences, Sector D1, Vasant Kunj, New Delhi, 110070, India
Tel + 91 11 46300000
Fax + 91 11 26123501
Email [email protected]
Purpose: Limited literature on the prevalence of baseline resistance associated substitutions (BL-RAS) among HCV–HIV co-infected patients and their association with treatment outcomes is available especially from India. Hence, the present study aimed to study naturally occurring RAS among non-cirrhotic HCV–HIV co-infected patients and their impact on the response to anti-HCV therapy.
Patients and Methods: In this retrospective study, archived blood samples of 80 HCV–HIV co-infected patients, before anti-HCV therapy initiation, were tested for substitutions at the drug acting sites (NS5a and NS5b) in the HCV genome by direct PCR sequencing.
Results: BL-RAS were seen in 19 (23.7%) patients. As well as BL-RAS, all patients were given sofosbuvir (SOF) 400 mg+ daclatasvir (DCV) 60 mg for 12 weeks. Overall, sustained virological response (SVR) was achieved in 63 (78.8%) patients, in 13 with BL-RAS and in 50 without BL-RAS. All the SVR failure cases (n=17) were retreated with SOF (400 mg) +DCV (60 mg)+ ribavirin (RBV) for 24 weeks. SVR was eventually attained in 14 (82.3%) patients, in 4/6 (66.6%) with BL-RAS and in 10/11 (91%) without BL-RAS. On univariate analysis, age more than 30 years (OR: 11.6; 95% CI: 3.0– 45.5, p-value< 0.001) and female gender (OR: 8.6; 95% CI: 1.1− 69, p-value < 0.009) were found to be significant factors associated with the attainment of SVR.
Conclusion: BL-RAS are common in HCV–HIV co-infected patients. The existence of BL-RAS, however, did not affect the attainment of SVR among non-cirrhotic, treatment naive HCV–HIV co-infected patients.
Keywords: HCV–HIV co-infection, drug resistance, direct acting antiviral, resistance associated substitutions
Introduction
Globally, around 2.3 million people are co-infected with the hepatitis C virus (HCV) and the human immunodeficiency virus (HIV).1 In India the prevalence of HCV–HIV co-infection is around 3–5% but it varies in different geographical regions and in different patient populations.2 The highest prevalence is seen in the Northeast region, around 11–13%, and among intravenous drug users (IVDU).2 HCV–HIV co-infection accelerates the rate of hepatitis C disease progression, leading to early cirrhosis and liver-related complications, thereby increasing mortality rates.3,4 With the advent of direct-acting antiviral (DAA) therapy for HCV, sustained virological response (SVR) is achieved at similar rates among co-infected patients as in HCV mono-infected patients.5–7 Failure to attain SVR despite adequate DAA duration and strict adherence is a multi-factorial issue and is influenced by both host and viral factors.8 Naturally occurring resistance associated substitutions (RAS) in the HCV genome at the drug acting sites before treatment initiation, ie baseline (BL- RAS), are an important virological factor that might influence treatment outcome.9,10 Limited literature determining the prevalence of BL-RAS among HCV–HIV co-infected patients and their association with treatment outcome is available especially from India.11,12 Therefore, in the present study we evaluated the impact of BL-RAS among HCV–HIV co-infected patients on the attainment of SVR.
Patients and Methods
A total of 80 HCV–HIV co-infected non-cirrhotic adult patients were included in this retrospective study (Figure 1). All the patients before initiation of HCV therapy were given standard anti-retroviral treatment and had achieved HIV viral suppression as per the national guidelines.13 None of the patients had previously received anti-viral therapy for HCV. Irrespective of the genotype (Gt), all patients received sofosbuvir (SOF) 400 mg+ daclatasvir (DCV) 60 mg for 12 weeks as per the national guidelines.13 Follow-up records until completion of the treatment were obtained from the hospital information system (HIS). Virological cure was determined by performance of SVR at 12 weeks after the end of treatment by conducting an HCV RNA test on the blood (plasma) sample. Baseline (before initiation of HCV therapy) blood samples were retrieved from −80°C and RAS testing was done. Plasma samples of patients who did not attain SVR were also retrieved and tested for RAS. The study was approved by the Institutional Review Board of the Institute of Liver and Biliary Sciences (IEC No. IEC/2017/49/NA06) and conducted in accordance with the Declaration of Helsinki. The study was performed on anonymised, de-identified archived samples, hence the necessity for patient consent forms was waived by the ethics committee.
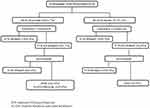 |
Figure 1 Details of the enrolled population. |
HCV RAS Testing
Once thawed, plasma samples were subjected to RAS testing and genotyping by direct PCR sequencing as per the protocol described earlier.14 Viral RNA isolation was done using the High Pure Viral Nucleic Acid Kit (Roche Diagnostics, GmbH, Mannheim, Germany). cDNA was prepared using Quantitect reverse transcription kit (Qiagen, GmbH, Germany). cDNA was subjected to PCR using in-house designed primers. Parts of NS5a and NS5b DAA target regions incorporating all important sites at which known substitutions are present were amplified by single-round PCR reaction using in-house designed primers.14 For the NS5a region, different primer sets were used to amplify samples of Gt1 (F 5ʹ-ACRCACTAYGTGCCBGAGAG-3ʹ, R 5ʹ- RAYCTGGCAHGGGCAYTTNA-3ʹ) and Gt3 (F 5ʹ-CRACNCAYTAYGTYCCYGA-3ʹ, R 5ʹ- CGRTGRAGYCTBACYCCRTC-3ʹ) generating a PCR amplicon of size 554bp and 602bp, respectively. For the NS5b region, pan-genotypic primer set (F 5ʹ-ACYACCATYATGGCNAARARYGAGGT-3ʹ and R 5ʹ- TAYCTRGTCATRGCCTCCGTGAAGRC-3ʹ) were used generating an amplicon size of 631bp. PCR was performed using a Phusion high-fidelity DNA polymerase (Thermoscientific Inc., Waltham, MA). Amplified products were purified by gel-excision using a QIAquick Gel Extraction Kit (QIAGEN, GmbH, Mannheim, Germany). Sanger sequencing was performed with primers as denoted in the Big Dye Terminator v3.1 Cycle Sequencing Kit on an ABI Prism 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA).
RAS Analysis
Sequences were proofread and aligned using the ClustalX version in the BioEdit program v7.2.5 (Carlsbad, CA). RAS analysis was done using a geno2pheno (HCV) online tool.15 When no RAS were detected, amplicons were designated as wild-type and, wherever RAS was detected, it was denoted at that particular amino acid position. Substitutions were reported as per the tool, specific for the genotype, subtype and against the drug used in the study.15
Statistical Analysis
Statistical analysis was done using the Statistical SPSS software, version 21.0 (Chicago, IL, USA). Continuous variables were expressed as mean ± SD or median where appropriate and categorical variables were expressed as a percentage. Univariate analysis was performed with various factors and final attainment of SVR in the study group. The odds ratio (OR) was calculated with a 95% confidence interval (CI). All statistical tests were two-tailed, and results with a p-value of <0.05 were considered statistically significant.
Results
Baseline characteristics of the study population (n=80) are described in Table 1. Mean HIV viral load in all patients was <50 copies/mL at the time of initiation of anti-HCV treatment. Median CD4 count was 341 (IQR: 218–506) cells/mm3. Gt6 was the commonest genotype seen in the study, followed by Gt3; 3b 6xa were the commonest subtypes seen in Gt3 and Gt6, respectively. BL-RAS was seen in 19 (23.7%) patients.
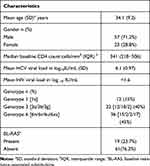 |
Table 1 Baseline Characteristics of the Studied Population (n=80) |
Overall, SVR was attained in 63 (78.8%) patients. Comparison of several factors in the SVR attained and non-attained groups was carried out (Table 2). On univariate analysis, age more than 30 years (OR: 11.6; 95% CI: 3.0–45.5, p-value <0.001) and female gender (OR: 8.6; 95% CI: 1.1−69, p-value <0.009) were found to be significant factors associated with the attainment of SVR. However, no significant association was seen in the study between HCV Gt, existence of BL-RAS and CD4 counts with respect to the attainment of SVR.
 |
Table 2 Factors Associated with Treatment Failure |
SVR failure was seen in 17 (21.2%) cases; 11 did not have any BL-RAS, while 6 had BL-RAS (Figure 1). SVR failure cases belonged to Gt3 in 10 (58.8%) cases, subtype 3b in 4 cases and subtype 3a in 6 cases. Gt6 was seen in 5 (29.4%) cases, with subtype 6xa in 3 cases and subtype 6n in 2 cases. Gt1 was seen in 2 cases and both were subtype 1a. All the SVR failure cases were reinitiated on SOF (400 mg) +DCV (60 mg)+ weight-based ribavirin (RBV) for 24 weeks, following which SVR was eventually achieved in 14 (82.3%) cases; 2 patients died and 1 was lost to follow-up.
Different types of BL–RAS seen in the study as per Gt and subtype are shown in Table 3. All BL-RAS were seen in the NS5a region and none were observed in the NS5b region of the virus. Thirteen (68.4%) patients, despite BL-RAS, attained SVR. In 6 patients with non-attainment of SVR after retreatment, eventually SVR was attained in 4 (66.6%). BL-RAS were not seen in 61 patients and, of those, 50 (81.9%) attained SVR.
 |
Table 3 Distribution of Baseline Resistance Associated Substitutions (BL-RAS) |
Of patients with BL-RAS, 17/19 (89.4%) belonged to Gt3, with 15/17 (88.2%) of subtype 3b and 2 of subtype 3a. The most common BL-RAS was dual RAS at 30K and 31M position observed in 16 (84.21%) patients. Among patients with BL-RAS, SVR was not achieved in 6 (31.5%), and BL-RAS continued to be seen until SVR failure in 4 (66.6%) of them. No new RAS developed at the time of SVR failure in any of the patients included in the study (Table 3).
Discussion
The present study, which is the first of its kind from India, demonstrated a 23.7% prevalence of BL-RAS in an HCV–HIV co-infected group. Earlier studies conducted with this co-infected group showed a prevalence of around 3–16.9%.10,11,16 Success of DAA therapy is usually seen at similar rates among HCV–HIV co-infected patients as in HCV mono-infected patients. In approximately 2–5% cases, where SVR is not attained, many factors have been associated with treatment failure.8 Non-adherence to treatment, drug interactions between HIV and HCV drugs, severity of underlying liver disease, genotype of the HCV virus and substitutions in the HCV viral genome at the drug acting site are a few of the important causative factors.8 There is a lack of literature concerning BL-RAS in HCV–HIV co-infected patients being compared with a cure for HCV.17 Therefore, in the present study we evaluated BL-RAS and their association with treatment outcomes in this group. Although in the present study we did find that a greater percentage of patients without BL-RAS attained a cure in comparison to those who had BL-RAS (Figure 1), it was not a significant association. This may be because fewer cases failed the treatment (n=17) than attained a cure (n=63). Similar results are reported for the HCV mono-infected patients.10,18
We found that all BL-RAS were seen in the NS5a region of the genome and none in the NS5b region. Earlier studies had shown the existence of BL-RAS even in the NS5b region. The absence of BL-RAS in the NS5b region in our study could be attributed to the fact that most of our patients belonged to Gt6 and Gt3 and only known substitutions were taken into consideration based on the information available in the geno2pheno tool. Gt6 has shown an inherent property of very minimal occurrence of BL-RAS in HCV mono-infected patients.19 In Gt3 of HCV, non Gt3a subtypes have shown more occurrence of naturally occurring substitutions in the viral genome.20 A similar finding was also observed in our study, where most of the BL-RAS were seen in the Gt3b subtype.
Overall, the SVR rate seen in our study was 78.8% smaller than we anticipated based on the existing literature. The limitation of the study was its retrospective nature, whereby several factors like strict adherence to treatment or development of any other co-morbidity during the course of treatment could not be taken into account. In the present study two factors – patient age >30 years and male gender – were found to be significantly associated with treatment failure. Male gender has already been reported as an important factor in treatment failure in HCV mono-infected cases.9,16
We also did not find any significant association between Gt of the virus and treatment failure; maximum treatment failure in our study belonged to Gt3. Gt3 has already been considered the most challenging Gt to treat. But retreatment with an SOF-based regimen was successful in most of our patients. In the present study, all SVR failure cases (n=17) were reinitiated on SOF+DCV+ weight-based RBV for 24 weeks and SVR 12 was attained in 14; 2 patients died and one was lost to follow-up. Our results highlight the importance of retreatment with SOF+DCV+ weight-based RBV for 24 weeks in SVR failure cases. Higher SVR rates have been shown to result from SOF-based retreatment in non-responders to prior SOF-based therapy.21
There are certain limitations in our study. As it was a retrospective study, information available from hospital records was analysed and only known RAS were considered. We also used a simple population sequencing technique to look for RAS, which restricted the detection rate to only those present at a rate of >15% in a patient. But as there is a scarcity of data regarding RAS testing and its usefulness in a HCV–HIV co-infected group, findings from our study could contribute a great dea.
Conclusion
Our study highlights that BL-RAS are common in HCV–HIV co-infected patients and mostly seen in Gt3, especially subtype 3b. However, the existence of BL-RAS did not affect the attainment of SVR among non-cirrhotic, treatment naive patients, and retreatment among SVR failures with SOF+DCV+ weight- based RBV for 24 weeks can make them attain SVR easily.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Disclosure
All authors disclose no potential conflicts of interest (financial, professional, or personal) that are relevant to the manuscript.
References
1. Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016;16(7):797–808. doi:10.1016/S1473-3099(15)00485-5
2. Goel A, Seguy N, Aggarwal R. Burden of hepatitis C virus infection in India: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2019;34(2):321–329. doi:10.1111/jgh.14466
3. Soto B, Sanchez-Quijano A, Rodrigo L, et al. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26(1):1–5. doi:10.1016/S0168-8278(97)80001-3
4. Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. Hepatology. 1999;30(4):1054–1058. doi:10.1002/hep.510300409
5. Vermehren J, Park JS, Jacobson IM, Zeuzem S. Challenges and perspectives of direct antivirals for the treatment of hepatitis C virus infection. J Hepatol. 2018;69(5):1178–1187. doi:10.1016/j.jhep.2018.07.002
6. Schlabe S, Rockstroh JK. Advances in the treatment of HIV/HCV coinfection in adults. Expert Opin Pharmacother. 2018;19(1):49–64. doi:10.1080/14656566.2017.1419185
7. Piekarska A, Jablonowska E, Garlicki A, et al. Real life results of direct acting antiviral therapy for HCV infection in HIV-HCV-coinfected patients: Epi-Ter2 study. AIDS Care. 2020;32(6):762–769. doi:10.1080/09540121.2019.1645808
8. Salmon D, Trimoulet P, Gilbert C, et al. Factors associated with DAA virological treatment failure and resistance-associated substitutions description in HIV/HCV coinfected patients. World J Hepatol. 2018;10(11):856–866. doi:10.4254/wjh.v10.i11.856
9. Huang W, Wang M, Gong Q, et al. Comparison of naturally occurring resistance-associated substitutions between 2008 and 2016 in Chinese patients with chronic hepatitis C virus infection. Microb Drug Resist. 2019;25(6):944–950. doi:10.1089/mdr.2018.0360
10. Paolucci S, Fiorina L, Mariani B, et al. Naturally occurring resistance mutations to inhibitors of HCV NS5A region and NS5B polymerase in DAA treatment-naive patients. Virol J. 2013;10:355. doi:10.1186/1743-422X-10-355
11. Chen M, Ma Y, Chen H, et al. Multiple Introduction and naturally occuring drug resistance of HCV among HIV-infected intravenous drug users in Yunnan: an origin of China’s HIV/HCV epidemics. PLoS One. 2015;10(11):e0142543. doi:10.1371/journal.pone.0142543
12. Ramezani A, Baesi K, Banifazl M, et al. Naturally occurring NS5A and NS5B resistant associated substitutions in HCV and HCV/HIV patients in iranian population. Clin Res HepatolGastroenterol. 2019;43(5):594–602. doi:10.1016/j.clinre.2019.01.011
13. Ministry of Health and Family Welfare; Government of India. National Guidelines for Diagnosis & Management of Viral hepatitis. 2018. Available from: https://www.inasl.org.in/diagnosis-management-viral-hepatitis.pdf.
14. Gupta E, Choudhary MC, Upadhyay N, et al. Lower rates of naturally occurring resistance-associated substitutions (RASs) in hepatitis C virus (HCV)-infected chronic kidney disease (CKD) patients than in HCV-infected patients with only liver disease. Infect Drug Resist. 2019;12:3635–3640. doi:10.2147/IDR.S220335
15. Kalaghatgi P, Sikorski AM, Knops E, et al. Geno2pheno[HCV] - a web-based interpretation system to support hepatitis C treatment decisions in the era of direct-acting antiviral agents. PLoS One. 2016;11(5):e0155869. doi:10.1371/journal.pone.0155869
16. Dietz J, Susser S, Vermehren J, et al. Patterns of resistance-associated substitutions in patients with chronic HCV infection following treatment with direct-acting antivirals. Gastroenterology. 2018;154(4):976–988 e974. doi:10.1053/j.gastro.2017.11.007
17. Malta F, Gaspareto KV, Lisboa-Neto G, Carrilho FJ, Mendes-Correa MC, Pinho JRR. Prevalence of naturally occurring NS5A resistance-associated substitutions in patients infected with hepatitis C virus subtype 1a, 1b, and 3a, co-infected or not with HIV in Brazil. BMC Infect Dis. 2017;17(1):716. doi:10.1186/s12879-017-2817-7
18. Hezode C, Reau N, Svarovskaia ES, et al. Resistance analysis in patients with genotype 1-6 HCV infection treated with sofosbuvir/velpatasvir in the Phase III studies. J Hepatol. 2018;68(5):895–903. doi:10.1016/j.jhep.2017.11.032
19. Li Z, Liu Y, Zhang Y, et al. Naturally occurring resistance-associated variants to hepatitis C virus direct-acting antiviral agents in treatment-naive HCV genotype 6a-infected patients. Biomed Res Int. 2017;2017:9849823.
20. Bagaglio S, Messina E, Hasson H, Galli A, Uberti-Foppa C, Morsica G. Geographic distribution of HCV-GT3 subtypes and naturally occurring resistance associated substitutions. Viruses. 2019;11(2):148. doi:10.3390/v11020148
21. Dalgard O, Weiland O, Noraberg G, et al. Sofosbuvir based treatment of chronic hepatitis C genotype 3 infections-a Scandinavian real-life study. PLoS One. 2017;12(7):e0179764. doi:10.1371/journal.pone.0179764
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
