Back to Journals » International Journal of Nanomedicine » Volume 15
Nanoparticle-Based Vaccines for Brucellosis: Calcium Phosphate Nanoparticles-Adsorbed Antigens Induce Cross Protective Response in Mice
Authors Sadeghi Z, Fasihi-Ramandi M, Bouzari S
Received 15 February 2020
Accepted for publication 10 May 2020
Published 29 May 2020 Volume 2020:15 Pages 3877—3886
DOI https://doi.org/10.2147/IJN.S249942
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Zohre Sadeghi,1 Mahdi Fasihi-Ramandi,2 Saeid Bouzari1
1Department of Molecular Biology, Pasteur Institute of Iran, Tehran, Iran; 2Molecular Biology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran
Correspondence: Saeid Bouzari
Department of Molecular Biology, Pasteur Institute of Iran, Tehran, Iran
Tel +98 21 6696 9297
Fax +98 21 66492619
Email [email protected]
Introduction: Vaccine formulation with appropriate adjuvants is an attractive approach to develop protective immunity against pathogens. Calcium phosphate nanoparticles (CaPNs) are considered as ideal adjuvants and delivery systems because of their great potential for enhancing immune responses. In the current study, we have designed nanoparticle-based vaccine candidates to induce immune responses and protection against B. melitensis and B. abortus.
Materials and Methods: For this purpose, we used three Brucella antigens (FliC, 7α-HSDH, BhuA) and two multi-epitopes (poly B and poly T) absorbed by CaPNs. The efficacy of each formulation was evaluated by measuring humoral, cellular and protective responses in immunized mice.
Results: The CaPNs showed an average size of about 90 nm with spherical shape and smooth surface. The CaPNs-adsorbed proteins displayed significant increase in cellular and humoral immune responses compared to the control groups. In addition, our results showed increased ratio of specific IgG2a (associated with Th1) to specific IgG1 (associated with Th2). Also, immunized mice with different vaccine candidate formulations were protected against B. melitensis 16M and B. abortus 544, and showed same levels of protection as commercial vaccines (B. melitensis Rev.1 and B. abortus RB51) except for BhuA-CaPNs.
Discussion: Our data support the hypothesis that these antigens absorbed with CaPNs could be effective vaccine candidates against B. melitensis and B. abortus.
Keywords: calcium phosphate nanoparticle, adjuvant, Brucella, vaccine, immune response
Introduction
Brucellosis as a worldwide zoonotic disease leads to abortion and infertility in domestic animals and Malta fever in humans. Vaccination provides the most effective way to control animal brucellosis. At present, the live attenuated vaccines including B. melitensis Rev. 1, B. abortus RB51 and B. abortus strain 19 are used for the prevention of brucellosis in domestic animals. Although live attenuated vaccines provide good protection against brucellosis through humoral and cellular immunity, they have been found to contain many limitations, such as abortion in pregnant animals, human pathogenicity, and cross-reactivity with natural infection during diagnosis.1–6 Therefore, scientific studies have focused on the development of subunit vaccines, including recombinant proteins, DNA vaccines, vectored vaccine vesicles.7–16 Due to poor immunogenicity as the main challenge of subunit vaccines, various groups of adjuvants have been used to achieve robust immunity, and nanoparticles as an adjuvant and delivery system have exhibited great potential in subunit vaccine development. To date, a wide variety of nanoparticles including inorganic compounds (gold, silicate) and polymers (chitosan, polyglutamic acid) have been used to improve the immunogenicity of subunit vaccines.17–21 Calcium phosphate nanoparticles (CaPNs) as inorganic nano-adjuvant were developed by He et al. The ability of CaPNs to efficiently deliver antigens to antigen-presenting cells (eg DC), activate DC and up-regulate co-stimulatory molecules and the MHC class I/II has been demonstrated. Therefore, it is able to stimulate strong cellular immunity as it is effectively taken up by dendritic cells and macrophages. CaPNs have some advantages that include biodegradability, biocompatibility, nontoxicity, and low-cost.22–25 Therefore, there is great interest in investigating the potential of CaPNs for vaccine development against brucellosis. In our previous studies, we introduced three Brucella antigens (FliC, 7α-HSDH, BhuA) and two multi-epitopes (poly B and poly T) vaccine candidates26,27 (under consideration for publication). Although we obtained high levels of humoral and cellular immunity, five vaccine candidates did not show higher levels of protection than commercial vaccines (B. melitensis Rev. 1, B. abortus RB51) in BALB/c mice. Thus, novel vaccine adjuvant candidates that can promote robust immune responses are urgently needed. Since CaPNs have shown promising activity as adjuvant and vaccine delivery vehicle in various infectious diseases, so in the present study for the first time, the function of adsorbed antigens (FliC, 7α-HSDH, BhuA, poly B and poly T) onto CaPNs in stimulating the immune response and protection against B. melitensis and B. abortus have been investigated.
Materials and Methods
Vaccine Candidates Preparation
Cloning, expression, purification and validation of the FliC, 7α-HSDH and BhuA antigens have been performed as described previously26 (under consideration for publication). Briefly, the Brucella genome was obtained using a DNA extraction kit and then FliC, 7α-HSDH and BhuA genes were amplified by the PCR method. Next, the amplified genes were cloned into expression plasmids (pET-28a) using restriction enzymes and T4 DNA ligase, and then recombinant plasmids were transformed to the expression host (E. coli BL21 (DE3)). Induction of protein expression in E. coli BL21 (DE3)) was performed using Isopropyl-β-D thiogalactopyranoside (IPTG). Proteins were then confirmed by SDS-PAGE and Western blot and purified using a protein purification column (Ni2+-NTA agarose column). The purified proteins were first dialyzed against PBS and then their concentration was determined using the Bradford protein assay. Poly B (fragments including most of B cell and T CD4+ epitopes) and poly T (fragments including most of T CD8+ cell and T CD4+ epitopes) from FliC, 7α-HSDH and BhuA proteins were designed using immunoinformatics tools and expressed, purified and validated as previously described.27 In order to design poly B and poly T by immunoinformatics tools, the protein sequences of these antigens were obtained from UniProt, and prediction of B and T epitopes was performed using online servers such as IEDB. Subsequently, the selected epitopes were fused by the appropriate linkers, and the physicochemical and structural properties, and antigenicity of these designed sequences were determined by different servers. Then, protein sequences were reverse translated into a nucleotide sequence and sent to the company for synthesis. Expression and purification of these two proteins were performed as described. The CaPNs were prepared as described previously.22 Briefly, 12.5 mM calcium chloride, 12.5 mM dibasic sodium phosphate, and 15.6 mM sodium citrate were slowly mixed and stirred for 48 h and then sonicated for 30 min. The zeta potential, size and morphology of nanoparticles were determined by dynamic light scattering (DLS) (Zetasizer Nano instrument Malvern 3000, UK), and scanning electron microscope (SEM). Proteins loaded with CaPNs were prepared by gently mixing 5 mg of the CaPNs and 1 mg of ROM4 (5:1 ratio) for 60 min. The loading efficiency (LE) was determined using the following equation:

The free protein concentration in the supernatant was determined by Bradford protein assay, according to the manufacturer’s instructions. For this assay, 10 fold dilutions of standard proteins (BSA) or our samples were mixed with Bradford reagent. Then, the optical density (OD) of controls and samples was determined by ELISA reader at 595 nm.
Determination of Protein Release from Nanoparticle
The release of different proteins from antigen-loaded CaPNs was evaluated using PBS (pH=7.4) at 37°C under magnetic stirring (100 rpm). To assess the amount of the released antigen from NPs, sampling was performed at the dedicated time intervals of 0, 0.5, 1, 1.5, 2, 4, 8, 12, 24, 48, 72 and 96 hours. In each time point, 500µL of the sample was elicited, centrifuged at 18,000 g for 15 min and then replaced by 500µL buffer. Finally, all samples were examined by making use of Bradford assay to determine the quantity of antigen in the supernatant. A sample with non-loaded CaPNs was applied as a blank.
Mice, Immunization and Challenge
Female BALB/c mice, six to eight weeks old, were obtained from Pasteur Institute of Iran (Karaj, Iran). All mice were maintained in a controlled environment, with access to food and water. The BALB/c mice were randomly classified into ten experimental groups (10 mice/group), including six test groups (FliC-, 7α-HSDH-, BhuA-, poly B-, poly T-, poly B+T-CaPNs) and four control groups (PBS, CaPNs, B. melitensis Rev.1, and B. abortus RB51). Mice were subcutaneously (s.c) immunized three times (days 0, 14 and 28) with 30µg of each proteins-CaPNs. Negative and positive control groups were received PBS, CaPNs and B. melitensis Rev.1, B. abortus RB51 respectively. Mice were challenged intraperitoneally at 4 weeks post-immunization with B. melitensis 16M and B. abortus 544. The study was approved by the Committee of Animal Ethics of the Pasteur Institute of Iran and conducted according to the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Assessment of Humoral Responses
Two weeks after each immunization, mice sera were obtained to determine humoral responses. The presence of specific antibodies, IgG1 and IgG2a isotypes, against proteins were measured through enzyme-linked immune sorbent assay (ELISA). In brief, 96-well microplates (SPL Life Sciences, Korea) were coated overnight at 4°C with purified proteins (10 µg/mL). The microplates were washed three times with PBST and then blocked with PBS containing 3% BSA for 2h at room temperature to prevent nonspecific binding. Serial dilutions of mice sera applied to wells for 2 h at 37°C. After washing with wash buffer, HRP-conjugated goat-anti-mouse antibody (Sigma, USA) (to determine whole specific antibodies) or secondary goat anti-mouse subtype antibody (Sigma, USA) and rabbit anti-goat HRP conjugate antibody (to determine IgG subclasses) were added to the wells. Finally, the microplates were incubated with TMB (tetramethylbenzidine) solution for 15 minutes and absorbance was read at 450 nm by an ELISA reader.
Determination of Cytokines Production
The spleens of mice from each group (3 mice/group) were homogenized aseptically and splenocytes were cultured in RPMI-1640 medium (Gibco, UK) (containing 10% FBS and penicillin/streptomycin) in the presence of 10 µg/mL of proteins. Phytohemagglutinin-A (PHA) (5 µg/mL, Sigma-Aldrich) and RPMI medium 1640 alone were used for positive and negative control, respectively. After 72 h stimulation with proteins, the supernatants were used for measurement of IL-2, IFN-γ, and IL-10 cytokines using ELISA kits (Peprotech, Inc. UK). In brief, microplates (SPL Life Sciences, Korea) were coated with capture antibodies overnight at room temperature (RT). The microplates were washed four times with wash buffer and then blocked with PBS containing 1% BSA for 1h at RT. After washing four times, serial dilutions of standard or samples applied to wells for at least 2 h at RT. After washing with wash buffer, detection antibodies were added to the wells for 2 h at RT. The plates were washed and avidin–HRP conjugate (or streptavidin–HRP conjugate) was added to each well for 30 minutes at RT. Finally, the microplates were incubated with TMB solution and color development was investigated by an ELISA reader.
Lymphocyte Proliferation Assay
The proliferative effects of isolated splenocytes in response to antigens were evaluated by MTT (3-(4, 5-dimethythiazol-2-yl)-2, 5-diphenyl tetrazolium bromide) assay. The MTT assay was performed using the method described previously.28 Briefly, the isolated splenocytes were cultured into 96-well microtiter plates. Then, splenocytes of experimental, positive and negative groups were treated with proteins (10 μg/mL), PHA and RPMI-1640, respectively. The plates were incubated at 37°C and 5% CO2 for 72 h and then 20μL MTT (5 mg/mL) was added to each well for 4 h. After separating the supernatant from each well, formazan crystals were solubilized in the dimethyl sulfoxide (DMSO) (Sigma) and the absorbance of each well was read at 570 nm by an ELISA reader. The stimulation index (SI) was calculated as the ratio of the mean absorbance of stimulated cells to the mean absorbance of unstimulated cells.
Protection Assay
The protectivity of induced immune responses was evaluated in immunized groups through intraperitoneal challenge with B. melitensis 16M and B. abortus 544. Briefly, one month after the final immunization, six mice from each immunized groups were challenged, and then thirty days later, the mice spleens removed aseptically. The homogenized spleens diluted and cultured on Brucella agar for 3–4 days at 37 °C to determine the CFU numbers per spleen. Units of protection were calculated by subtracting the mean log10 CFU of the experimental group from the mean log10 CFU of the PBS group.
Statistical Analysis
Data were statistically analyzed by one-way ANOVA and post hoc tests. Data were reported as mean ± SEM (standard error of the mean) and differences were considered to be statistically significant at P ≤0.05.
Results
Characterization of CaPNs
The CaPN particle size was determined by DLS and showed an average size of about 90 nm. The images taken by SEM showed a spherical shape and smooth surface of the nanoparticles (Figure 1). Zeta potential (surface charge) of nanoparticles was −20 mV. The stability of size, shape and zeta potential of CaPNs were also investigated and showed no significant changes after 21 days incubation at 4 °C or room temperature. Based on Bradford assay, the adsorption efficiency of antigens to nanoparticles was 50–75% in different antigens. In addition, the release profiles of antigens from CaPNs are shown in Figure 2.
 |
Figure 1 Scanning electron microscope of CaPNs. |
 |
Figure 2 Release profile of different antigens-CaPNs at pH 7.4 and 37 °C for 96 hours (data are mean± SD, n = 3). |
Induction of Humoral Immune Response
The ability of different vaccine formulations to induce specific antibodies in the serum of BALB/c mice were evaluated by ELISA method. The levels of total antibodies were significantly higher (P < 0.05) in all experimental groups in comparison to control groups. The increase in specific antibodies began after the first immunization, and the optical density values reached the highest level after the third immunization. As shown in Figure 3, the highest optical density was observed in the 1/1000 dilution in the serum of mice group injected with poly B+T-CaPNs and the lowest optical density in the dilution of 1/1000 in the serum of mice injected with BhuA-CaPNs (P< 0.05). However, no significant difference was observed between the groups immunized with FliC-CaPNs, poly B-CaPNs, and poly T-CaPNs (P> 0.05). In order to determine the type of immunity induced in immunized mice by various formulations (Th1 and/or Th2 response), the ratio of the IgG2a and IgG1 subclasses was measured two weeks after the last immunization. All vaccine formulations could induce specific IgG1 and IgG2a levels in mice following s.c immunization (Figure 4). The highest IgG2a to IgG1 ratio was observed in the mice immunized with multi-epitope vaccine candidates (poly B-, poly T- and poly B+T-CaPNs).
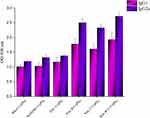 |
Figure 4 IgG subtypes in mice sera after immunization with different vaccine formulations. |
Induction of Cellular Immune Response
The cell-mediated immune responses of immunized mice with various formulations were investigated by lymphocyte proliferation and cytokine patterns. As shown in Figure 5, the splenocytes from mice immunized with FliC-, 7α-HSDH-, BhuA-, poly B-, poly T-, poly B+T-CaPNs indicated significantly higher stimulation index (S.I.) than negative control mice (PBS, CaPNs) (P< 0.05). Also, we evaluated the effect of various vaccine candidate formulations on cytokine levels. In splenocytes of all mice injected and stimulated with various proteins, we detected stronger IFN-γ and IL-2 responses than negative control groups (P< 0.05) (Figure 6). In comparison to all groups, these cytokines were secreted higher in Rev-1 and RB51 immunized mice (except for poly B+T-CaPNs compared to Rev-1 and RB51 and poly T-CaPNs compared to RB51 that produce similar levels of cytokines (P> 0.05)). Comparing the level of cytokine secretion in the vaccine candidate groups, we find that groups immunized with poly B+T-CaPNs and poly T-CaPNs had the highest (P< 0.05) and BhuA-CaPNs group had the lowest (P< 0.05) level of cytokine secretion, and there was no significant difference between FliC-CaPNs, 7α-HSDH-CaPNs, and poly B-CaPNs groups (P > 0.05). In addition, low levels of IL-10 were detected in all immunized mice, and however, there was no statistically significant difference in IL-10 cytokine production between groups (P > 0.05).
Induction of Protective Immunity
Protection against B. melitensis 16M and B. abortus 544 in immunized and control groups is shown in Table 1. The mice immunized with FliC-, 7α-HSDH-, BhuA-, poly B-, poly T-, poly B+T-CaPNs showed significant levels of protection compared to the negative control groups (P< 0.05). BhuA, 7α-HSDH, FliC, poly B, poly T and poly B+T antigens plus CaPNs induced 0.9, 1.05, 1.12, 1.18, 1.24, 1.5 log10 units of protection against B. melitensis and 0.85, 1.03, 1, 1.13, 1.21, 1.37 log10 units of protection against B. abortus, respectively. Mice immunized with 7α-HSDH-, FliC-, poly B-, poly T-and poly B+T-CaPNs exhibited the same levels of protection (P > 0.05). Although the protection obtained from BhuA-CaPNs was significantly lower than B. melitensis Rev.1 (1.74 log10 unit of protection) and B. abortus RB51 (1.53 log10 unit of protection) vaccine strain (p<0.05), other immunized groups did not show significant differences with B. melitensis Rev.1 and B. abortus RB51 (P > 0.05).
 |
Table 1 Protection Against B. melitensis 16M and B. abortus 544 Challenge in Immunized Mice |
Discussion
The limitations of current vaccines against infectious diseases have encouraged researchers to develop nanoparticle-based vaccines. Different nanoparticles have been investigated as adjuvant and antigen delivery systems. There are accumulating evidences that nanoparticles have the potential to strengthen the immune system against subunit vaccines.18,21,29-35 In order to select a suitable nano-adjuvant, we reviewed comparative studies of the various adjuvants used for intracellular bacteria or viruses that require cellular immunity. For example, Morcol et al, in his study on the vaccine candidate against Influenza A infection, suggested CaPNs as a more effective than alum,36 and the reasons given are as follows: Th2-oriented response, IgE-mediated allergic reactions, problems with stability during freeze-drying, and variability in production in alum.23,34,36 Also, a recent comparative study using the Brucella antigen (omp31) in the formulation of nano-vaccine candidates showed that CaPNs had better efficiency than chitosan nanoparticles and aluminum hydroxide due to their effects on IFN-γ and IgG2a induction and stimulation index.23 In a study conducted by Singh et al, on the vaccine candidate against Brucella using rL7/L12 protein as a model, PLGA (85:15) nanoparticles were introduced as a strong and safe adjuvant.37 However, the major drawback in developing PLGA-based nanoparticle formulations is the instability of encapsulated proteins due to chemical and mechanical stress during the manufacturing processes.38 Thus, among numerous studies, the CaPNs because of their unique properties such as low cost, simple manufacturing process, safety, no adverse effects, biocompatibility, high stability and effective immune induction are proposed.22–25 Therefore, we used CaPNs to promote the immunogenicity of FliC, 7α-HSDH, BhuA, poly B, poly T, and poly B+T antigens against brucellosis. In the first step, the physicochemical properties of CaPNs, such as shape, size, and charge were investigated as important factors in inducing immune response through NPs–APCs interaction. We found that the average particle size of CaPNs was <100 and had spherical shapes and smooth surfaces (Figure 1). Previous studies have shown that small size and spherical shapes are effective in endocytosis.39,40 In addition, our results showed that the size, shape and zeta potential of CaPNs were stable. Our results are consistent with the previous study, which have shown that different formulations of vaccine candidates containing CaPNs have been stable for a long time in different conditions.41 In the next step, the effects of different formulations containing CaPNs on immune responses, especially cell-mediated immunity were investigated. Due to the intracellular character of Brucella, Th1-type immune response plays an important role in the protection against brucellosis. The Th1 response, specifically characterized by IFN-γ, activates the bactericidal function of macrophages, cytotoxic function of CD8+ T-cells, and induces B cell isotype switching to IgG2a. These Th1 response functions are crucial for killing and clearing of Brucella.42–45 Our results demonstrated that these formulations (proteins adsorbed onto CaPNs) led to increase in IFN-γ and IL-2 levels in all immunized mice (Figure 6), and this increase in Th1 cytokine production was higher in mice immunized with poly B+T-CaPNs and poly T-CaPNs. We also performed lymphocyte proliferation assays as another indicator of cell-mediated immune response, and the results showed that mice vaccinated with FliC-, 7α-HSDH-, BhuA-, poly B-, poly T-, poly B+T-CaPNs could stimulate cell-mediated immunity in BALB/c mice (Figure 5). Similarly, Rahimi et al showed that the DNA vaccine with co-delivery of calcium phosphate nanoparticles was capable of enhancing the cellular immune response against T.gondii in BALB/c mice.46 According to Knuschke et al, the CaPNs strongly enhances antigen delivery to APCs, thereby enhancing cell-mediated immunity against retroviral FV infection.47 In another study, Huang et al demonstrated that the CaPNP/multipeptide vaccine could induce a T-cell response against all four Dengue virus (DV) serotypes.41 Moreover, the vaccine candidates formulated with nano-adjuvant also induced humoral immune response in all study groups compared to the control groups (Figure 3). The adjuvant effect of CaPNs for the stimulation of humoral immunity has been reported previously. He et al have demonstrated that mice were immunized intravaginally and intranasally with HSV-2 protein _CaPNs enhanced HSV-specific antibodies.48 Our results are consistent with studies that used CaPNs in the formulation of vaccine candidates shown that these formulations are able to increase the humoral immune response.49,50 The high titers of IgG2a, associated with Th1 responses, were observed in mice immunized with CaPNs-adjuvanted candidates, and the highest levels were observed in poly B-, poly T- and poly B+T-CaPNs groups (Figure 4). Therefore, high levels of IFN-γ and IgG2a production as Th1 immune response indicator and low levels of IL-10 and IgG1 production confirmed that the immune response shifted to Th1 in all test groups. Our results agree with the finding of previous studies that claimed CaPNs stimulate the Th1-Th2 immune response (Th1> Th2).22,24
Our data showed that FliC-, 7α-HSDH-, BhuA-, poly B-, poly T-, poly B+T-CaPNs provide protection against B. melitensis and B. abortus infection as compared to negative control groups (Table 1). The protection obtained after immunization with FliC-, 7α-HSDH-, poly B-, poly T-, poly B+T-CaPNs is comparable to the protection obtained with vaccine strains, i.e., B. melitensis Rev.1 and B. abortus RB51. The stronger protective effects of FliC-CaPNs, 7α-HSDH-CaPNs, poly B-CaPNs, and poly T-CaPNs formulations could be attributed to their better performance than BhuA antigen in stimulating cytokine secretion (IFN-γ and IL-2) and IgG2a antibody switching. Other challenge studies with different vaccine candidates have also demonstrated the protective ability of CaPNs in BALB/c mice.46,49,50 As anticipated, compared to our previous studies, CaPNs were able to effectively improve the immune responses of antigens. Since induction of strong protection is a leading factor in determining the efficacy of vaccine candidates, hence our formulations in this study could be considered as new vaccine candidates because of their protection level compared to B. melitensis Rev.1 and B. abortus RB51 vaccine strains. Moreover, our formulations contain recombinant proteins and CaPNs as safe compounds, instead of live attenuated microorganisms. Another advantage that can be considered is that all the antigens used in the vaccine formulations were conserved between the two different strains of Brucella (B. melitensis 16M and B. abortus 544); therefore, cross-protection could be obtained by a single vaccine.
Conclusion
In this study, we used nano-adjuvant to improve the antigenicity of Brucella antigens and determined whether adsorbed antigens (FliC, 7α-HSDH, BhuA, poly B and poly T) could be good vaccine formulation for brucellosis. Our data suggested that the use of various Brucella antigens in CaPNs formulation has the ability to stimulate humoral and cellular responses. High levels of IFN-γ and IgG2a production and low levels of IL-10 and IgG1 production confirmed the shift of immune response to Th1 (Th1>Th2). Different formulations of vaccine candidates containing CaPNs demonstrated protection against B. melitensis and B. abortus infection in BALB/c mice. Therefore, based on these observations, the authors proposed the potential of this adjuvant and recommend these formulations as new vaccine candidates for brucellosis.
Funding
This project was supported by Pasteur Institute of Iran (grant number: 1071).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Corbel MJ. Brucellosis: an overview. Emerg Infect Dis. 1997;3(2):213.
2. Hou H, Liu X, Peng Q. The advances in brucellosis vaccines. Vaccine. 2019;37(30):3981–3988. doi:10.1016/j.vaccine.2019.05.084
3. Aparicio ED. Epidemiology of brucellosis in domestic animals caused by Brucella melitensis, Brucella suis and Brucella abortus. Rev Sci Tech Off Int Epiz. 2013;32(1):53–60. doi:10.20506/rst.32.1.2187
4. de Figueiredo P, Ficht TA, Rice-Ficht A, et al. Pathogenesis and immunobiology of brucellosis: review of Brucella–Host Interactions. Am J Pathol. 2015;185(6):1505–1517. doi:10.1016/j.ajpath.2015.03.003
5. Avila-Calderón ED, Lopez-Merino A, Sriranganathan N, Boyle SM, Contreras-Rodríguez A. A history of the development of Brucella vaccines. Biomed Res Int. 2013;2013.
6. Dorneles EM, Sriranganathan N, Lage AP. Recent advances in Brucella abortus vaccines. Vet Res. 2015;46(1):76. doi:10.1186/s13567-015-0199-7
7. Velikovsky CA, Goldbaum FA, Cassataro J, et al. Brucella lumazine synthase elicits a mixed Th1-Th2 immune response and reduces infection in mice challenged with Brucella abortus 544 independently of the adjuvant formulation used. Infect Immun. 2003;71(10):5750–5755. doi:10.1128/IAI.71.10.5750-5755.2003
8. Cassataro J, Velikovsky CA, de la Barrera S, et al. A DNA vaccine coding for the Brucella outer membrane protein 31 confers protection against B. melitensis and B. ovis infection by eliciting a specific cytotoxic response. Infect Immun. 2005;73(10):6537–6546. doi:10.1128/IAI.73.10.6537-6546.2005
9. Pasquevich KA, Estein SM, Samartino CG, et al. Immunization with recombinant Brucella species outer membrane protein Omp16 or Omp19 in adjuvant induces specific CD4+ and CD8+ T cells as well as systemic and oral protection against Brucella abortus infection. Infect Immun. 2009;77(1):436–445. doi:10.1128/IAI.01151-08
10. Abkar M, Amani J, Sahebghadam Lotfi A, et al. Subcutaneous immunization with a novel immunogenic candidate (urease) confers protection against Brucella abortus and Brucella melitensisinfections. APMIS. 2015;123(8):667–675. doi:10.1111/apm.12400
11. Abkar M, Lotfi AS, Amani J, et al. Survey of Omp19 immunogenicity against Brucella abortus and Brucella melitensis: influence of nanoparticulation versus traditional immunization. Vet Res Commun. 2015;39(4):217–228. doi:10.1007/s11259-015-9645-2
12. Singh AK, Singh AK, Balakrishna K, et al. Studies on recombinant glucokinase (r-glk) protein of Brucella abortus as a candidate vaccine molecule for brucellosis. Vaccine. 2014;32(43):5600–5606. doi:10.1016/j.vaccine.2014.07.106
13. Ghasemi A, Jeddi-Tehrani M, Mautner J, et al. Immunization of mice with a novel recombinant molecular chaperon confers protection against Brucella melitensis infection. Vaccine. 2014;32(49):6659–6666. doi:10.1016/j.vaccine.2014.09.013
14. Hop HT, Reyes AWB, Simborio HLT, et al. Immunization of mice with recombinant Brucella abortus organic hydroperoxide resistance (Ohr) protein protects against a virulent Brucella abortus 544 Infection. J Microbiol Biotechnol. 2016;26(1):190–196. doi:10.4014/jmb.1505.05028
15. Mailybayeva A, Yespembetov B, Ryskeldinova S, et al. Improved influenza viral vector based Brucella abortus vaccine induces robust B and T-cell responses and protection against Brucella melitensis infection in pregnant sheep and goats. PLoS One. 2017;12(10).
16. Avila-Calderón ED, Lopez-Merino A, Jain N, et al. Characterization of outer membrane vesicles from Brucella melitensis and protection induced in mice. Clin Dev Immunol. 2011;2012.
17. Maughan CN, Preston SG, Williams GR. Particulate inorganic adjuvants: recent developments and future outlook. J Pharm Pharmacol. 2015;67(3):426–449. doi:10.1111/jphp.12352
18. Vartak A, Sucheck S. Recent advances in subunit vaccine carriers. Vaccines. 2016;4(2):12.
19. Nevagi RJ, Khalil ZG, Hussein WM, et al. Polyglutamic acid-trimethyl chitosan-based intranasal peptide nano-vaccine induces potent immune responses against group A streptococcus. Acta Biomater. 2018;80:278–287. doi:10.1016/j.actbio.2018.09.037
20. Abkar M, Fasihi-Ramandi M, Kooshki H, et al. Oral immunization of mice with Omp31-loaded N-trimethyl chitosan nanoparticles induces high protection against Brucella melitensis infection</em>. Int J Nanomedicine. 2017;12:8769. doi:10.2147/IJN.S149774
21. Pati R, Shevtsov M, Sonawane A. Nanoparticle vaccines against infectious diseases. Front Immunol. 2018;9:2224. doi:10.3389/fimmu.2018.02224
22. He Q, Mitchell AR, Johnson SL, et al. Calcium phosphate nanoparticle adjuvant. Clin Diagn Lab Immunol. 2000;7(6):899–903. doi:10.1128/CDLI.7.6.899-903.2000
23. Abkar M, Alamian S, Sattarahmady N. A comparison between adjuvant and delivering functions of calcium phosphate, aluminum hydroxide and chitosan nanoparticles, using a model protein of Brucella melitensis Omp31. Immunol Lett. 2019;207:28–35. doi:10.1016/j.imlet.2019.01.010
24. Lin Y, Wang X, Huang X, et al. Calcium phosphate nanoparticles as a new generation vaccine adjuvant. Expert Rev Vaccines. 2017;16(9):895–906. doi:10.1080/14760584.2017.1355733
25. Paneque-Quevedo AA. Inorganic compounds as vaccine adjuvants. Biotecnol Apl. 2013;30(4):243–249.
26. Sadeghi Z, Fasihi-Ramandi M, Azizi M, et al. Mannosylated chitosan nanoparticles loaded with FliC antigen as a novel vaccine candidate against Brucella melitensis and Brucella abortus infection. J Biotechnol. 2020;310:89–96. doi:10.1016/j.jbiotec.2020.01.016
27. Sadeghi Z, Fasihi-Ramandi M, Bouzari S. Evaluation of immunogenicity of novel multi-epitope subunit vaccines in combination with poly I: C against Brucella melitensis and Brucella abortus infection. Int Immunopharmacol. 2019;75:105829. doi:10.1016/j.intimp.2019.105829
28. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi:10.1016/0022-1759(83)90303-4
29. Dykman LA, Staroverov SA, Fomin AS, et al. Gold nanoparticles as an adjuvant: influence of size, shape, and technique of combination with CpG on antibody production. Int Immunopharmacol. 2018;54:163–168. doi:10.1016/j.intimp.2017.11.008
30. Haryono A, Salsabila K, Restu WK, Harmami SB, Safari D. Effect of chitosan and liposome nanoparticles as adjuvant codelivery on the immunoglobulin G subclass distribution in a mouse model. J Immunol Res. 2017;2017.
31. Dhakal S, Lu F, Ghimire S, et al. Corn-derived alpha-D-glucan nanoparticles as adjuvant for intramuscular and intranasal immunization in pigs. Nanomedicine. 2019;16:226–235.
32. Li P, Asokanathan C, Liu F, et al. PLGA nano/micro particles encapsulated with pertussis toxoid (PTd) enhances Th1/Th17 immune response in a murine model. Int J Pharm. 2016;513(1–2):183–190. doi:10.1016/j.ijpharm.2016.08.059
33. Najminejad H, Kalantar SM, Mokarram AR, et al. Bordetella pertussis antigens encapsulated into N-trimethyl chitosan nanoparticulate systems as a novel intranasal pertussis vaccine. Artif Cells Nanomed Biotechnol. 2019;47(1):2605–2611. doi:10.1080/21691401.2019.1629948
34. Morcol T, Nagappan P, Bell SJD, et al. Influenza A (H5N1) virus subunit vaccine administered with CaPNP adjuvant induce high virus neutralization antibody titers in mice. AAPS PharmSciTech. 2019;20(8):315. doi:10.1208/s12249-019-1530-9
35. Amini Y, Moradi B, Tafaghodi M, et al. TB trifusion antigen adsorbed on calcium phosphate nanoparticles stimulates strong cellular immunity in mice. Biotechnol Bioprocess Eng. 2016;21(5):653–658. doi:10.1007/s12257-016-0326-y
36. Morçӧl T, Hurst BL, Tarbet EB. Calcium phosphate nanoparticle (CaPNP) for dose-sparing of inactivated whole virus pandemic influenza A (H1N1) 2009 vaccine in mice. Vaccine. 2017;35(35):4569–4577. doi:10.1016/j.vaccine.2017.07.016
37. Singh D, Somani VK, Aggarwal S, et al. PLGA (85: 15) nanoparticle based delivery of rL7/L12 ribosomal protein in mice protects against Brucella abortus 544 infection: a promising alternate to traditional adjuvants. Mol Immunol. 2015;68(2):272–279. doi:10.1016/j.molimm.2015.09.011
38. Mohammadi-Samani S, Taghipour B. PLGA micro and nanoparticles in delivery of peptides and proteins; problems and approaches. Pharm Dev Technol. 2015;20(4):385–393. doi:10.3109/10837450.2014.882940
39. Foged C, Brodin B, Frokjaer S, et al. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm. 2005;298(2):315–322. doi:10.1016/j.ijpharm.2005.03.035
40. Ma N, Ma C, Li C, et al. Influence of nanoparticle shape, size, and surface functionalization on cellular uptake. J Nanosci Nanotechnol. 2013;13(10):6485–6498. doi:10.1166/jnn.2013.7525
41. Huang X, Karabudak A, Comber JD, et al. A novel immunization approach for dengue infection based on conserved T cell epitopes formulated in calcium phosphate nanoparticles. Hum Vaccin Immunother. 2017;13(11):2612–2625. doi:10.1080/21645515.2017.1369639
42. Dorneles EM, Teixeira-Carvalho A, Araújo MSS, et al. Immune response triggered by Brucella abortus following infection or vaccination. Vaccine. 2015;33(31):3659–3666. doi:10.1016/j.vaccine.2015.05.057
43. Elfaki MG, Alaidan AA, Al-Hokail AA. Host response to Brucella infection: review and future perspective. J Infect Developing Countries. 2015;9(07):697–701. doi:10.3855/jidc.6625
44. Skendros P, Boura P. Immunity to brucellosis. Rev Sci Tech. 2013;32(1):137–147. doi:10.20506/rst.32.1.2190
45. Sung KY, Yoo HS. Host immune responses during Brucella infection: a brief review. J Preventive Vet Med. 2014;38(1):26–34. doi:10.13041/jpvm.2014.38.1.26
46. Rahimi MT, Sarvi S, Sharif M, et al. Immunological evaluation of a DNA cocktail vaccine with co-delivery of calcium phosphate nanoparticles (CaPNs) against the Toxoplasma gondii RH strain in BALB/c mice. Parasitol Res. 2017;116(2):609–616. doi:10.1007/s00436-016-5325-6
47. Knuschke T, Bayer W, Rotan O, et al. Prophylactic and therapeutic vaccination with a nanoparticle-based peptide vaccine induces efficient protective immunity during acute and chronic retroviral infection. Nanomedicine. 2014;10(8):1787–1798. doi:10.1016/j.nano.2014.06.014
48. He Q, Mitchell A, Morcol T, Bell SJ. Calcium phosphate nanoparticles induce mucosal immunity and protection against herpes simplex virus type 2. Clin Diagn Lab Immunol. 2002;9(5):1021–1024. doi:10.1128/cdli.9.5.1021-1024.2002
49. Ahmadpour E, Sarvi S, Hashemi Soteh MB, et al. Enhancing immune responses to a DNA vaccine encoding Toxoplasma gondii GRA14 by calcium phosphate nanoparticles as an adjuvant. Immunol Lett. 2017;185:40–47. doi:10.1016/j.imlet.2017.03.006
50. Joyappa DH, Kumar CA, Banumathi N, et al. Calcium phosphate nanoparticle prepared with foot and mouth disease virus P1-3CD gene construct protects mice and guinea pigs against the challenge virus. Vet Microbiol. 2009;139(1–2):58–66. doi:10.1016/j.vetmic.2009.05.004
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



