Back to Journals » International Journal of Nanomedicine » Volume 17
Nanomaterial-Based Drug Delivery Systems: A New Weapon for Cancer Immunotherapy
Authors Jiang Z, Zhang W, Zhang J, Liu T , Xing J, Zhang H, Tang D
Received 26 May 2022
Accepted for publication 9 September 2022
Published 3 October 2022 Volume 2022:17 Pages 4677—4696
DOI https://doi.org/10.2147/IJN.S376216
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yan Shen
Zhengting Jiang,1,* Wenjie Zhang,1,* Jie Zhang,1 Tian Liu,1 Juan Xing,1 Huan Zhang,1 Dong Tang2
1Clinical Medical College, Yangzhou University, Yangzhou, 225000, People’s Republic of China; 2Department of General Surgery, Institute of General Surgery, Northern Jiangsu Province Hospital, Clinical Medical College, Yangzhou University, Yangzhou, 225000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dong Tang, Department of General Surgery, Institute of General Surgery, Northern Jiangsu Province Hospital, Clinical Medical College, Yangzhou University, Yangzhou, 225000, People’s Republic of China, Email [email protected]
Abstract: Cancer immunotherapy, a major breakthrough in cancer treatment, has been successfully applied to treat a number of tumors. However, given the presence of factors in the tumor microenvironment (TME) that impede immunotherapy, only a small proportion of patients achieve a good clinical response. With the ability to increase permeability and cross biological barriers, nanomaterials have been successfully applied to deliver immunotherapeutic agents, thus realizing the anti-cancer therapeutic potential of therapeutic agents. This has driven a wave of research into systems for the delivery of immunotherapeutic agents, which has resulted in widespread interest in nanomaterial-based drug delivery systems. Nanomaterial-based drug delivery systems are able to overcome the challenges from TME and thus achieve good results in cancer immunotherapy. If it can make a breakthrough in improving biocompatibility and reducing cytotoxicity, it will be more widely used in clinical practice. Different types of nanomaterials may also have some subtle differences in enhancing cancer immunotherapy. Moreover, delivery systems made of nanomaterials loaded with drugs, such as cytotoxic drugs, cytokines, and adjuvants, could be used for cancer immunotherapy because they avoid the toxicity and side effects associated with these drugs, thereby enabling their reuse. Therefore, further insights into nanomaterial-based drug delivery systems will provide more effective treatment options for cancer patients.
Keywords: drug repurposing, delivery system, immunotherapy, tumor microenvironment, nanomaterial
Introduction
Cancer immunotherapy, a major breakthrough in cancer treatment, aims to improve the anti-tumor immune response by enhancing the body’s defenses to eliminate malignant cells.1 Over the years, it has been successfully used in the treatment of a number of tumors, particularly in hematological malignancies and solid tumors.2,3 Currently, immune checkpoint inhibitors (ICIs) are the clinically approved and most widely tested immunotherapies in which the anti-tumor activity of immune cells is enhanced by their blockade of specific immune checkpoints, such as programmed death receptor 1/programmed death ligand 1 (PD-1/PD-L1) and cytotoxic T lymphocyte antigen 4 (CTLA-4).4 However, although cancer immunotherapy has been shown to be durable and effective in treating tumors, only a small percentage of patients (about 10%) achieve a good clinical response.5 To date, several challenges remain in improving the quality of response to cancer immunotherapy, including suppression of antigen presenting cell (APC) function, formation of an immunosuppressive environment, extracellular matrix (ECM) fibrosis, and abnormal cellular metabolism. Therefore, there is an urgent need for a new approach to cancer immunotherapy that delivers the therapeutic potential of therapeutic agents in a safer and more controlled manner, and with reduced drug toxicity. Nanomaterial-based drug delivery systems, a novel delivery platform currently under investigation, could increase the accumulation of immunotherapeutic drugs in the patient’s body, thereby enabling more effective targeting of desired immune and cancer cells and avoiding off-target adverse effects.6
Nanomaterials, the core of the new drug delivery system, are typically in the size range of 1–100 nanometers, and are mainly used in the manufacture of therapeutic drugs and devices.7 Given their reduced size to the nanoscale, typical nanomaterials share several common properties: enhanced electrical conductivity, spectral shifts in optical absorption, high surface-to-volume ratios, superparamagnetic behavior, and unique fluorescent properties.8–10 Nanomaterials are notable in the medical field largely due to their increased permeability, crossing of biological barriers, and their improved ability to be applied to drug transport and their controlled drug release. In addition, the biocompatibility of most nanomaterials employed for therapeutic purposes has improved significantly, although there is still incompatibility with the long-term accumulation of some nanomaterials in healthy organs.11 Consequently, nanomaterial-based drug delivery systems are being used for targeted delivery of anticancer agents to desired cancer cells, which has been shown to significantly improve immunotherapeutic efficacy and control the production of off-target toxicity.12 It is also worth noting that targeted delivery is one of the main advantages of nanomaterial-based immunotherapy compared to free drugs, which is achieved by either passive targeting or active targeting to precisely target specific cancer cells.13
This review aims exploring the mechanisms by which nanomaterial-based drug delivery systems enhance cancer immunotherapy. Specifically, we explore several factors that impede cancer immunotherapy and discuss how the inhibitory effect on immunotherapy can be altered with novel drug delivery systems. In addition, we examine the role of nanomaterial-based drug delivery systems in drug repurposing, and highlight the challenges it faces to achieve more controlled and precise cancer immunotherapy.
Effectiveness of Cancer Immunotherapy Blocked
Studies have shown that cellular and non-cellular components in the TME, consisting of immune cells, extracellular matrix, and stromal cells, are able to surround cancer cells.14 Therefore, for cancer immunotherapy to have a satisfactory effect, several obstructive factors from the TME must be overcome, including inhibition of APC function, formation of an immunosuppressive environment, extracellular matrix fibrosis, and abnormal cellular metabolism (Figure 1). These factors not only promote tumor proliferation and metastasis, but also provide challenges in the transport of therapeutic materials to the site of action, thereby blocking immunotherapy.
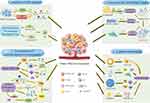 |
Figure 1 Several barriers to cancer immunotherapy from TME. (1) Inhibition of APC function. A range of immunosuppressive factors in TME block APC recruitment through the initiation of the transcriptional activator STAT3 and signal transduction. DCs are mediated by tumors and produce dysfunction, becoming semi-mature or even immature DCs that eventually lose the ability to provide the necessary activation signals. Under these effects, antigen presentation is more likely to trigger T-cell tolerance, including peripheral T-cell clonal deficiency, T-cell incompetence, and the production of regulatory T cells (Tregs). (2) The formation of an immunosuppressive environment. TME shapes the immunosuppressive environment in two ways: 1. by suppressing the function of immune cells; TME suppress anti-tumor T cell responses to disrupt T cell responses. TIBs derived from TME were able to suppress T cell-mediated immune responses by secreting soluble mediators. The down-regulation of NK cell function and the immunosuppressive function of TAM were closely associated with the secretion of prostaglandin E2 and cytokines. 2. By recruiting immunosuppressive cells. Tregs secrete a variety of substances that bind to immune cells and ultimately inhibit the function of anti-tumor immunity and immune effector cells. Binding to molecules on the surface of effector cells such as CD80/CD86 is also a way for Tregs to exert immunosuppressive effects. MDSC recruited from the blood stream by TME can promote tumorigenesis and metastasis. At the same time, reduced STAT3 activity in MDSC led to rapid differentiation of M-MDSC from TAM. Moreover, MDSC attracts Treg cells to the tumor site in order to enhance their immunosuppressive function, while suppressing the immune function of DCs, B cells and NK cells. (3) Fibrosis of the extracellular matrix. Proteoglycan and HA reinforce the compressive properties in ECM tissues through their GAG chain-bound water. The fibrillar collagen contributes to the tensile strength of the tissue and CAFs are a key factor in increasing ECM stiffness. (4) Abnormal cellular metabolism. High levels of lipid, is positively correlated with CD8+ T-cell depletion. The elevated ROS in local TME can enhance the pro-tumor effects of TAM, MDSC and CAF. High levels of lactic acid is responsible for impeding the function of immune cells. The angiogenic response induced by high concentrations of VEGFA promotes an immature phenotype of the vascular system. The Figure was created by Figdraw (www.figdraw.com). Abbreviations: APC, antigen presenting cell; DC, dendritic cell; Tregs, regulatory T cells; TIB, tumor-infiltrating B cell; TAM, tumor-associated macrophage; MDSC, myeloid-derived suppressor cell; sEV, small extracellular vesicle; HA, hyaluronic acid; ECM, extracellular matrix; CAF, cancer-associated fibroblast; ROS, reactive oxygen species; VEGFA, vascular endothelial growth factor A. |
Inhibition of APC Function
Given that APC is able to fully activate adaptive immunity, it is key to the initiation and regulation of anti-cancer immunity.15 However, a range of immunosuppressive factors in TME, such as GM-CSF, S100A9 M-CSF, CXCL8, IL-10, and gangliosides, block APC recruitment through initiation of the transcriptional activator STAT3 and signal transduction.16 Subsequently, the induction of endogenous anti-tumor immunity by APC is limited due to severe interference by the TME. It is significant that dendritic cells (DCs), the most powerful APC, are mediated by tumors to induce dysfunction, thereby becoming semi-mature or even immature DCs that eventually lose the ability to provide the necessary activation signals.17,18 Under these effects, antigen presentation is more likely to trigger T-cell tolerance, including peripheral T-cell clonal deficiency, T-cell incompetence and the production of regulatory T cells (Tregs).19–21
Formation of an Immunosuppressive Environment
TME shapes the immunosuppressive environment in two ways: 1) By suppressing the function of immune cells. Originally, immune cells perform the function of recognizing, responding to, and inhibiting tumor progression. However, after being affected by TME, they infiltrate and secrete inflammatory cytokines, thereby creating an inflammatory microenvironment conducive to tumor growth. The TME suppresses anti-tumor T cell responses by regulating molecular production, such as interleukin (IL)-10 and transforming growth factor-β (TGF-β), which are used to evade detection, ultimately leading to the formation of an immunosuppressive microenvironment and disruption of T cell responses.22 A previous study reported that tumor-infiltrating B cells (TIBs), derived from TME, were able to suppress T cell-mediated immune responses by secreting soluble mediators that stimulated proangiogenic and protumorigenic hormone function in bone marrow cells, thereby promoting tumor progression.23 In addition, several studies have demonstrated that the down regulation of NK cell function and the immunosuppressive function of tumor-associated macrophages (TAM) are closely associated with the secretion of prostaglandin E2 and cytokines (such as IL-1, TNF, and IL-6) by tumor cells in the TME, respectively.24–27 2) By recruiting immunosuppressive cells. In the TME, immunosuppressive cells indirectly block immunotherapy by secreting factors that establish immune tolerance. As an important immunosuppressive population, Tregs secrete a variety of substances that bind to immune cells and ultimately inhibit the function of anti-tumor immunity and immune effector cells, including perforin, granzyme B, and inhibitory cytokines such as IL-10, IL-35, and transforming growth factor (TGF)-β.28,29 Tregs also exert immunosuppressive effects through binding to molecules on the surface of effector cells, such as CD80/CD86.30 Myeloid-derived suppressor cell (MDSC), recruited from the blood stream by TME, can promote tumorigenesis and metastasis by secreting IL-6, matrix metalloproteinases (MMPs), VEGF, and exosomes.31 It has also reported that reduced STAT3 activity in MDSC leads to rapid differentiation of M-MDSC from TAM, thereby indirectly contributing to the formation of an immunosuppressive environment.32–34 Moreover, MDSC attract Treg cells to the tumor site to enhance their immunosuppressive function, while at the same time suppressing the immune function of DCs, B cells, and NK cells.35,36
Fibrosis of the Extracellular Matrix
As the non-cellular component of the cells in all tissues, ECM makes up the largest component of TME which consists of proteins such as collagen, laminin, hyaluronic acid, and proteoglycan.37 Notably, proteoglycan and hyaluronic acid reinforce the compressive properties in ECM tissues through their glycosaminoglycan (GAG) chain-bound water. The heterotypic protofibrils of collagen I, III, and V make up the fibrillar collagen that contributes to the tensile strength of the tissue.38 These proteins make the ECM in tumor tissue to be inherently hyperdense and stiff, which prevents drug penetration, eventually leading to low accumulation and impeding therapeutic efficacy.39 A number of studies have demonstrated that cancer-associated fibroblasts (CAFs) are a key factor in increasing ECM stiffness and ultimately leading to ECM fibrosis.40,41 It should be noted that CAF is recruited into the tumor stroma and deposits ECM proteins via cytokines and growth factors secreted by tumor cells. Subsequently, CAF reorganizes and crosslinks collagen to enhance the density of collagen fibers, tensile properties, and elevate the compressive force of cells within the tissue, which induces fibrosis in the ECM.38 Numerous studies have revealed that the fibrotic ECM contributes to regulation of the activity of the immune cell population, including recruitment of Tregs, MDSCs, and TAMs, and suppression of T cells, B cells, DCs, and NK cells.42–45 Importantly, the fibrotic ECM is able to block high molecular weight drugs, thereby reducing the efficacy of cancer immunotherapy.46
Abnormal Cellular Metabolism
High production of cellular metabolites such as lipids, reactive oxygen species (ROS), and lactic acid may be harmful to immune cells and cause immunosuppression, ultimately resulting in limitations to immunotherapy.47 For example, high levels of cholesterol, a type of lipid, are positively correlated with depletion of CD8+ T cells.48 Stromal cells and tumor cells in the TME produce large amounts of ROS. The elevated ROS in local TME can in turn promote proliferation of cancer cells and enhance the pro-tumor effects of TAM, MDSC, and CAF.49–51 As one of the most important metabolites in TME, high levels of lactic acid are responsible for impeding the function of immune cells by a variety of mechanisms, including inhibiting the proliferation of immune cells in TME, inducing the dedifferentiation of immune cells in TME and acting as a signaling molecule.52–54 It has also been reported that the angiogenic response induced by high concentrations of vascular endothelial growth factor A (VEGFA) promotes an immature phenotype of the vascular system, thereby leading to development of tumors with vascular dysfunction.55,56 In addition, a handful of studies have found that hypoxia promotes the development of cancer cells and resists immunotherapy by suppressing immune cells, recruiting immunosuppressive cells, and upregulating immune checkpoint molecules.57–60
Nanomaterial-Based Drug Delivery System is Capable of Enhancing Cancer Immunotherapy
Mechanisms Through Which Nanomaterials Enhance Cancer Immunotherapy
Although clinical trials have achieved good results, there are still significant drawbacks and barriers to cancer immunotherapy. Several barriers from the TME limit the ability to deliver the drug, which results in a vast majority of patients not responding to immunotherapy even after taking the drug. Currently, new delivery platforms are being investigated to modify the weak immune efficacy. The boom in nanotechnology offers further opportunities for nanomaterials in cancer immunotherapy. Nanomaterials selectively target tumor sites and prolong the circulation time and blood retention of the drug. Nanomaterial-based drug delivery systems could be more widely used in cancer immunotherapy if the biocompatibility of nanomaterials could be further improved and cytotoxicity could be reduced to a low level.61,62
First, accumulating evidence has proven that nanomaterial-based drug delivery systems can target immune cells, such as effector T cells, dendritic cells, and NK cells, to activate adaptive immunity and enhance their anti-tumor activity, thereby altering the immunosuppressive environment of the TME.12,63,64 Nanomaterial delivery systems carrying antigens and adjuvants can specifically deliver antigens to DCs, activate CTL, and promote DC maturation with the help of antigen presentation or adjuvants. The DCs then present antigen fragments to naive T cells to activate CD4 and CD8 T cells, thereby acquiring cytotoxic capacity to enhance the immune response and fight cancer cells.64 Moreover, one study found that addition of TLR ligands to nanomaterial-based drug delivery systems can induce DC maturation to activate NK cells, thereby leading to a strong immune response.65
Second, given that the formation of an immunosuppressive environment mainly results from accumulation of immunosuppressive cells (for instance Tregs and MDSC) and secretion of immunosuppressive factors (for instance VEGF and TGF-β) and M2 phenotypic polarization of macrophages, nanomaterial-based drug delivery systems can target these components in TME and reshape the immunosuppressive environment into an immune-supportive state, thereby enhancing the effectiveness of cancer immunotherapy.66 A previous study reported that nanomaterials modified with iron oxide were able to reverse the polarization state of macrophages, ultimately causing a phenotypic shift from M2 to M1, which resulted in the loss of TAM activity. This can be attributed to the fact that iron oxide promotes macrophage recruitment, which in turn induces upregulation of M1-associated TNF-α and CD86, and decreases M2-associated IL-10 and CD206.67
Third, the nanomaterial-based drug delivery systems break through the dense and rigid fibrotic barrier of the ECM by combining collagenase and hyaluronidase, which increases the anti-tumor efficiency of the drug.68,69 Collagen and hyaluronic acid are the main ECM structural components associated with fibrosis, promoting tumor metastasis and angiogenesis by virtue of low elasticity and high gelation pressure.70,71 Notably, collagenase breaks down collagen to help the drug-carrying nanomaterial delivery system penetrate the tumor tissue, whereas hyaluronidase degrades hyaluronic acid in tumor ECM to achieve tumor penetration and anti-tumor efficacy of nanomaterial delivery systems. In addition, Tenascin-C, galactose lectin-1, and fibronectin are important components of nanomaterial-based drug delivery systems because they help to break through fibrosis.72 Similarly, induction of apoptosis in CAFs by nanomaterial-based drug delivery systems diminishes the tumor-promoting effect of CAFs and their ability to shape the ECM.73
Finally, nanomaterial-based drug delivery systems could further facilitate stimulation of the immune system, thereby exploiting the synergistic effects of cancer immunotherapy and nanomaterials.74 For example, stimulation of nanomaterials with added stimulus response units, including internal stimuli (pH, adenosine triphosphate (ATP), redox potential, and enzymes) and external stimuli (light, X-rays, ultrasound, and magnetic and electric fields), can enable the versatility of nanomaterials in immunotherapy and further improve the pharmacological properties of the loaded immunomodulators.75,76 Collectively, the above findings suggest that nanomaterial-based drug delivery systems have shown great advantages in cancer immunotherapy (Figure 2).
