Back to Journals » Clinical Ophthalmology » Volume 14
Myopic Traction Maculopathy: Diagnostic and Management Strategies
Authors Frisina R , Gius I, Palmieri M, Finzi A, Tozzi L, Parolini B
Received 18 September 2020
Accepted for publication 14 October 2020
Published 2 November 2020 Volume 2020:14 Pages 3699—3708
DOI https://doi.org/10.2147/OPTH.S237483
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Rino Frisina,1 Irene Gius,1 Michele Palmieri,2 Alessandro Finzi,3 Luigi Tozzi,1 Barbara Parolini2
1Department of Ophthalmology of University of Padova, Padova, Italy; 2Eyecare Clinic, Brescia, Italy; 3Villa Erbosa Di Bologna, Bologna, Emilia-Romagna, Italy
Correspondence: Rino Frisina
Department of Ophthalmology of University of Padova, