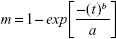Back to Journals » International Journal of Nanomedicine » Volume 13
Nanohybrid hydrogels designed for transbuccal anesthesia
Authors Ribeiro LNM , Franz-Montan M, Breitkreitz MC , Rodrigues da Silva GH , Castro SR, Guilherme VA, de Araújo DR, de Paula E
Received 13 July 2018
Accepted for publication 23 August 2018
Published 15 October 2018 Volume 2018:13 Pages 6453—6463
DOI https://doi.org/10.2147/IJN.S180080
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Lígia Nunes de Morais Ribeiro,1 Michelle Franz-Montan,2 Márcia Cristina Breitkreitz,3 Gustavo Henrique Rodrigues da Silva,1 Simone Ramos de Castro,1 Viviane Aparecida Guilherme,1 Daniele Ribeiro de Araújo,4 Eneida de Paula1
1Department of Biochemistry and Tissue Biology, Institute of Biology, University of Campinas (Unicamp), Campinas, São Paulo, Brazil; 2Department of Physiological Sciences, Piracicaba Dental School, Unicamp, Piracicaba, São Paulo, Brazil; 3Department of Analytical Chemistry, Institute of Chemistry, Unicamp, Campinas, São Paulo, Brazil; 4Human and Natural Science Center, ABC Federal University, Santo André, São Paulo, Brazil
Background: Local anesthesia in dentistry is by far the most terrifying procedure for patients, causing treatment interruption. None of the commercially available topical formulations is effective in eliminating the pain and phobia associated to the needle insertion and injection.
Materials and methods: In this work we prepared a nanostructured lipid-biopolymer hydrogel for the sustained delivery of lidocaine–prilocaine (LDC-PLC) for transbuccal preanesthesia. The lipid was composed of optimized nanostructured lipid carriers (NLC) loaded with 5% LDC-PLC (NLC/LDC-PLC). The biopolymer counterpart was selected among alginate, xanthan (XAN), and chitosan matrices. The XAN-NLC hydrogel presented the most uniform aspect and pseudoplastic rheological profile, as required for topical use; therefore, it was selected for subsequent analyses. Accelerated stability tests under critical conditions (40°C; 75% relative humidity) were conducted for 6 months, in terms of drug content (mg/g), weight loss (%), and pH.
Results: In vitro LDC-PLC release profile through Franz diffusion cells revealed a bimodal kinetics with a burst effect followed by the sustained release of both anesthetics, for 24 hours. Structural analyses (fourier transform infrared spectroscopy, differential scanning calorimetry and scanning electron microscopy) gave details on the molecular organization of the hybrid hydrogel, confirming the synergic interaction between the components. Safety and efficacy were evaluated through in vitro cell viability (3T3, HaCat, and VERO cells) and in vivo antinociceptive (tail-flick, in mice) tests, respectively. In comparison to a control hydrogel and the eutectic mixture of 5% LDC-PLC cream (EMLA®), the XAN-NLC/LDC-PLC hybrid hydrogel doubled and quadrupled the anesthetic effect (8 hours), respectively.
Conclusion: Considering such exciting results, this multifaceted nanohybrid system is now ready to be further tested in clinical trials.
Keywords: hybrid hydrogel, NLC, xanthan, lidocaine–prilocaine, topical buccal anesthesia, dentistry
Introduction
One of the most expected innovations in dentistry is the development of an effective topical anesthetic formulation. This is a relevant concern due to the patient fear to the injection and local anesthetic (LA) administration. Currently, “needle phobia” is the cause of treatment nonadherence, aggravating the dental problems. Moreover, there is a lack of efficient noninvasive pain relief formulations.1 The most desirable marketed topical anesthetic cream is composed of the eutectic mixture of lidocaine and prilocaine (LDC-PLC) 5% w/w (EMLA®). However, this formulation has been designed for dermatological purposes, and it causes ulceration when applied at the oral mucosa and does not minimize the palatal anesthesia pain, in humans.2,3 Furthermore, systemic toxicity symptoms, such as fever after the eutectic mixture of LDC-PLC cream buccal application, were also reported.4
The nanoencapsulation of LA is a versatile approach, which has been successfully found in protecting the drug against degradation, providing nanostructured drug delivery systems (DDS) with physicochemical stability, biocompatibility, and optimized efficacy.5 Nanostructured lipid carriers (NLC) are a DDS formed by a lipid blend inner core coated with surfactants, and they provide efficient loading of hydrophobic molecules.6 In an attempt to develop an efficient pre-anesthetic for transbuccal application, we have previously described an optimized NLC/LDC-PLC (5%) formulation, selected from factorial design, with excellent structural properties, stability, and in vitro sustained release profile for both LAs.7 Despite the excellent properties of that DDS, the fluidness and low adhesion8 inherent to most of the colloidal formulations prevented its application as a pre-anesthetic in dentistry.
Hydrogels are macroscopic and water-soluble pharmaceutical forms formed by a tridimensional polymeric network, widely used for topical administration of several classes of drugs.9 Hydrogels can be processed from synthetic or natural polymers.10,11 Processing hydrogels from biopolymeric matrices is especially interesting due to their biocompatibility and biodegradability properties, abundance, low cost, and absence of organic solvents or weak acids in most of the preparation methods.12 Xanthan gum (XAN) is an anionic biopolymer synthesized from strains of Xanthomonas campestris that is broadly used in pharmaceutical and food industries.13 Its interest for use as hydrogel matrices for oral mucosa is also given by the free carboxylic acid available groups, which form hydrogen bonds with the mucus layer, improving the specificity of drug-release profile and efficacy.14,15
The hybridization process of materials is an ancient practice, reported from the antique Egyptians.16 In the DDS field, these advanced hybrid materials combine structural, physicochemical, mechanical, and therapeutic properties of each excipient in a single final form. Hybridization enables the development of smart formulations or pharmaceutical forms that are able to specifically interact with biological barriers,17 such as the mucosal tissues and skin. There are some reports of lipid-biopolymer hydrogels for the topical sustained release of ibuprofen,18 ketoprofen,19 ofloxacin,20 Resina draconis,21 clobetasol,22 and LAs,23–25 which exhibited optimized properties in comparison to their lipid-related DDS.
In this sense, the development of lipid-biopolymer hydrogels based on NLC/LDC-PLC (5%) formulation was described,7 incorporated in XAN, alginate (ALG), or chitosan (CHT) matrices. The resultant hybrid hydrogels combined the sustained release of the LA provided by the NLC, with the desirable consistency, viscosity, and adhesion afforded by the biopolymer matrix. The NLC decreased the hydrophilic character of the biopolymers, improving the overall formulation affinity for the encapsulated anesthetics. The results obtained with the optimized (XAN-NLC) bio-hybrid hydrogel strongly support its future application for transbuccal anesthesia.
Materials and methods
Materials
LDC and PLC were donated by Cristália Prod. Quim. Farm. Ltda (Itapira, Brazil). Pluronic® 68 (P68), ALG, XAN, and CHT were supplied by Sigma (St Louis, MO, USA). Cetyl palmitate (CP) was provided by Dhaymers Química Fina (Taboao Da Serra, Brazil) and capric/caprylic triglycerides (GC) were from Lipo do Brasil Ltda (São Bernardo do Campo, Brazil). High-performance liquid chromatography (HPLC)-grade acetonitrile was from J.T. Baker, Phillipsburg, NJ, USA. Deionized water (18 MΩ) was obtained from an Elga USF Maxima Ultra-Pure water purifier (Elga LabWater, High Wycombe, UK).
NLC preparation method
NLC/LDC-PLC was prepared through the emulsification–ultrasonication method. Briefly, a blend of CP (16%; w/v) and GC (3.8%; w/v) was heated in a water bath to 10.0°C above the melting point of CP (55°C). Then, a eutectic mixture of LDC-PLC (5%; w/v) was incorporated in the oily phase until complete dissolution.7 Next, an aqueous phase containing 3.5% P68 (w/v) and equally heated was added dropwise to the oily phase, under high-speed agitation (10,000 rpm) for 2 minutes, in an Ultra-Turrax blender (IKA WerkeStaufen, Staufen im Breisgau, Germany). The obtained microemulsion was ultrasonicated for 30 minutes at 500 W and 20 kHz, with intermittent 30 seconds (on/off) cycles in a Vibracell tip sonicator (Sonics & Mat. Inc., Danbury, CT, USA); the obtained samples were cooled to reach room temperature.
Biopolymer solution and hydrogel preparation
Aliquots of 2 g of ALG, XAN, and CHT were dispersed in 50 mL of deionized water or acetic acid 0.1% (only for CHT), under stirring and until complete homogenization. Then, LDC-PLC (5%) hydroalcoholic solutions were dispersed in ALG, XAN, and CHT suspensions and stirred for 2 hours at room temperature. For the hybrid hydrogel, NLC formulations replaced the water used for dissolution of XAN.26 The proportion of NLC/LDC-PLC and XAN in the hydrogel was 1:1 (w/w). In all types of prepared hydrogels, the final concentration of LDC-PLC was 25 mg/g. Crosslinking of the ALG-based and CHT-based hydrogels was achieved, respectively, by dropwise addition of 1 mL of 2% (w/v) CaCl2 solution or 1% (w/v) tripolyphosphate solution, under magnetic stirring for 1 hour. The prepared hydrogels were stabilized overnight at 4°C, and visually inspected prior to use.
In vitro LDC-PLC release from hydrogels
The release of LDC-PLC from the biopolymer-based or lipid-biopolymer hydrogels was studied using a Franz-cell system, composed of a donor compartment (400 μL) and an acceptor compartment (4 mL), filled with 5 mM Tween/PBS, pH 7.4. A membrane with 10,000 Da molecular exclusion pore size separated the compartments. The systems were maintained under sink condition. Aliquots were withdrawn from the acceptor compartment at periodic intervals for HPLC quantification (λ=220 nm), and the volume was maintained constant by replacement of buffer solution (200 μL). All measurements were performed in sextuplicate. The two peak areas in the chromatograms were used to determine the percentage of LDC and PLC released. The KinetDS 3.0 software (Aleksander Mendyk, Kraków, Poland) was employed to analyze the kinetic curves. Among the kinetic models tested, the Weibull model was the best fit considering the coefficient of determination (R2).
|
where: m is the amount of LDC-PLC released at the time t, b is the release exponent, and a is the time scale of release.
Accelerated stability of lipid-biopolymer hydrogels
The accelerated stability of the XAN-based hydrogels was performed in triplicate at predetermined times, 0, 3, and 6 months, in terms of pH changes, weight loss (<5%), and LA content (mg/g, quantified by HPLC). The hydrogels were stored in an adapted stability chamber at 40°C±3°C and 75%±3% relative humidity (ICH Expert Working Group, 2003).49 Statistical analyses were carried out by unpaired Student’s t-test and one-way analysis of variance (ANOVA) and Tukey post hoc test (p<0.05).
Structural characterization
The XAN-based hydrogels viscosity (mPa s) was determined with a rheometer (Haake RheoStress 1, Thermo Fisher Scientific, Waltham, MA, USA), in a low amplitude oscillatory strain, from 0.1 to 10 Hz, at 37°C. The analysis time for ascending and descending curve was 170 seconds, and the shear gradient ranged from 0 [1/s] to 170 [1/s].
ATR-FTIR spectra were recorded with an infrared spectrometer equipped with ATR (Bruker IFS, Bruker, Billerica, MA, USA), in the range of 4500–500 cm−1.
DSC measurements were carried out in a TA Q20 calorimeter (TA Instruments, New Castle, DE, USA) equipped with a cooling system. The samples (5 mg) were placed in aluminum pans and the thermal profiles were obtained from 0°C to 250°C, at 10°C/min heating rate, under a flow of nitrogen.
FE-SEM equipment (FEI-NOVA NanoSEM 230, Sydney, NSW, Australia) was used to elucidate the textural properties of the hydrogels. The samples were adhered on a carbon tape and subjected to a gold conducive coating on the surface.
Bioassays
In vitro cell viability
The cytotoxicity of XAN-based hydrogels was determined by MTT assay, in cultures of Balb/c 3T3 fibroblasts, immortalized human keratinocytes cells (HaCat), and monkey kidney fibroblasts (VERO). All the cell lines were commercially purchased from the Laboratório BCRJ, Universidade Federal do Rio de Janeiro, UFRJ, Brazil. Briefly, the cells were seeded in 96-well culture plates and incubated for 2 hours (37°C, 5% CO2). The Roswell Park Memorial Institute culture medium was replaced by 200 μL of fresh medium, with different concentrations of hydrogels. After the exposure period (2 hours), the medium was removed, and the plate was washed with PBS (pH 7.4). Then, 200 μL of medium (without serum) containing 0.5 mg/mL of MTT reagent was added to each well and incubated for 2 hours at 37°C. After that, the MTT solution was removed and 200 μL of ethanol was added to each well, dissolving the formed formazan crystals. The formazan absorbance was quantified in a microplate reader (570 nm). Results were expressed as the mean viability percentage ± standard error means (SEM) (n=3). The statistical analysis was carried out by two-way ANOVA and Tukey post hoc test.
Tail-flick test
Male adult Swiss mice (25–30 g) were obtained from the Centro Multidisciplinar para Investigação Biológica na área de Ciência em Animais de Laboratório (CEMIB-UNICAMP, Campinas, Brazil). The protocol was approved by the UNICAMP Institutional Animal Care and Use Committee (4468-1/2017), and the methodologies following the recommendations of the Guide for the Care and Use of Laboratory Animals. The animals were maintained (5/cage) with free access to food and water and were placed in a restraint over an analgesimeter, with a portion of the tail (5 cm from its top) exposed to heat from a projector lamp (55°C±1°C). Thirty seconds cut-off time was used to avoid thermal injury and the baseline (normal response to the noxious stimulus) was noted. For the blockage of the caudal nerve, hydrogels (0.025 g) containing 5% LDC-PLC were applied on the back of the mice tail, and occluded. The analysis started 30 minutes after hydrogel administration and the data were recorded every 30 minutes during the first 1 hour, and every 60 minutes up to the end of the experiment (8 hours). Data were expressed as percentage of maximum effect (%MPE), and the areas under the curves were calculated. The statistical analyses were performed by two-way ANOVA plus Tukey post hoc, n=5.
Results
Visual inspection of lipid-biopolymer hydrogels
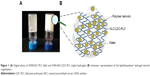
Different hybrid hydrogels were prepared by blending the lipid (NLC/LDC-PLC) and biopolymer (CHT, ALG, and XAN) phases, ionically crosslinked when necessary. The most successful hybrid hydrogel composition was prepared with NLC and XAN (XAN-NLC/LDC-PLC) excipients, which showed homogenous aspect and suitable consistency (Figure 1). The ALG-based hydrogels presented heterogeneous aspect, with dispersed microgranules in the final form. Regarding the CHT hydrogel matrix, separation was observed immediately after the preparation. Therefore, the XAN-based NLC hydrogel was selected for further in vitro and in vivo tests.
In vitro LDC-PLC release test
The in vitro LDC-PLC release test was carried out in a Franz-type vertical diffusion cell for 24 hours, and quantified by HPLC. Control hydrogels, containing only the biopolymer and the anesthetics (XAN-LDC and XAN-PLC), reached 100% release of both LA after 4–5 hours of experiment (Figure 2). The hybrid (lipid-biopolymer) XAN-NLC hydrogels exhibited a sustained bimodal release profile: after 3 and 24 hours of study, 35% and 57% of lidocaine and 43% and 81% of prilocaine were released, respectively.
The KinetD 3.0 software was used to fit the release curves in Figure 2, using several mathematical models.27 The best fit (higher R2 values) for LDC and PLC release from the hydrogels were found with the Weibull model (Table S1). In all curves, the b coefficient (Weibull model) that determines the release mechanism, ranged from 0.03 to 0.14, denoting Fickian diffusion.28
Accelerated stability test
In the accelerated stability test (conducted under critical stress condition) of semisolid formulations, the physicochemical parameters that are followed over time anticipate processes such as drug degradation and physicochemical changes, that can compromise the formulation performance.29 Therefore, the XAN-based hydrogel was followed during 6 months at 40°C and 75% relative humidity, in terms of pH, weight loss, and LA content (Figure 3).29 No significant changes (p>0.05) in any of the analyzed parameters were detected for the XAN-NLC/LDC-PLC hydrogel that preserved its pH (~7.4), initial weight (loss <2%), and LDC-PLC content (~25 mg/g) for 6 months. However, for the control hydrogel prepared without the lipid nanoparticles (XAN/LDC-PLC), the stability was only kept up to 3 months. After that (6 months) the formulation exhibited a significant increase in weight loss (>5%) and a decrease in pH (p<0.05) and content of both anesthetics (p<0.05).
Structural characterization
For the selected formulation, the interactions between the polymer and NLC/LDC-PLC excipients were assessed by rheology, FE-SEM, FTIR-ATR, and DSC analyses, providing information about the molecular organization of the novel nanohybrid system.
Figure 4A shows rheological properties of XAN-based hydrogels, given by the viscosity vs frequency (37°C) curve, which is currently required for any potential topical formulation.30 A pseudoplastic behavior was observed for all the analyzed XAN hydrogels, where the increase in frequency was followed by a decrease in viscosity.31 Indeed, all XAN-based hydrogels presented the rheological profiles of the pristine material.
The FE-SEM (Figure 4B) images were used to compare the textural properties of XAN/LDC-PLC (control) and XAN-NLC/LDC-PLC hydrogels. The microscopy of control hydrogel showed the typical XAN flake-like architecture. The hybrid (XAN-NLC/LDC-PLC) hydrogel exhibited rougher surfaces, plus evident XAN-coated nanoparticles, with a completely different texture than the control.
The observed FTIR-ATR bands and assignment are provided in Figure 4C and Table S2, respectively. The infrared spectroscopic profile of the lipid nanoparticles (NLC/LDC-PLC) and their excipients has been previously determined.7 Figure 4C shows typical XAN polysaccharides bands, observed in the 1100–920 and 3250–2885 cm−1 regions of the spectra. The bands centered at 1021 and 1061 cm−1 are noticed in XAN and XAN-NLC/LDC-PLC spectra, respectively, and can be attributed to the νC=O vibrations of XAN glucose residues. The bands comprised in the wide (3250–2885 cm−1) region in the XAN spectrum, and those bands shifted to higher wavenumber in the XAN-NLC/LDC-PLC spectrum (3310 cm−1), correspond to XAN structural νOH.32 As expected, in the hybrid-polymer spectrum, bands centered at 2917 and 2851 cm−1, consistent with C-H and O-CH2 stretching vibrations of the NLC structural lipid (CP), were observed.33 Interestingly, the set of bands in the 3303–2800 cm−1 region, assigned to the LA, was not observed in the hybrid hydrogel spectrum.
Finally, the thermodynamic features of the excipients and XAN-based hydrogels were assessed by DSC analysis (Figure 4D). The thermogram of pure LDC and PLC evidenced endothermic peaks centered at 69°C and 43°C, respectively, related to the respective melting points of the LA.7 The calorimetric behavior of pristine XAN showed a wide endothermic peak at 115°C, due to evaporation of the water physically adsorbed in the biopolymer.34 In the XAN/LDC-PLC thermogram, the endothermic peaks of both LA were less intense and shifted to lower temperatures. Finally, in the hybrid (XAN-NLC/LDC-PLC) sample, a single endothermic peak was noticed at 115°C, assigned to the evaporation of physically adsorbed -water of the XAN biopolymer.
Once the structural properties of XAN-NLC/LDC-PLC were elucidated, this hybrid hydrogel could be evaluated in terms of cytotoxicity and biological activity.
Bioassays
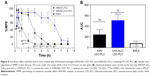
The MTT-cell viability assay was conducted using 3T3, HaCat, and VERO cultured cells, for 2 hours. Since pure XAN hydrogel is widely known as noncytotoxic, even after 21 days of analysis,35 it was used as a control. None of the concentrations tested affected the cell viability of the three cultured cell lines (p>0.05), in any of tested hydrogel concentrations (up to 250 mg/mL) (Figure 5). The concentration-dependent decrease in cell viability of the three cell lines observed in Figure 5 exclusively for XAN/LDC-PLC samples is a result of the well-known intrinsic cytotoxicity of LA.36
Last but not least, the anesthetic effect of the XAN-based hydrogels was assessed by the tail-flick test in mice (n=5). Figure 6 shows the %MPE and total anesthesia, given by the area under the curve for XAN-NLC/LDC-PLC (~505), XAN/LDC-PLC (~238) hydrogels and the eutectic mixture of LDC-PLC cream (~76). In both the %MPE and area under the curve parameters, the hybrid hydrogel exhibited an anesthetic effect almost twice and four times higher than control hydrogel and the eutectic mixture of LDC-PLC cream, respectively. The caudal nerve block lasted for 2, 4, and 8 hours, in the animals treated with the commercial cream, XAN/LDC-PLC, and XAN-NLC/LDC-PLC, respectively.
Discussion
As mentioned above, we have previously developed through factorial design an NLC/LDC-PLC formulation with optimized properties. However, the fluidity of that formulation was a serious limitation for its successful topical application.16 Aiming to accomplish major advances in transbuccal anesthesia, we hybridized this NLC formulation with ALG, XAN, or CHT hydrogels. In the hybridization approach, benefits from the nanostructured particles and polymers were combined into a single pharmaceutical form: the sustained release profile of NLC-encapsulated LA and the appropriate viscosity, consistency, and adhesion of the biopolymer matrix, to provide efficient topical anesthesia of the oral mucosa.17
Despite the best mucoadhesion of CHT,37 its incompatibility with NLC formulations led to separation,38 probably due to the tripolyphosphate ionic crosslinking. Moreover, the use of weak acid in the polymer preparation and its undesirable flavor39 contraindicate CHT administration at the oral mucosa. ALG hydrogels were crosslinked with a cationic solution of CaCl240 in order to attain the desirable viscosity. However, their final form was not homogeneous, probably due to the interaction of calcium ions with the NLC. Together, these findings supported the choice of XAN as the biopolymer counterpart for the hybrid formulations. XAN was also the cheapest biopolymer tested, and its polymerization only requires water, which is extremely suitable for industrial scale production.
The blending procedure (hybridization) seemed not to affect the structural organization of the NLC, since XAN-NLC/LDC-PLC exhibited a bimodal LA release profile – with an initial high burst effect followed by sustained release – decisive to achieve an optimized LA action.41 The release of LDC was longer sustained than that of PLC in the hybrid hydrogels, in a similar way to the release from NLC suspensions.7 The higher hydrophobic character and encapsulation efficiency by NLC could explain the differential release profile of LDC.
In addition, the presence of a biopolymer matrix in the formulation extended the release time of both anesthetics, probably due to the double physical barrier that the LA should overcome to be released. The time for total release of the anesthetics increased from 8 hours in suspensions of NLC7 to 18 hours in XAN-NLC/LDC-PLC. The continuous sustained release observed for hybrid formulation was also a result of the weak interactions between nonencapsulated LA molecules (in the aqueous of NLC) and XAN free hydroxyl groups.42 The kinetic of hydrogels was best fitted by the Weibull model, where Fickian diffusion describes the LA release from inside the nanoparticles through the swelled XAN chains. This result is also in agreement with the bimodal LDC-PLC release profile also observed in polyacrylate hydrogels.43
A set of biophysical methods provided valuable molecular information, confirming that a novel topical formulation was produced, which was stable in critical conditions for 6 months, as required by regulatory issues. The final pH of lipid-biopolymer hydrogel (7.4) was the same as the oral mucosa, contrasting those of the control hydrogel (XAN/LDC-PLC, pH ~5) and commercial cream (pH ~9). Since the pH attained after topical administration is strictly related to the buccal mucosa’s susceptibility to injury, the pH of XAN-NLC/LDC-PLC is an outstanding advantage. Furthermore, the formulation showed a pseudoplastic behavior due to XAN, a natural improver of rheological properties in pharmaceuticals and food industries.44 The pseudoplastic behavior assured: 1) the desirable viscosity prior to the hybrid hydrogel application (keeping it in the site of administration) and 2) a subsequent decrease in viscosity, favoring hydrogel spreadability and fixation in the oral mucosa.31
It is worth mentioning that the control (XAN/LDC-PLC) hydrogel, despite the pseudoplastic XAN-related behavior, was not found appropriate for transbuccal anesthesia, as revealed by the accelerated stability study and structural characterization tests. In fact, DSC analysis anticipated the lack of stability, by the presence of the two endothermic peaks related to pristine LA. Therefore, in XAN/LDC-PLC samples, the expected formation of drug–polymer conjugates was not observed, which could explain the lack of stability in all parameters evaluated over 6 months of storage and the less sustained LDC-PLC release profile. These facts reinforce the relevance of the material hybridization.
The hybrid hydrogel combined advantages from the lipid and biopolymer components. FTIR-ATR data confirmed the interaction between them, by the coexistence of mutual bands in the XAN-NLC/LDC-PLC spectrum (eg, the C-H and O-CH2 lipid vibrations and the shift of typical anhydrous glucose bands of xanthan to higher wavenumbers). DSC thermogram demonstrated that the XAN-NLC/LDC-PLC maintained the thermodynamic features of NLC (control), without any endothermic event related to pristine LA. FE-SEM images proved that a new assembly was formed, with a completely different texture than that of the control hydrogel, in which the excipients could not be distinguished. Based on the abovementioned information, it was suggested that the new hybrid material is able to protect LA against hydrolysis, corroborating the slower release profile and good stability exhibited by the XAN-NLC/LDC-PLC hydrogel.
Bioassays confirmed the safety of XAN hydrogels, even at the highest quantity (250 mg/mL), against different cell lines. This was possible due to the fact that XAN is a biocompatible matrix, currently applied as a thickener and gelling agent that only requires the addition of water to form a stable gel.45 Moreover, in the XAN/LDC-PLC hydrogel, the biopolymer somehow neutralized the intrinsic toxicity of the anesthetics, probably due to available XAN-hydroxyl groups that interact with the dispersed free LA through hydrogen bonds, decreasing the potential toxicity of the anesthetics. Additionally, the safety behavior observed for XAN-NLC/LDC-PLC hydrogel was predictable, since NLC formulations were previously described as noncytotoxic for 3T3 and HaCat cells.36,46–48
Finally, the anesthesia duration was determined through tail-flick assay in mice. XAN-NLC/LDC-PLC exhibited an anesthetic effect two times higher than the control hydrogel (XAN/LDC-PLC) and four times higher than the commercially available cream. The prolonged analgesia effect is a result of combined action of NLC plus the hydrophilic hydrogel matrix. The massive superficial contact area of NLC favors the anesthetic release from the lipid core to the swelled adhesive biopolymer network, toward the biological barrier. This promising lipid-biopolymer hydrogel will be tested for the minimization of pain (prior to LA injection) in humans, and thus can also be useful in the treatment of other oral painful conditions.
Concluding remarks
The high interest in the development of an effective preanesthetic formulation to be applied in dentistry has moved this current work. We have pointed out the preparation of a pre-anesthetic of complex molecular organization. The nanohybrid hydrogel for the delivery of LDC-PLC (5%) was composed of XAN as biopolymer and NLC/LDC-PLC as the lipid matrix, comprising the use of abundant, biocompatible, and cheap biomaterials. This strategy provided an advanced pharmaceutical form with excellent compatibility between the excipients, desirable rheological properties, and prolonged delivery profile. The hydrogel was stable (for 6 months on critical conditions), had suitable mechanical properties to be orally administered, was safe, and doubled and quadrupled, the anesthesia duration in comparison to a control hydrogel formulation (without the nanostructured lipid counterpart) and the commercial cream, respectively. The nanohybrid hydrogel should now be clinically evaluated to enable the formulation to reach the market.
Acknowledgment
The authors thank FAPESP (#14/25372-0, #14/14457-5) and Cristália Ind. Quim. Farm. Ltda, for kindly providing lidocaine and prilocaine.
Disclosure
The authors report no conflicts of interest in this work.
References
Franz-Montan M, Ribeiro LNM, Volpato MC, et al. Recent advances and perspectives in topical oral anesthesia. Expert Opin Drug Deliv. 2017;14(5):673–684. | ||
Franz-Montan M, Ranali J, Ramacciato JC, de Andrade ED, Volpato MC, Groppo FC. Ulceration of gingival mucosa after topical application of EMLA: report of four cases. Br Dent J. 2008;204(3):133–134. | ||
Franz-Montan M, de Paula E, Groppo FC, Silva AL, Ranali J, Volpato MC. Liposomal delivery system for topical anaesthesia of the palatal mucosa. Br J Oral Maxillofac Surg. 2012;50(1):60–64. | ||
Kamata M, Tada Y, Yazawa N, Watanabe T, Kikuchi K, Sato S. Drug fever caused by eutectic mixture of local anesthetic cream. J Investig Allergol Clin Immunol. 2011;21(5):421. | ||
de Paula E, Cereda CM, Fraceto LF, et al. Micro and nanosystems for delivering local anesthetics. Expert Opin Drug Deliv. 2012;9(12):1505–1524. | ||
Müller RH, Alexiev U, Sinambela P, Keck CM. Nanostructured lipid carriers (NLC): the second generation of solid lipid nanoparticles. In: Dragicevic N, Maibach H, editors. Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement. Berlin: Springer; 2016;11:161–185. | ||
Ribeiro LNM, Franz-Montan M, Breitkreitz MC, et al. Nanostructured lipid carriers as robust systems for topical lidocaine-prilocaine release in dentistry. Eur J Pharm Sci. 2016;93:192–202. | ||
Guilherme VA, Ribeiro LNM, Tofoli GR, Franz-Montan M, de Paula E, de Jesus MB. Current Challenges and Future of Lipid Nanoparticles Formulations for Topical Drug Application to Oral Mucosa, Skin, and Eye. Curr Pharm Des. 2018;23(43):6659–6675. | ||
Patel VF, Liu F, Brown MB. Advances in oral transmucosal drug delivery. J Control Release. 2011;153(2):106–116. | ||
Wang S, Zhao J, Hu F, et al. Phase-changeable and bubble-releasing implants for highly efficient HIFU-responsive tumor surgery and chemotherapy. J Mater Chem B. 2016;4(46):7368–7378. | ||
Wu C, Zhao J, Hu F, et al. Design of injectable agar-based composite hydrogel for multi-mode tumor therapy. Carbohydr Polym. 2018;180:112–121. | ||
Wegst UG, Bai H, Saiz E, Tomsia AP, Ritchie RO. Bioinspired structural materials. Nat Mater. 2015;14(1):23–36. | ||
Rajesh N, Siddaramaiah. Feasibility of xanthan gum-sodium alginate as a transdermal drug delivery system for domperidone. J Mater Sci Mater Med. 2009;20(10):2085–2089. | ||
Chinna Reddy P, Chaitanya KS, Madhusudan Rao Y. A review on bioadhesive buccal drug delivery systems: current status of formulation and evaluation methods. Daru. 2011;19(6):385–403. | ||
Salamat-Miller N, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev. 2005;57(11):1666–1691. | ||
Ribeiro LNM, Alcântara ACS, Rodrigues da Silva GH, et al. Advances in Hybrid Polymer-Based Materials for Sustained Drug Release. Int J Polym Sci. 2017;2017(11):1–16. | ||
Franz-Montan M, de Araújo DR, Ribeiro LNM, de Melo NFS, de Paula E. Nanostructured systems for transbuccal drug delivery. In: Andronescu E, Grumezescu A, editors. Nanostructures for Oral Medicine. 1st ed. New York: Elsevier; 2017:87–114. | ||
Casadei MA, Cerreto F, Cesa S, et al. Solid lipid nanoparticles incorporated in dextran hydrogels: a new drug delivery system for oral formulations. Int J Pharm. 2006;325(1–2):140–146. | ||
Cirri M, Bragagni M, Mennini N, Mura P. Development of a new delivery system consisting in “drug – in cyclodextrin – in nanostructured lipid carriers” for ketoprofen topical delivery. Eur J Pharm Biopharm. 2012;80(1):46–53. | ||
Ustündağ-Okur N, Gökçe EH, Bozbıyık Dİ, Eğrilmez S, Ozer O, Ertan G. Preparation and in vitro-in vivo evaluation of ofloxacin loaded ophthalmic nano structured lipid carriers modified with chitosan oligosaccharide lactate for the treatment of bacterial keratitis. Eur J Pharm Sci. 2014;63:204–215. | ||
Hao J, Wang X, Bi Y, et al. Fabrication of a composite system combining solid lipid nanoparticles and thermosensitive hydrogel for challenging ophthalmic drug delivery. Colloids Surf B Biointerfaces. 2014;114:111–120. | ||
Silva LA, Andrade LM, de Sá FA, et al. Clobetasol-loaded nanostructured lipid carriers for epidermal targeting. J Pharm Pharmacol. 2016;68(6):742–750. | ||
Sivakumaran D, Maitland D, Hoare T. Injectable microgel-hydrogel composites for prolonged small-molecule drug delivery. Biomacromolecules. 2011;12(11):4112–4120. | ||
Franz-Montan M, Baroni D, Brunetto G, et al. Liposomal lidocaine gel for topical use at the oral mucosa: characterization, in vitro assays and in vivo anesthetic efficacy in humans. J Liposome Res. 2015;25(1):11–19. | ||
Cereda CMS, Guilherme VA, Alkschbirs MI. Liposomal Butamben Gel Formulations: Toxicity assays and topical anesthesia in an animal model. J Lip Res. 2016;28:1–9. | ||
Melo NFS, Campos EVR, Franz-Montan M, et al. Characterization of Articaine-Loaded Poly(ε-caprolactone) Nanocapsules and Solid Lipid Nanoparticles in Hydrogels for Topical Formulations. J Nanosci Nanotechnol. 2018;18(6):4428–4438. | ||
Mendyk A, Jachowicz R. Unified methodology of neural analysis in decision support systems built for pharmaceutical technology. Expert Syst Appl. 2007;32(4):1124–1131. | ||
Papadopoulou V, Kosmidis K, Vlachou M, Macheras P. On the use of the Weibull function for the discernment of drug release mechanisms. Int J Pharm. 2006;309(1–2):44–50. | ||
ICH Expert Working Group. Stability Testing of New Drug Substances and Products. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. Vol. 24; 2003. Available from: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q1A_R2/Step4/Q1A_R2__Guideline.pdf. Accessed October 4, 2018. | ||
Alves MP, Raffin RP, Fagan SB. Rheological behavior of semisolid formulations containing nanostructured systems. In: Beck R, Guterres S, Pohlmann A, editors. Nanocosmetics and Nanomedicines New Approaches for Skin Care. Berlin: Springer; 2011:37–48. | ||
Pereira GG, Dimer FA, Guterres SS, Kechinski CP, Granada JE, Cardozo NSM. Formulation and characterization of poloxamer 407®: thermoreversible gel containing polymeric microparticles and hyaluronic acid. Química Nova. 2013;36(8):1121–1125. | ||
Shalviri A, Liu Q, Abdekhodaie MJ, Wu XY. Novel modified starch–xanthan gum hydrogels for controlled drug delivery: Synthesis and characterization. Carbohydr Polym. 2010;79(4):898–907. | ||
Pokharkar VB, Jolly MR, Kumbhar DD. Engineering of a hybrid polymer-lipid nanocarrier for the nasal delivery of tenofovir disoproxil fumarate: physicochemical, molecular, microstructural, and stability evaluation. Eur J Pharm Sci. 2015;71:99–111. | ||
Bhattacharya SS, Shukla S, Banerjee S, Chowdhury P, Chakraborty P, Ghosh A. Tailored IPN Hydrogel Bead of Sodium Carboxymethyl Cellulose and Sodium Carboxymethyl Xanthan Gum for Controlled Delivery of Diclofenac Sodium. Polym Plast Technol Eng. 2013;52(8):795–805. | ||
Bueno VB, Silva AM, Barbosa LR, et al. Hybrid composites of xanthan and magnetic nanoparticles for cellular uptake. Chem Commun. 2013;49(85):9911. | ||
You P, Yuan R, Chen C. Design and evaluation of lidocaine- and prilocaine-coloaded nanoparticulate drug delivery systems for topical anesthetic analgesic therapy: a comparison between solid lipid nanoparticles and nanostructured lipid carriers. Drug Des Devel Ther. 2017;11:2743–2752. | ||
Ribeiro LNM, Alcântara AC, Darder M, Aranda P, Araújo-Moreira FM, Ruiz-Hitzky E. Pectin-coated chitosan-LDH bionanocomposite beads as potential systems for colon-targeted drug delivery. Int J Pharm. 2014;463(1):1–9. | ||
Baloğlu E, Karavana SY, Hyusein IY, Köse T. Design and formulation of mebeverine HCl semisolid formulations for intraorally administration. AAPS PharmSciTech. 2010;11(1):181–188. | ||
Hazzah HA, Farid RM, Nasra MM, Hazzah WA, El-Massik MA, Abdallah OY. Gelucire-Based Nanoparticles for Curcumin Targeting to Oral Mucosa: Preparation, Characterization, and Antimicrobial Activity Assessment. J Pharm Sci. 2015;104(11):3913–3924. | ||
García-Astrain C, Avérous L. Synthesis and evaluation of functional alginate hydrogels based on click chemistry for drug delivery applications. Carbohydr Polym. 2018;190:271–280. | ||
Rodrigues da Silva GH, Ribeiro LNM, Mitsutake H, et al. Optimised NLC: a nanotechnological approach to improve the anaesthetic effect of bupivacaine. Int J Pharm. 2017;529(1–2):253–263. | ||
Consumi M, Leone G, Pepi S. Xanthan Gum-Chitosan: Delayed, prolonged, and burst-release tablets using same components in different ratio. Adv Polym Technol. 2017;2018:1–10. | ||
Calixto G, Yoshii AC. Rocha E Silva H, Stringhetti Ferreira Cury B, Chorilli M. Polyacrylic acid polymers hydrogels intended to topical drug delivery: Preparation and characterization. Pharm Dev Technol. 2015;20(4):490–496. | ||
Shatwell KP, Sutherland IW, Ross-Murphy SB. Influence of acetyl and pyruvate substituents on the solution properties of xanthan polysaccharide. Int J Biol Macromol. 1990;12(2):71–78. | ||
Rowe R, Sheskey P, Owen S. Handbook of Pharmaceutical Excipients. 5th ed. London: Pharmaceutical Press; 2006. | ||
Severino P, Chaud MV, Shimojo A, et al. Sodium alginate-cross-linked polymyxin B sulphate-loaded solid lipid nanoparticles: Antibiotic resistance tests and HaCat and NIH/3T3 cell viability studies. Colloids Surf B Biointerfaces. 2015;129:191–197. | ||
Ribeiro LNM, Breitkreitz MC, Guilherme VA, et al. Natural lipids-based NLC containing lidocaine: from pre-formulation to in vivo studies. Eur J Pharm Sci. 2017;106:102–112. | ||
Barbosa RM, Silva CMG, Bella TS, et al. Cytotoxicity of solid lipid nanoparticles and nanostructured lipid carriers containing the local anesthetic dibucaine designed for topical application. Journal of Physics: Conference Series. 2013;429(1):12035. | ||
ICH Expert Working Group. Final Concept Paper, Q8: Pharmaceutical Development. International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. 2003. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q8_R1/Concept_papers/Q8_Concept_Paper.pdf. Accessed October 4, 2018. |
Supplementary materials
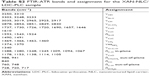  | Table S2 FTIR-ATR bands and assignment for the XAN-NLC/LDC-PLC sample |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.