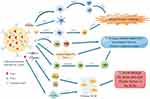 |
Figure 2 Nanomaterial-based drug delivery systems to enhance cancer immunotherapy. (1) Nanomaterial-based drug delivery systems can target immune cells such as effector T cells, dendritic cells and NK cells to gain a strong immune response. (2) Given that the formation of immunosuppressive TME mainly results from the accumulation of immunosuppressive cells and secretion of immunosuppressive factors (for instance VEGF and TGF-β) and M2 phenotypic polarization of macrophages, nanomaterial-based drug delivery systems can target these components in TME and reshape immunosuppressed TME into an immune-supportive state. (3) Nanomaterial-based drug delivery systems break through the dense and rigid fibrotic barrier of the ECM by combining collagenase and hyaluronidase, which increases the anti-tumor efficiency of the drug. Similarly, the tumor-promoting effect of CAFs and their ability to shape the ECM is diminished due to the induction of apoptosis in CAFs by nanomaterial-based drug delivery systems. The Figure was created by Figdraw (www.figdraw.com). Abbreviations: DC, dendritic cell; Tregs, regulatory T cells; TAM, tumor-associated macrophage; MDSC, myeloid-derived suppressor cell; HA, hyaluronic acid; ECM, extracellular matrix; CAF, cancer-associated fibroblast; CTL, cytotoxic T lymphocyte; M2, M2 macrophage; M1, M1 macrophage. |
Different Types of Nanomaterials Target TME to Enhance Cancer Immunotherapy
Inorganic Nanomaterials
Inorganic nanomaterials are mainly classified into metallic nanomaterials, mesoporous silica nanomaterials, and carbon nanomaterials, with each group having unique structural form and intrinsic properties. Owing to their unique optical, magnetic, and photothermal characteristics, metallic nanomaterials hold great promise for research in drug delivery and bioimaging.77 On one hand, metal nanomaterial-based drug delivery systems have shown promising results in enhancing immune responses and reducing immunosuppression. Ultra-small Fe3O4 nanomaterials used as delivery systems in combination with ovalbumin can promote DC maturation and enhance immune response.78 Considering the characteristics of Fe3O4 transforming M2 Tam into M1 phenotype based on Fenton reaction, Fe3O4 nanomaterial delivery system is able to target tumor antigens, immunostimulatory adjuvants and immunomodulators to TAM, thereby transforming the immunosuppressive TME fraction into an immune supportive one, ultimately achieving the conversion of “cold” to “hot” tumors.79 On the other hand, metal nanomaterial-based drug delivery systems can inhibit fibrosis by interacting with proteins in the TME.80 Gold nanomaterials isolate several key autocrine and paracrine signaling factors secreted by CAFs by adsorbing VEGF, heparin binding growth factor and fibroblast growth factor, which disrupts the crosstalk ability of CAFs and cancer cells, ultimately preventing angiogenesis and fibrosis.81 Gold nanomaterials also sufficiently bind IL-8 and TGF-β, key factors involved in paracrine and autocrine signaling in the TME, to alter the interaction between cancer and fibroblasts, thereby inhibiting tumor growth and remodeling the TME.82 Notably, given their ability to interact with most cellular components of proteins and mitochondria, metal nanomaterials can trigger toxic effects, including DNA damage, ATP alteration, ROS production, mitochondrial dysfunction, apoptosis, and cell membrane damage.83 However, surface coating and surface passivation have been shown to be simple and controlled methods of preventing toxicity, which are suitable for almost every metallic nanomaterial.84,85 Thus, metallic nanomaterials should be carefully examined before they are administered to patients to avoid cytotoxicity.
By virtue of the disulfide bonds that are integral to the manufacture of silica networks, mesoporous silica nanomaterials produce size tunable and biocompatible characteristics that enable loading of a wide range of drugs, and ultimately rapid degradation and release of controlled drugs.86 The use of mesoporous silica as a delivery system can overcome challenges from cellular metabolism and immunosuppression. Li et al87 reported a hollow mesoporous silica nanomaterial capable of responding to glutathione (GSH) by interfering with monocarboxylate transporter protein 4 (siMCT-4) of RNA and loading hydroxycamptothecin (HCPT), thereby driving increased intracellular lactate and apoptosis in tumor cells. Besides, after doping Ce6 on mesoporous silica, Kim et al designed a mesoporous silica nanomaterial that could effectively respond to hypoxia to act as an immune adjuvant delivery system, thereby enhancing the activity of DCs and restoring the effectiveness of tumor immunotherapy.88
Carbon nanomaterials (CNMs) are effective drug delivery platforms largely due to their inherent hydrophobicity and the loading of chemical drugs through hydrophobic interactions or π-π stacking.89 In comparison, CNMs are considered to be superior to metal-based nanomaterials with regard to safety and biocompatibility, which is more beneficial for cancer immunotherapy.90 Numerous studies have found that carbon nanomaterials, such as graphene, carbon nanotubes (CNTs), and carbon nanohorns (CNHs), play an active role as delivery systems in immunotherapy. The delivery system made of graphene prevents the development of a fibrotic and immunosuppressive environment. By mimicking the anti-angiogenic structural domain of the histidine-proline-rich glycoprotein (HPRG) in human neuroblastoma (SH-SY5Y) and prostate cancer cells (PC-3), graphene oxide (GO) nanocarriers, made from graphene with its large number of oxygen-containing tributaries and high surface-to-volume ratio, were able to effectively inhibit PC-3 toxicity, block cell migration, and prevent prostaglandin-mediated inflammation in PC-3, which avoid fibrosis.91 In addition, a drug delivery system consisting of reduced graphene oxide (rGO) was able to increase infiltration of tumor-specific CD8+ T cells and reduce infiltration of regulatory T cells (Tregs) in distal tumors by delivering the transforming growth factor β (TGF-β) inhibitor SB-431542 and the chemotherapeutic agent mitoxantrone (MTX), thereby enhancing immunotherapy.92 In recent years, the water solubility and strong toxicity of CNTs have been addressed by surface functionalization and material modifications, which has resulted in CNT-based drug delivery systems gradually being more bioavailable and intensively investigated. Coating CNT with folic acid (FA) and drug-coupled sugar block copolymers synthesized from anti-cancer drugs (doxorubicin, Dox), creates an efficient drug delivery platform for dual targeting of folate receptor (FR) and glucose transporter protein (GLUT5) in breast cancer.93 Single-walled carbon nanotubes (SWCNT) form a promising drug delivery system for the targeted delivery of paclitaxel (PTX) and cis-platinum (CDDP), which allows these drugs to be transported in cells and maintain their therapeutic effect.94,95 In addition, the drug delivery system based on CNHs has also become a force that cannot be ignored. Modified by π-π stacking interactions, Yang et al loaded DOX and cisplatin onto modified nanohorn to fabricate a dual chemotherapeutic drug-loaded single-walled CNH system, which served to eradicate primary breast tumors and lung metastases.96
Liposomal Nanomaterials
Liposomes, spherical vesicles composed of single or multilayered phospholipids, are thought to be the first closed microscopic phospholipid bilayer nanosystems, typically ranging in size from 20 nm to over 1 µm.97 Liposomal nanomaterials can be widely applied in cancer immunotherapy by associating with a variety of cancer drug molecules, and controlling the release of immunomodulators and antigens.98 It is worth noting that liposomal nanomaterial-based drug delivery systems are capable of enhancing the effectiveness of cancer immunotherapy using a variety of approaches.
First, liposome nanomaterial-based drug delivery systems can achieve the ability to trigger or enhance the immune response by delivering stimulatory molecules, thereby successfully enhancing the efficacy of cancer immunotherapy.99 For example, to overcome the toxicity of “free” drugs such as IL-2 Fc fusion protein and immunostimulatory anti-CD137, Zhang et al showed that immunoliposomes in the tumor tissue of a mouse melanoma model facilitated rapid accumulation of the drug and activation of the immune response due to surface coupling to its delivery of anti-CD137 and IL-12, ultimately achieving strong anti-tumor activity without toxicity.100 Similarly, incorporation of the highly toxic adjuvant monophosphoryl lipid A (MPLA) into liposomes reduced toxicity while at the same time activating DCs and enhancing CD8+ T-cell responses. Interestingly, liposomes carrying MPLA also enhanced the expression of CD83 on DCs to induce pro-inflammatory cytokine production (IL1β, IL6, and IL8), ultimately inducing a more effective immune response and efficiently enhancing immune monitoring.101
Second, liposomal nanomaterial-based drug delivery systems can overcome immunosuppressive networks and restore a state of TME suppression driven by inhibitory mediators by delivering immune checkpoint blocking molecules focused on shutting down negative feedback pathways to increase immune responses.102,103 Currently, PEGylated liposomes containing CTLA-4 antibodies are approved for use against reduced T-cell content and activity largely due to competitive inhibition of CD28 and CD80 by CTLA-4.104 Compared to “free” CTLA-4 antibodies, liposomes reduce toxicity in other organs and accumulate in large numbers at the tumor site, thereby resulting in an improved immune response.105 In contrast to the anti-immune mechanism of CTLA-4, PD-1 maintains peripheral tolerance and avoids autoimmunity by inhibiting downstream signaling of the TCR.106 Lang et al discovered a system for the delivery of PD-1 inhibitors thioridazine and HY19991 by liposomal nanomaterials. This drug delivery system was able to incorporate the drugs into the bilayer structure of liposomes and subsequently deliver them to metalloproteinase-rich regions of the tumor, ultimately increasing the accumulation of thioridazine and HY19991 in the tumor by 7.23 and 3.65 fold, respectively, compared to the “free” drugs.107
Furthermore, liposomal nanomaterial-based drug delivery systems are capable of delivering soluble mediators with complex mechanisms, including TGF-β inhibitors and Indoleamine 2.3-dioxygenase (IDO) inhibitors, to selectively modulate TME. With the help of a pancreatic ductal adenocarcinoma model, Meng et al found that PEGylated liposomes loaded with TGF-β inhibitors were able to enter the tumor more easily and reduce the pericyte coverage of the tumor vascular system.108 Similarly, a PEGylated liposome delivery system delivering a TGF-β inhibitor with IL-2 significantly retarded tumor growth by increasing activated CD8 T cell infiltration and NK cell activity in a B16/B6 melanoma mouse model.109 Notably, IDO derived from cells in the TME, such as cancer cells and MDSC, not only restricts T-cell function, but also attracts Tregs, thereby significantly inhibiting the anti-tumor response. Recent studies have shown that systemic toxicity and enhanced anti-tumor immune response can be reduced by using liposomes to deliver immunogenic cell death-inducing chemotherapeutic agents to the desired site.110
Liposomal nanomaterials as delivery platforms still need to address the issues of controlled release and drug loading of liposomes. Its bioavailability also needs to be further improved. In addition, solid liposomal nanomaterials need to overcome the intrinsic low incorporation rate and uncertain tendency to gelation due to their crystalline structure.111 Overall, liposomal nanomaterial-based drug delivery systems have made great achievements in cancer immunotherapy where they have enabled improved cancer treatment through different immunotherapeutic mechanisms, including delivery of stimulatory molecules, immune checkpoint blocking molecules, and soluble mediators.
Polymeric Nanomaterials
Polymeric nanomaterials mainly refer to polymeric nanoparticles (PNPs) with a size of 10–1000 nm. Polymeric nanomaterials serve as delivery platforms for the targeted transport of various drugs and adjuvants to specific sites in cancer, and the composition has undergone continuous modification and refinement.112 Initially, the use of non-biodegradable polymers, such as polystyrene, polymethyl methacrylate (PMMA), and polyacrylamide, to manufacture nanomaterials left the body susceptible to chronic inflammation and toxic reactions.113 Consequently, biodegradable polymers were created to avoid the disadvantages of the previous materials, including poly(caprolactone) (PCL), poly(lactic-co-glycolic acid) (PLGA), poly(lactic acid) (PLA), poly(amino acid), and natural polymers.114 It should be noted that such improved polymeric nanomaterials not only improve the stability of volatile drugs, but also enrich the delivery methods of chemical drugs. Most notably, they can also minimize the adverse toxicity of drugs to normal tissues.115 Polymeric nanomaterial-based drug delivery systems can reactivate the anti-tumor immunity of APCs, thereby resulting in enhanced efficacy of cancer immunotherapy. Different cell surface molecules on DCs, such as CD11c, CD40, and DEC-205, could be targeted and modulated by a PLGA nanomaterial delivery system loaded with Toll-like receptor (TLR) 3 ligands, TLR7 ligands, and ovalbumin, which promotes anti-tumor immunity. Among them, CD40 is the most highly bound molecule to the nanomaterials and makes an outstanding contribution to enhancement of the immune response.116 Polymeric nanomaterial-based drug delivery systems also address the adverse effects of cellular metabolism. A perfect example is the polymeric nanomaterial delivery system invented by Zhou et al which carries doxorubicin and the epidermal growth factor receptor (EGFR) inhibitor erlotinib. Given that doxorubicin achieves a slow and sustained release with the aid of erlotinib, this delivery system can also target and vascularly degrade abnormal blood vessels in the tumor tissue, thereby resulting in a significant reduction in vessel density and ultimately improving the abnormal tumor vascular system in the TME.117 The most important contribution of polymeric nanomaterial-based drug delivery systems is to counteract the immunosuppressive environment. Smith et al found that a poly (beta amino ester) nanomaterial delivery system loaded with encoded DNA triggered production of active CAR T cells and established memory cells in a B-cell leukemia mouse model, which had a positive effect on the destruction of cancer cells.118 Likewise, the polycaprolactone (PCL)-b-polyethylene glycol (PEG) delivery system can effectively activate CTL due to the presence of an oligodeoxynucleotide (ODN)-based immune adjuvant and the endogenous tumor antigen heat shock protein 70 (HSP70), ultimately leading to the generation of a long-term memory immune response.119 Furthermore, a recent study found that a polydopamine nanomaterial delivery system could induce repolarization of M2-TAMs to M1-TAMs by delivering Fe3+, which prevented tumor progression and metastasis in mice models of breast and colorectal cancers.120,121 It is worth noting that some polymeric nanomaterials may have damaging effects on the organism because they may show different effects at different sizes. For example, although PCL of smaller size could enhance immune effects by increasing expression of IL-10 and IL-12 in macrophages, PCL with nanoporous characteristics could also increase in vivo inflammation in fibrous capsules, thereby promoting tumor development.122 A few challenges remain to be addressed for polymeric nanomaterials: evidence shows that toxic monomer aggregation and toxic degradation are possible undesirable conditions for some polymeric nanomaterials.123 Therefore, improving their fabrication and chemical properties is the next focus of research. If these hindrances can be overcome, polymeric nanomaterial delivery systems will have a broader future in cancer immunotherapy.
Small Extracellular Vesicles (sEVs) Nanomaterials
Small extracellular vesicles (30–150 nm in diameter) are found in almost all cells, including endothelial cells, epithelial cells, fibroblasts, neuronal cells, immune cells, and cancer cells.124 sEVs carry typical biomolecules, such as DNA, RNA, and proteins and can participate in long-range communication.125,126 Compared to other nanomaterials, sEVs effectively avoid phagocytosis by circulating monocytes due to the presence of CD47, thereby facilitating effective drug delivery.127 Therefore, sEVs nanomaterials can be involved in cancer immunotherapy as good delivery systems. sEVs are used as carriers for the delivery of anti-cancer drugs to initiate anti-cancer immune responses. Studies involving renal cancer have shown that sEVs delivery systems carrying the glycolipid-anchored-IL-12 (GPI-IL-12) could gene significantly promote T cell proliferation, and efficiently activate CTL via the FasL/Fas signaling pathway by expressing the tumor-associated antigen MAGE-1 and the tumor rejection antigen G250.128,129 In lung cancer, by using exosomes as drug delivery vehicles to package paclitaxel, the limitations of poor water solubility of paclitaxel are effectively avoided and the function of immune cells is re-engaged to kill tumor cells.130 Similarly, exosomes containing chemotherapeutic agents, such as cisplatin, methotrexate, and curcumin, have exhibited outstanding immunotherapeutic effects in the treatment of different cancers.131–133 Moreover, numerous studies have shown that sEVs RNA (such as miR-125a-3p, miR-27a, miR-130a, lncRNA BLACAT1) can be used as diagnostic biomarkers.134–137 Drug delivery systems based on sEVs allow for prognostic diagnostics alongside targeted drug delivery, which significantly enriches the use of nanomaterials. However, considering challenges such as heterogeneity, difficulty in preservation and lack of uniform standards for isolation and purification, research into sEVs nanomaterials needs to be further developed before they can be promoted.138,139
Other Nanomaterials
With the advent of nanotechnology, an increasing number of nanomaterial-based drug delivery systems have been well developed and involved in clinical applications. This section will also briefly introduce several other productive nanomaterials, including monoclonal antibody nanomaterials, nanoemulsions, and dendritic media.77 Monoclonal antibodies (mAbs) are widely used in cancer immunotherapy largely due to their anti-tumor effects and excellent targeting ability. Recently, they have been used in the manufacture of drug delivery systems.140 As a monoclonal antibody capable of treating breast cancer, trastuzumab (Tmab) with paclitaxel forms a drug delivery system that shows great promise for both human epidermal growth factor receptor 2 (HER2)-positive and negative breast cancers, with greater efficacy than either alone.141,142 Nanoemulsions (NE), colloidal nanoparticles ranging from 10 to 1000 nm in size, are made from emulsifiers, aqueous phases, and oils.143 Given their several outstanding properties: large surface area, optical transparency, biodegradability, ease of manufacture, and an ideal drug release profile, Nes have been widely used as drug nanocarriers.144 The NE delivery system carrying IFN-γ not only remains stable under extreme temperature disturbances, but also activates phagocytosis and inhibits the function of MCF-7 human breast cancer cells, thereby demonstrating potential in immunotherapy.145 The highly branched dendritic media, ranging between 1 and 10 nm in size, are highly regular polymers which have a repeating branched structure and an abundance of cavities.146 Considering their special structure that gives them versatile and tunable branched chains, bioavailability to hydrophobic drugs and superior solubility, dendritic polymers can play a crucial role in drug delivery.147 Dendrimer-based nanomaterials allow delivery of DOX to the TME for the treatment of colon cancer, which exhibits enhanced cancer immune efficacy compared to the drug alone.148 In Additional, polyamidoamine (PAMAN)-based drug delivery systems, a type of dendrimer, have an excellent role in combination therapy for the treatment of liver cancer.149 Nanomaterials such as magnetic nanomaterials and nanogels are also beginning to play an increasingly important role in drug delivery systems. It is expected that more nanomaterials will be used for drug delivery applications in the future. Here, we summarize the structural maps of different types of nanomaterials (Figure 3) and the mechanisms of how they can enhance cancer immunotherapy (Table 1).
 |
Table 1 Mechanisms Through Which Different Types of Nanomaterials Enhance Cancer Immunotherapy |
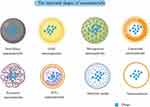 |
Figure 3 The structural shapes of nanomaterials. The Figure was created by Figdraw (www.figdraw.com). |
Nanomaterial-Based Drug Delivery System for Drug Repurposing in Cancer Immunotherapy
Drug repurposing, also known as repositioning or redirection, can serve a new purpose through rescuing a drug which has failed in one indication to deliver a potential drug candidate to another indication.150 With the aid of characteristics of actively targeted tumor sites and retention (EPR) effect of nanomaterial-based drug delivery systems, cytotoxic drugs, cytokines, and adjuvants overcome their inherent toxicity and side effects, ultimately enabling drug repurposing in cancer immunotherapy151 (Table 2). 1) Cytotoxic drug reuse. Nanomaterial-based delivery system of cytotoxic drugs that cause high off-target toxicity in immune cells and systemic toxicity alters the immunosuppressive conditions in tumor tissue by enhancing immunogenic cell death (ICD) and prolongs the retention of cytotoxic drugs at the tumor site. For example, a study involving a mouse model of mammary tumors found that a manganese dioxide nanomaterial delivery system carrying polymeric lipid and DOX could change the immunosuppressive environment by strengthening T-cell activity and attenuating acidosis, thereby resulting in enhanced immune response.152 Mesoporous silica nanomaterials delivering oxaliplatin (OX) and IDO were found to improve in situ pancreatic ductal adenocarcinoma by inducing ICD that recruits CTL from TME and releases high mobility group protein B1 (HMGB-1) required for DC.153 2) Cytokine reuse. Nanomaterial-based drug delivery systems can maximize the effectiveness of cytokines in cancer immunotherapy by overcoming cytokine resistance, off-target effects, short half-lives, toxicities, inflammatory immune responses, and generally low clinical efficacy. Incorporation of hydroxyethyl starch nanomaterials results in IL-2, a T-cell growth factor, binding more tightly to the IL-2 receptor, ultimately promoting more efficient targeting of the T-cell population.154,155 In addition, the ligands on the hydroxyethyl starch nanomaterials indirectly promote proliferation of activated CD4 CD25 T cells, thereby enhancing the immune response.156 3) Adjuvant reuse. Nanomaterial-based drug delivery systems have been shown to co-deliver adjuvants and antigens for effective antigen cross-presentation largely because they overcome the disadvantages of severe adjuvant toxicity and the small number of patients for whom they are suitable. Kim et al157 developed an interesting delivery system using two different nanomaterials carrying adjuvants, Toll-like receptor 3 (TLR3) agonist (poly I:C) and ovalbumin (OVA), model tumor antigens, respectively. This system enhanced secretion of type I IFN-α and IFN-β through uptake of antigen-presenting cells, thereby activating anti-tumor immunity.157 Nanomaterials delivering antigen (AlbiAg) and albumin-binding adjuvant (AlbiCpG) induced antigen-specific T-cell responses in mice, which were critical for altering immunosuppression in the TME and enhancing immunotherapeutic efficacy.158,159 Moreover, nanomaterial-based delivery of antigen adjuvants is available to direct the immune response to specific sites, such as type 1 T helper cells (Th1) or type 2 T helper cells (Th2).160
 |
Table 2 Summary of the Three Categories of Nanomaterial-Based Delivery Systems for Drug Repurposing |
Patents Related to Nanomaterial-Based Drug Delivery Systems
The use of nanomaterials in drug delivery and cancer therapy is critical to the pursuit of better clinical practice, and researchers have developed and patented a large number of nanomaterial inventions. With advantages such as small size, high penetration and better targeting of cancer cells, the number of patented technologies for nanomaterial-based drug delivery systems continues to grow.163 Many different types of nanomaterials have been patented for drug delivery, including metal, carbon, silica, lipid, and polymeric nanomaterials. Patent WO2014047318Al for targeted combination therapy of cancer attaches therapeutic agents to iron oxide nanomaterials with superparamagnetic properties in a non-covalent manner, thereby maintaining the original structure and function of the drug, and it also significantly improves the bioavailability and stability of the loading agent. The invention of patent WO2018102921Al utilizes ultra-stable gold nanomaterials that can withstand cold or heat treatment and remain unchanged. The invention is primarily used to deliver therapeutic agents to mucous membranes and to treat mucous membrane-related diseases. Patent US8535726B2 relates to graphitic nanomaterials, which comprise complexes composed of carbon nanotubes, in particular SWNTs.164 The system enables targeted delivery of drugs to the interior of specific cells by linking hydrophilic polymers, which remain stable in aqueous suspension form. Patent WO2019113184Al describes the clinical application of hollow silica nanomaterials for specific delivery of therapeutic agents to nerves. This invention consists of a multilayer silicone shell with mesopores in each film, and it enhances the ability of the drug to penetrate the nerve, increases the rate at which the therapeutic effect is achieved and prolongs the therapeutic effect of the nerve blocking agent.165 Patent WO2018031782A1 related to liposomal nanomaterials uses SLN to deliver and enhance the stability of glycopeptide drugs. Liposomal nanomaterials can integrate the glycopeptide and prevent the peptide chain of the drug from being broken down by the enzyme peptidase until the glycopeptide binds to the target thereby improving drug stability, tolerance and producing lower cytotoxicity. Patent US8945629B2 is a clinical application of polymeric nanomaterials for enhancing the targeting of chemotherapeutic drugs to cancer cells and prolonging the drug’s effectiveness through the delivery of anti-cancer drugs. The system consists of a polymer soluble in cancer cells and an inner core containing an anti-cancer drug, which protects the entire system by preventing recognition and attack by the reticuloendothelial system. However, the safety, biocompatibility and potential toxicity aspects of nanomaterials still have damaging results for humans. Therefore, more research on nanomaterials should be conducted and more relevant patents should be applied for clinical use, so as to provide better treatment effects for various diseases including cancer.
Challenges for Nanomaterial-Based Drug Delivery Systems
Despite the growing use of nanomaterial-based drug delivery systems in cancer immunotherapy, a number of challenges remain. First, there are significant differences between humans and animals or between patients that cannot be ignored.166 Unlike mice used in clinical studies that lack the complexity of human tumors, human tumors are heterogeneous and mutations can occur in cells within the same tumor.167 This results in the impossibility of an identical TME for each tumor, which significantly reduces the efficiency of nanomaterial-based drug delivery. While the humanization of mouse models may be helpful in addressing such issues, the complexity of the clinical setting is not something it can fully predict and change.168 In the future, conducting more clinical trials and using biomarker assays may provide better results for nanomaterial-based drug delivery systems into cancer immunotherapy. Second, given the many gaps in TME research, the complexity and heterogeneity of TMEs has made it difficult to estimate the targeting efficiency of nanomaterial-based drug delivery systems, which ultimately compromises the effectiveness of cancer immunotherapy. Reversing the immunosuppressive environment of the TME to an immune-supportive environment also has the potential risk of promoting tumor metastasis. Therefore, further studies on the immune interactions and long-term effects of regulatory strategies in TME appear to be necessary. Moreover, toxicity remains a major drawback of nanomaterials. Nanomaterials of very small size can be potentially harmful when penetrating physiological barriers, including endocrine disruption, reduced fertility and metabolic diseases.169 It is also worth mentioning that the techniques for determining the toxicity of nanomaterials are not yet sufficiently mature. This may be attributed to the fact that when nanomaterials react with biological substances in vivo, their own properties may change, and there is a great deal of uncertainty about their final form and the toxicity they will manifest.170,171 Reducing the toxicity of nanomaterials to low levels is a key factor in getting it into clinical treatment on a large scale. The root cause approach to reducing toxicity is to start with a comprehensive toxicology study. By characterizing nanomaterials in detail, researchers can make the data obtained in toxicological assays reliable, reproducible and comparable. In addition, modifying specific material characteristics is also a better way to design lesser toxic nanomaterials, such as controlling the length of nanotubes, applying surfactant coatings, and removing impurities.172 Finally, there is a major question of whether the biological properties, stability and manufacturability of nanomaterial-based drug delivery systems are guaranteed in terms of clinical translation and large-scale manufacturing. The quality control and manufacturing reproducibility of delivery systems has been studied and improved by academic researchers and pharmaceutical companies to address this issue. For example, by using a monodisperse approach to precisely control key quality attributes (such as particle size, drug loading, and targeted ligand coating), the quality and yield of nanomaterials have been improved. This manufacturing control allows the drug to be targeted to specific sites of cancer treatment while ensuring safety and quality.
Conclusion
In this review, we first focus on the specific mechanisms by which nanomaterial-based drug delivery systems overcome barriers to enhance immunotherapy. And we discuss in detail how the different types of nanomaterials currently in existence can facilitate cancer immunotherapy by playing their specific role in TME. Furthermore, the paper discusses nanomaterial-based drug delivery systems for drug repurposing in cancer immunotherapy. By delivering immunotherapeutic drugs (cytotoxic drugs, cytokines, antigens, and adjuvants), nanomaterial-based drug delivery systems can effectively repurpose these drugs, thereby improving cancer immunotherapy. Notably, it is undeniable that there are still limitations and challenges with nanomaterial-based drug delivery systems. It is expected that in the near future, considering the improvements in nanomaterials and advances in nanobiotechnology, nanomaterial-based drug delivery systems will also be available for precision targeting approaches and combination therapies, which will provide new and promising directions for cancer immunotherapy.
Abbreviations
AlbiAg, Albumin-binding antigen; AlbiCpG, Albumin-binding adjuvant; APC, Antigen presenting cell; ATP, Adenosine triphosphate; CAF, Cancer-associated fibroblast; CDDP, Cis-platinum; CNHs, Carbon nanohorns; CNMs, Carbon nanomaterials; CNTs, Carbon nanotubes; CRT, Calreticulin; CTL, Cytotoxic T lymphocyte; CTLA-4, Cytotoxic T lymphocyte antigen 4; DC, Dendritic cell; Dox, Doxorubicin; ECM, Extracellular matrix; EGFR, Epidermal growth factor receptor; FA, Folic acid; FR, Folate receptor; GAG, Glycosaminoglycan; GLUT5, Glucose transporter protein; GO, Graphene oxide; GSH, Glutathione; HA, Hyaluronic Acid; HCPT, Hydroxycamptothecin; HER-2, Human epidermal growth factor receptor 2; HMGB-1, High mobility group protein B1; HPRG, Histidine-proline-rich glycoprotein; HSP70, Heat shock protein 70; ICD, Immunogenic cell death; ICIs, Immune checkpoint inhibitors; IDO, Indoleamine 2.3-dioxygenase; mAbs, monoclonal Antibodies; MDSC, Myeloid-derived suppressor cell; MMPs, Matrix metalloproteinases; MPLA, Monophosphoryl lipid A; MTX, Mitoxantrone; NE, Nanoemulsions; ODN, Oligodeoxynucleotide; OVA, Ovalbumin; PAMAN, Polyamidoamine; PC-3, Prostate cancer cells; PCL, Poly (caprolactone); PD-1M, Programmed death receptor 1; PD-L1, Programmed death ligand 1; PLA, Poly (lactic acid); PLGA, Poly (lactic-co-glycolic acid); PMMA, Polymethyl methacrylate; PNPs, Polymeric nanoparticles; PTX, Paclitaxel; rGO, reduced Graphene oxide; ROS, Reactive oxygen species; sEVs, Small extracellular vesicle; SH-SY5Y, Human neuroblastoma; siMCT-4, Monocarboxylate transporter protein 4; SWCNT, Single-walled carbon nanotubes; Tmab, Trastuzumab; TAM, Tumor-associated macrophage; TIB, Tumor-infiltrating B cell; TGF-β, Transforming growth factor-β; TLR, Toll-like receptor; TME, Tumor microenvironment; TRAIL, Tumor necrosis factor related apoptosis-inducing ligand; Tregs, regulatory T cells; VEGFA, Vascular endothelial growth factor A.
Acknowledgments
The Figures were created by Figdraw (www.figdraw.com).
Author Contributions
Wenjie Zhang and Zhengting Jiang share first authorship. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the Training Project of Key Talents of Youth Medicine in Jiangsu province, China [No. QNRC2016330], the Graduate Research- Innovation Project in Jiangsu province [No. SJCX21_1644], the Social Development-Health Care Project of Yangzhou, Jiangsu Province [No. YZ2018087], the Social Development-Health Care Project of Yangzhou, Jiangsu Province [No. YZ2021075], and High-level talent “six one projects” top talent scientific research project of Jiangsu Province [No. LGY2019034]. The funding bodies had no role in the design of the study; in the collection, analysis, and interpretation of the data; and in writing the manuscript.
Disclosure
The authors declare that there is no conflict of interest.
References
1. Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192(12):5451–5458. doi:10.4049/jimmunol.1490019
2. Im A, Pavletic SZ. Immunotherapy in hematologic malignancies: past, present, and future. J Hematol Oncol. 2017;10(1):94.
3. Nixon NA, Blais N, Ernst S, et al. Current landscape of immunotherapy in the treatment of solid tumours, with future opportunities and challenges. Curr Oncol. 2018;25(5):e373–e384.
4. Scott AM, Allison JP, Wolchok JD. Monoclonal antibodies in cancer therapy. Cancer Immun. 2012;12:14.
5. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385.
6. Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18(3):175–196.
7. Ali ES, Sharker SM, Islam MT, et al. Targeting cancer cells with nanotherapeutics and nanodiagnostics: current status and future perspectives. Semin Cancer Biol. 2021;69:52–68.
8. Song S, Qin Y, He Y, Huang Q, Fan C, Chen HY. Functional nanoprobes for ultrasensitive detection of biomolecules. Chem Soc Rev. 2010;39(11):4234–4243.
9. Osaki T, Yokoe I, Sunden Y, et al. Efficacy of 5-aminolevulinic acid in photodynamic detection and photodynamic therapy in veterinary medicine. Cancers. 2019;11(4):495.
10. Gao W, Wang Z, Lv L, et al. Photodynamic therapy induced enhancement of tumor vasculature permeability using an upconversion nanoconstruct for improved intratumoral nanoparticle delivery in deep tissues. Theranostics. 2016;6(8):1131–1144. doi:10.7150/thno.15262
11. Kyriakides TR, Raj A, Tseng TH, et al. Biocompatibility of nanomaterials and their immunological properties. Biomed Mater. 2021;16(4):042005. doi:10.1088/1748-605X/abe5fa
12. Irvine DJ, Dane EL. Enhancing cancer immunotherapy with nanomedicine. Nat Rev Immunol. 2020;20(5):321–334.
13. Rosenblum D, Joshi N, Tao W, Karp JM, Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun. 2018;9(1):1410.
14. Wang M, Zhao J, Zhang L, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8(5):761–773.
15. Martin K, Schreiner J, Zippelius A. Modulation of APC function and anti-tumor immunity by anti-cancer drugs. Front Immunol. 2015;6:501.
16. Ma Y, Aymeric L, Locher C, Kroemer G, Zitvogel L. The dendritic cell-tumor cross-talk in cancer. Curr Opin Immunol. 2011;23(1):146–152.
17. Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4(12):941–952.
18. Hargadon KM. Tumor-altered dendritic cell function: implications for anti-tumor immunity. Front Immunol. 2013;4:192.
19. Lutz MB, Kurts C. Induction of peripheral CD4+ T-cell tolerance and CD8+ T-cell cross-tolerance by dendritic cells. Eur J Immunol. 2009;39(9):2325–2330.
20. Hoyne GF. Mechanisms that regulate peripheral immune responses to control organ-specific autoimmunity. Clin Dev Immunol. 2011;2011:294968.
21. Redmond WL, Marincek BC, Sherman LA. Distinct requirements for deletion versus anergy during CD8 T cell peripheral tolerance in vivo. J Immunol. 2005;174(4):2046–2053.
22. Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31(6):220–227.
23. Sarvaria A, Madrigal JA, Saudemont A. B cell regulation in cancer and anti-tumor immunity. Cell Mol Immunol. 2017;14(8):662–674.
24. Böttcher JP, Bonavita E, Chakravarty P, et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172(5):1022–1037.e1014.
25. Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266.
26. Szebeni GJ, Vizler C, Kitajka K, Puskas LG. Inflammation and cancer: extra- and intracellular determinants of tumor-associated macrophages as tumor promoters. Mediators Inflamm. 2017;2017:9294018.
27. Lee JH, Lee GT, Woo SH, et al. BMP-6 in renal cell carcinoma promotes tumor proliferation through IL-10-dependent M2 polarization of tumor-associated macrophages. Cancer Res. 2013;73(12):3604–3614.
28. Cao X, Cai SF, Fehniger TA, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27(4):635–646.
29. Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–532.
30. Walker LS. Treg and CTLA-4: two intertwining pathways to immune tolerance. J Autoimmun. 2013;45(100):49–57.
31. Tcyganov E, Mastio J, Chen E, Gabrilovich DI. Plasticity of myeloid-derived suppressor cells in cancer. Curr Opin Immunol. 2018;51:76–82.
32. Safarzadeh E, Orangi M, Mohammadi H, Babaie F, Baradaran B. Myeloid-derived suppressor cells: important contributors to tumor progression and metastasis. J Cell Physiol. 2018;233(4):3024–3036.
33. Condamine T, Dominguez GA, Youn JI, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol. 2016;1(2):aaf8943.
34. Tian X, Shen H, Li Z, Wang T, Wang S. Tumor-derived exosomes, myeloid-derived suppressor cells, and tumor microenvironment. J Hematol Oncol. 2019;12(1):84.
35. Shime H, Kojima A, Maruyama A, et al. Myeloid-derived suppressor cells confer tumor-suppressive functions on natural killer cells via polyinosinic: polycytidylic acid treatment in mouse tumor models. J Innate Immun. 2014;6(3):293–305.
36. Shu CC, Pan SW, Feng JY, et al. The clinical significance of programmed death-1, regulatory T cells and myeloid derived suppressor cells in patients with nontuberculous mycobacteria-lung disease. J Clin Med. 2019;8(5):736.
37. Henke E, Nandigama R, Ergün S. Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Front Mol Biosci. 2019;6:160.
38. Piersma B, Hayward MK, Weaver VM. Fibrosis and cancer: a strained relationship. Biochim Biophys Acta Rev Cancer. 2020;1873(2):188356.
39. Reid SE, Kay EJ, Neilson LJ, et al. Tumor matrix stiffness promotes metastatic cancer cell interaction with the endothelium. EMBO j. 2017;36(16):2373–2389.
40. Liu T, Zhou L, Li D, Andl T, Zhang Y. Cancer-associated fibroblasts build and secure the tumor microenvironment. Front Cell Dev Biol. 2019;7:60.
41. Albacete-Albacete L, Navarro-Lérida I, López JA, et al. ECM deposition is driven by caveolin-1-dependent regulation of exosomal biogenesis and cargo sorting. J Cell Biol. 2020;219(11):e202006178.
42. Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348(6230):74–80.
43. Mao X, Xu J, Wang W, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20(1):131.
44. Serrels A, Lund T, Serrels B, et al. Nuclear FAK controls chemokine transcription, Tregs, and evasion of anti-tumor immunity. Cell. 2015;163(1):160–173.
45. Bae YH, Mui KL, Hsu BY, et al. A FAK-Cas-Rac-lamellipodin signaling module transduces extracellular matrix stiffness into mechanosensitive cell cycling. Sci Signal. 2014;7(330):ra57.
46. Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci U S A. 2011;108(7):2909–2914.
47. Cairns RA, Mak TW. Fire and water: tumor cell adaptation to metabolic conditions. Exp Cell Res. 2017;356(2):204–208.
48. Ma L, Hernandez MO, Zhao Y, et al. Tumor cell biodiversity drives microenvironmental reprogramming in liver cancer. Cancer Cell. 2019;36(4):418–430.e416.
49. Weinberg F, Hamanaka R, Wheaton WW, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107(19):8788–8793.
50. Sena LA, Li S, Jairaman A, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38(2):225–236.
51. Costa A, Scholer-Dahirel A, Mechta-Grigoriou F. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin Cancer Biol. 2014;25:23–32.
52. Huber V, Camisaschi C, Berzi A, et al. Cancer acidity: an ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin Cancer Biol. 2017;43:74–89.
53. Zhang D, Tang Z, Huang H, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574(7779):575–580.
54. Feichtinger RG, Lang R. Targeting L-lactate metabolism to overcome resistance to immune therapy of melanoma and other tumor entities. J Oncol. 2019;2019:2084195.
55. De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17(8):457–474.
56. Klein D. The tumor vascular endothelium as decision maker in cancer therapy. Front Oncol. 2018;8:367.
57. Sarkar S, Germeraad WT, Rouschop KM, et al. Hypoxia induced impairment of NK cell cytotoxicity against multiple myeloma can be overcome by IL-2 activation of the NK cells. PLoS One. 2013;8(5):e64835.
58. Labiano S, Palazon A, Melero I. Immune response regulation in the tumor microenvironment by hypoxia. Semin Oncol. 2015;42(3):378–386.
59. Facciabene A, Peng X, Hagemann IS, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475(7355):226–230.
60. Silva VL, Al-Jamal WT. Exploiting the cancer niche: tumor-associated macrophages and hypoxia as promising synergistic targets for nano-based therapy. J Control Release. 2017;253:82–96.
61. Zhang X, Zhang S, Kang Y, Huang K, Gu Z, Wu J. Advances in Long-Circulating Drug Delivery Strategy. Curr Drug Metab. 2018;19(9):750–758.
62. Shi Y, Lammers T. Combining nanomedicine and immunotherapy. Acc Chem Res. 2019;52(6):1543–1554.
63. Bukhari SI, Imam SS, Ahmad MZ, et al. Recent progress in lipid nanoparticles for cancer theranostics: opportunity and challenges. Pharmaceutics. 2021;13(6):840.
64. Cabral H, Kinoh H, Kataoka K. Tumor-targeted nanomedicine for immunotherapy. Acc Chem Res. 2020;53(12):2765–2776.
65. Deng G, Sun Z, Li S, et al. Cell-membrane immunotherapy based on natural killer cell membrane coated nanoparticles for the effective inhibition of primary and abscopal tumor growth. ACS Nano. 2018;12(12):12096–12108.
66. Overchuk M, Zheng G. Overcoming obstacles in the tumor microenvironment: recent advancements in nanoparticle delivery for cancer theranostics. Biomaterials. 2018;156:217–237.
67. Ngambenjawong C, Gustafson HH, Pun SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev. 2017;114:206–221.
68. Xu F, Huang X, Wang Y, Zhou S. A size-changeable collagenase-modified nanoscavenger for increasing penetration and retention of nanomedicine in deep tumor tissue. Adv Mater. 2020;32(16):e1906745.
69. Abdolahinia ED, Nadri S, Rahbarghazi R, Barar J, Aghanejad A, Omidi Y. Enhanced penetration and cytotoxicity of metformin and collagenase conjugated gold nanoparticles in breast cancer spheroids. Life Sci. 2019;231:116545.
70. Eikenes L, Tufto I, Schnell EA, Bjørkøy A, De Lange Davies C. Effect of collagenase and hyaluronidase on free and anomalous diffusion in multicellular spheroids and xenografts. Anticancer Res. 2010;30(2):359–368.
71. Jaracz S, Chen J, Kuznetsova LV, Ojima I. Recent advances in tumor-targeting anticancer drug conjugates. Bioorg Med Chem. 2005;13(17):5043–5054.
72. Upreti M, Jyoti A, Johnson SE, et al. Radiation-enhanced therapeutic targeting of galectin-1 enriched malignant stroma in triple negative breast cancer. Oncotarget. 2016;7(27):41559–41574.
73. Kovács D, Igaz N, Marton A, et al. Core-shell nanoparticles suppress metastasis and modify the tumour-supportive activity of cancer-associated fibroblasts. J Nanobiotechnology. 2020;18(1):18.
74. Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun. 2016;7:13193.
75. Pacifici N, Bolandparvaz A, Lewis JS. Stimuli-responsive biomaterials for vaccines and immunotherapeutic applications. Adv Ther. 2020;3:2000129.
76. Wang J, Tao W, Chen X, Farokhzad OC, Liu G. Emerging advances in nanotheranostics with intelligent bioresponsive systems. Theranostics. 2017;7(16):3915–3919.
77. Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. 2021;14(1):85.
78. Luo L, Iqbal MZ, Liu C, et al. Engineered nano-immunopotentiators efficiently promote cancer immunotherapy for inhibiting and preventing lung metastasis of melanoma. Biomaterials. 2019;223:119464.
79. Zanganeh S, Hutter G, Spitler R, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11(11):986–994.
80. Miao L, Newby JM, Lin CM, et al. The binding site barrier elicited by tumor-associated fibroblasts interferes disposition of nanoparticles in stroma-vessel type tumors. ACS Nano. 2016;10(10):9243–9258.
81. Melamed JR, Riley RS, Valcourt DM, Day ES. Using gold nanoparticles to disrupt the tumor microenvironment: an emerging therapeutic strategy. ACS Nano. 2016;10(12):10631–10635.
82. Saha S, Xiong X, Chakraborty PK, et al. Gold Nanoparticle reprograms pancreatic tumor microenvironment and inhibits tumor growth. ACS Nano. 2016;10(12):10636–10651.
83. Attarilar S, Yang J, Ebrahimi M, et al. The toxicity phenomenon and the related occurrence in metal and metal oxide nanoparticles: a brief review from the biomedical perspective. Front Bioeng Biotechnol. 2020;8:822.
84. Osmond-McLeod MJ, Osmond RI, Oytam Y, et al. Surface coatings of ZnO nanoparticles mitigate differentially a host of transcriptional, protein and signalling responses in primary human olfactory cells. Part Fibre Toxicol. 2013;10(1):54.
85. Cai X, Lee A, Ji Z, et al. Reduction of pulmonary toxicity of metal oxide nanoparticles by phosphonate-based surface passivation. Part Fibre Toxicol. 2017;14(1):13.
86. Peng S, Xiao F, Chen M, Gao H. Tumor-microenvironment-responsive nanomedicine for enhanced cancer immunotherapy. Adv Sci. 2022;9(1):e2103836.
87. Li K, Lin C, He Y, et al. Engineering of cascade-responsive nanoplatform to inhibit lactate efflux for enhanced tumor chemo-immunotherapy. ACS Nano. 2020;14(10):14164–14180.
88. Im S, Lee J, Park D, Park A, Kim YM, Kim WJ. Hypoxia-triggered transforming immunomodulator for cancer immunotherapy via photodynamically enhanced antigen presentation of dendritic cell. ACS Nano. 2019;13(1):476–488.
89. Krishna KV, Ménard-Moyon C, Verma S, Bianco A. Graphene-based nanomaterials for nanobiotechnology and biomedical applications. Nanomedicine. 2013;8(10):1669–1688.
90. Fadeel B, Bussy C, Merino S, et al. Safety assessment of graphene-based materials: focus on human health and the environment. ACS Nano. 2018;12(11):10582–10620.
91. Quagliarini E, Di Santo R, Pozzi D, Tentori P, Cardarelli F, Caracciolo G. Mechanistic insights into the release of doxorubicin from graphene oxide in cancer cells. Nanomaterials. 2020;10(8):1482.
92. Zhou F, Wang M, Luo T, Qu J, Chen WR. Photo-activated chemo-immunotherapy for metastatic cancer using a synergistic graphene nanosystem. Biomaterials. 2021;265:120421.
93. Omurtag Ozgen PS, Atasoy S, Zengin Kurt B, Durmus Z, Yigit G, Dag A. Glycopolymer decorated multiwalled carbon nanotubes for dual targeted breast cancer therapy. J Mater Chem B. 2020;8(15):3123–3137.
94. Al Garalleh H, Algarni A. Modelling of paclitaxel conjugated with carbon nanotubes as an antitumor agent for cancer therapy. J Biomed Nanotechnol. 2020;16(2):224–234.
95. He Z, Jiang R, Long W, et al. The combination of Diels-Alder reaction and redox polymerization for preparation of functionalized CNTs for intracellular controlled drug delivery. Mater Sci Eng C Mater Biol Appl. 2020;109:110442.
96. Yang J, Su H, Sun W, et al. Dual chemodrug-loaded single-walled carbon nanohorns for multimodal imaging-guided chemo-photothermal therapy of tumors and lung metastases. Theranostics. 2018;8(7):1966–1984.
97. Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48.
98. Zununi Vahed S, Salehi R, Davaran S, Sharifi S. Liposome-based drug co-delivery systems in cancer cells. Mater Sci Eng C Mater Biol Appl. 2017;71:1327–1341.
99. Cruz LJ, Rueda F, Simón L, Cordobilla B, Albericio F, Domingo JC. Liposomes containing NY‑ESO‑1/tetanus toxoid and adjuvant peptides targeted to human dendritic cells via the Fc receptor for cancer vaccines. Nanomedicine. 2014;9(4):435–449.
100. Zhang Y, Li N, Suh H, Irvine DJ. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat Commun. 2018;9(1):6. doi:10.1038/s41467-017-02251-3
101. Boks MA, Bruijns SCM, Ambrosini M, et al. In situ delivery of tumor antigen- and adjuvant-loaded liposomes boosts antigen-specific T-cell responses by human dermal dendritic cells. J Invest Dermatol. 2015;135(11):2697–2704. doi:10.1038/jid.2015.226
102. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi:10.1056/NEJMoa1200694
103. Da Silva CG, Peters GJ, Ossendorp F, Cruz LJ. The potential of multi-compound nanoparticles to bypass drug resistance in cancer. Cancer Chemother Pharmacol. 2017;80(5):881–894. doi:10.1007/s00280-017-3427-1
104. Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood. 2018;131(1):58–67. doi:10.1182/blood-2017-06-741033
105. Nikpoor AR, Tavakkol-Afshari J, Sadri K, Jalali SA, Jaafari MR. Improved tumor accumulation and therapeutic efficacy of CTLA-4-blocking antibody using liposome-encapsulated antibody: in vitro and in vivo studies. Nanomedicine. 2017;13(8):2671–2682. doi:10.1016/j.nano.2017.08.010
106. Fife BT, Pauken KE, Eagar TN, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10(11):1185–1192. doi:10.1038/ni.1790
107. Lang T, Liu Y, Zheng Z, et al. Cocktail strategy based on spatio-temporally controlled nano device improves therapy of breast cancer. Adv Mater. 2019;31(33):e1903844. doi:10.1002/adma.201903844
108. Meng H, Zhao Y, Dong J, et al. Two-wave nanotherapy to target the stroma and optimize gemcitabine delivery to a human pancreatic cancer model in mice. ACS Nano. 2013;7(11):10048–10065. doi:10.1021/nn404083m
109. Park J, Wrzesinski SH, Stern E, et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater. 2012;11(10):895–905. doi:10.1038/nmat3355
110. Huang Z, Wei G, Zeng Z, et al. Enhanced cancer therapy through synergetic photodynamic/immune checkpoint blockade mediated by a liposomal conjugate comprised of porphyrin and IDO inhibitor. Theranostics. 2019;9(19):5542–5557. doi:10.7150/thno.35343
111. Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12(1):62–76. doi:10.1208/s12249-010-9563-0
112. Masood F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater Sci Eng C Mater Biol Appl. 2016;60:569–578. doi:10.1016/j.msec.2015.11.067
113. Fu Y, Kao WJ. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin Drug Deliv. 2010;7(4):429–444. doi:10.1517/17425241003602259
114. Elsabahy M, Wooley KL. Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev. 2012;41(7):2545–2561. doi:10.1039/c2cs15327k
115. Martín-Saldaña S, Palao-Suay R, Aguilar MR, Ramírez-Camacho R, San Román J. Polymeric nanoparticles loaded with dexamethasone or α-tocopheryl succinate to prevent cisplatin-induced ototoxicity. Acta Biomater. 2017;53:199–210. doi:10.1016/j.actbio.2017.02.019
116. Cruz LJ, Rosalia RA, Kleinovink JW, Rueda F, Löwik CW, Ossendorp F. Targeting nanoparticles to CD40, DEC-205 or CD11c molecules on dendritic cells for efficient CD8(+) T cell response: a comparative study. J Control Release. 2014;192:209–218. doi:10.1016/j.jconrel.2014.07.040
117. Zhou Z, Kennell C, Jafari M, et al. Sequential delivery of erlotinib and doxorubicin for enhanced triple negative Breast cancer treatment using polymeric nanoparticle. Int J Pharm. 2017;530(1–2):300–307. doi:10.1016/j.ijpharm.2017.07.085
118. Smith TT, Stephan SB, Moffett HF, et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat Nanotechnol. 2017;12(8):813–820. doi:10.1038/nnano.2017.57
119. Chang HC, Zou ZZ, Wang QH, et al. Targeting and specific activation of antigen-presenting cells by endogenous antigen-loaded nanoparticles elicits tumor-specific immunity. Adv Sci. 2020;7(1):1900069. doi:10.1002/advs.201900069
120. Song M, Liu T, Shi C, Zhang X, Chen X. Bioconjugated manganese dioxide nanoparticles enhance chemotherapy response by priming tumor-associated macrophages toward M1-like phenotype and attenuating tumor hypoxia. ACS Nano. 2016;10(1):633–647.
121. Rong L, Zhang Y, Li WS, Su Z, Fadhil JI, Zhang C. Iron chelated melanin-like nanoparticles for tumor-associated macrophage repolarization and cancer therapy. Biomaterials. 2019;225:119515.
122. Padmanabhan J, Kyriakides TR. Nanomaterials, inflammation, and tissue engineering. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(3):355–370.
123. Lockman PR, Mumper RJ, Khan MA, Allen DD. Nanoparticle technology for drug delivery across the blood-brain barrier. Drug Dev Ind Pharm. 2002;28(1):1–13.
124. Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125.
125. Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17.
126. Greening DW, Gopal SK, Mathias RA, et al. Emerging roles of exosomes during epithelial-mesenchymal transition and cancer progression. Semin Cell Dev Biol. 2015;40:60–71.
127. Koh E, Lee EJ, Nam GH, et al. Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials. 2017;121:121–129.
128. Zhang Y, Luo CL, He BC, Zhang JM, Cheng G, Wu XH. Exosomes derived from IL-12-anchored renal cancer cells increase induction of specific antitumor response in vitro: a novel vaccine for renal cell carcinoma. Int J Oncol. 2010;36(1):133–140.
129. Zhang J, Zhang Y, Luo C, Xia Y, Chen H, Wu X. Glycosyl-phosphatidylinositol-anchored interleukin-2 expressed on tumor-derived exosomes induces antitumor immune response in vitro. Tumori. 2010;96(3):452–459.
130. Wang J, Li W, Zhang L, et al. Chemically edited exosomes with dual ligand purified by microfluidic device for active targeted drug delivery to tumor cells. ACS Appl Mater Interfaces. 2017;9(33):27441–27452.
131. Jia G, Han Y, An Y, et al. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018;178:302–316.
132. Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol. 2018;15(10):617–638.
133. Ma J, Zhang Y, Tang K, et al. Reversing drug resistance of soft tumor-repopulating cells by tumor cell-derived chemotherapeutic microparticles. Cell Res. 2016;26(6):713–727.
134. Wang J, Yan F, Zhao Q, et al. Circulating exosomal miR-125a-3p as a novel biomarker for early-stage colon cancer. Sci Rep. 2017;7(1):4150.
135. Dong SJ, Cai XJ, Li SJ. The clinical significance of MiR-429 as a predictive biomarker in colorectal cancer patients receiving 5-fluorouracil treatment. Med Sci Monit. 2016;22:3352–3361.
136. Xu J, Zhao J, Zhang R. Prognostic significance of serum miR-193b in colorectal cancer. Int J Clin Exp Pathol. 2017;10(9):9509–9514.
137. Dai M, Chen X, Mo S, et al. Meta-signature LncRNAs serve as novel biomarkers for colorectal cancer: integrated bioinformatics analysis, experimental validation and diagnostic evaluation. Sci Rep. 2017;7:46572.
138. Wei W, Ao Q, Wang X, et al. Mesenchymal stem cell-derived exosomes: a promising biological tool in nanomedicine. Front Pharmacol. 2020;11:590470.
139. Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514.
140. Markman JL, Rekechenetskiy A, Holler E, Ljubimova JY. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv Drug Deliv Rev. 2013;65(13–14):1866–1879.
141. Nieto C, Vega MA. Trastuzumab: more than a guide in HER2-positive cancer nanomedicine. Nanomaterials. 2020;10(9):1674.
142. Abedin MR, Powers K, Aiardo R, Barua D, Barua S. Antibody-drug nanoparticle induces synergistic treatment efficacies in HER2 positive breast cancer cells. Sci Rep. 2021;11(1):7347.
143. Jaiswal M, Dudhe R, Sharma PK. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech. 2015;5(2):123–127.
144. Gorain B, Choudhury H, Nair AB, Dubey SK, Kesharwani P. Theranostic application of nanoemulsions in chemotherapy. Drug Discov Today. 2020;25(7):1174–1188.
145. Ribeiro EB, de Marchi PGF, Honorio-França AC, França EL, Soler MAG. Interferon-gamma carrying nanoemulsion with immunomodulatory and anti-tumor activities. J Biomed Mater Res A. 2020;108(2):234–245.
146. Svenson S. The dendrimer paradox–high medical expectations but poor clinical translation. Chem Soc Rev. 2015;44(12):4131–4144.
147. Durocher I, Girard D. In vivo proinflammatory activity of generations 0-3 (G0-G3) polyamidoamine (PAMAM) nanoparticles. Inflamm Res. 2016;65(9):745–755.
148. Pishavar E, Ramezani M, Hashemi M. Co-delivery of doxorubicin and TRAIL plasmid by modified PAMAM dendrimer in colon cancer cells, in vitro and in vivo evaluation. Drug Dev Ind Pharm. 2019;45(12):1931–1939.
149. Tarach P, Janaszewska A. Recent advances in preclinical research using PAMAM dendrimers for cancer gene therapy. Int J Mol Sci. 2021;22(6):2912.
150. Verbaanderd C, Meheus L, Huys I, Pantziarka P. Repurposing drugs in oncology: next steps. Trends Cancer. 2017;3(8):543–546.
151. Antoszczak M, Markowska A, Markowska J, Huczyński A. Old wine in new bottles: drug repurposing in oncology. Eur J Pharmacol. 2020;866:172784.
152. Amini MA, Abbasi AZ, Cai P, et al. Combining tumor microenvironment modulating nanoparticles with doxorubicin to enhance chemotherapeutic efficacy and boost antitumor immunity. J Natl Cancer Inst. 2019;111(4):399–408.
153. Lu J, Liu X, Liao YP, et al. Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat Commun. 2017;8(1):1811.
154. Christian DA, Hunter CA. Particle-mediated delivery of cytokines for immunotherapy. Immunotherapy. 2012;4(4):425–441.
155. Frick SU, Domogalla MP, Baier G, et al. Interleukin-2 functionalized nanocapsules for T cell-based immunotherapy. ACS Nano. 2016;10(10):9216–9226.
156. Nair PM, Flores H, Gogineni A, et al. Enhancing the antitumor efficacy of a cell-surface death ligand by covalent membrane display. Proc Natl Acad Sci U S A. 2015;112(18):5679–5684.
157. Kim SY, Noh YW, Kang TH, et al. Synthetic vaccine nanoparticles target to lymph node triggering enhanced innate and adaptive antitumor immunity. Biomaterials. 2017;130:56–66.
158. Zhu G, Lynn GM, Jacobson O, et al. Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nat Commun. 2017;8(1):1954.
159. Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, Moon JJ. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat Mater. 2017;16(4):489–496.
160. Wilson JT, Keller S, Manganiello MJ, et al. pH-Responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides. ACS Nano. 2013;7(5):3912–3925.
161. Wang P, Wang H, Huang Q, et al. Exosomes from M1-polarized macrophages enhance paclitaxel antitumor activity by activating macrophages-mediated inflammation. Theranostics. 2019;9(6):1714–1727.
162. Yuan H, Jiang W, von Roemeling CA, et al. Multivalent bi-specific nanobioconjugate engager for targeted cancer immunotherapy. Nat Nanotechnol. 2017;12(8):763–769.
163. Ali Hazis NU, Aneja N, Rajabalaya R, David SR. Systematic patent review of nanoparticles in drug delivery and cancer therapy in the last decade. Recent Adv Drug Deliv Formul. 2021;15(1):59–74.
164. Khan AU, Khan M, Cho MH, Khan MM. Selected nanotechnologies and nanostructures for drug delivery, nanomedicine and cure. Bioprocess Biosyst Eng. 2020;43(8):1339–1357.
165. Bharti C, Nagaich U, Pal AK, Gulati N. Mesoporous silica nanoparticles in target drug delivery system: a review. Int J Pharm Investig. 2015;5(3):124–133.
166. Ojha T, Pathak V, Shi Y, et al. Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors. Adv Drug Deliv Rev. 2017;119:44–60.
167. Zhao X, Li L, Starr TK, Subramanian S. Tumor location impacts immune response in mouse models of colon cancer. Oncotarget. 2017;8(33):54775–54787.
168. Sanmamed MF, Chester C, Melero I, Kohrt H. Defining the optimal murine models to investigate immune checkpoint blockers and their combination with other immunotherapies. Ann Oncol. 2016;27(7):1190–1198.
169. Yao Y, Tang M. Advances in endocrine toxicity of nanomaterials and mechanism in hormone secretion disorders. J Appl Toxicol. 2022;42(7):1098–1120.
170. Shams F, Golchin A, Azari A, et al. Nanotechnology-based products for cancer immunotherapy. Mol Biol Rep. 2022;49(2):1389–1412.
171. Juárez-Maldonado A, Tortella G, Rubilar O, Fincheira P, Benavides-Mendoza A. Biostimulation and toxicity: the magnitude of the impact of nanomaterials in microorganisms and plants. J Adv Res. 2021;31:113–126.
172. Yuan X, Zhang X, Sun L, Wei Y, Wei X. Cellular toxicity and immunological effects of carbon-based nanomaterials. Part Fibre Toxicol. 2019;16(1):18.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
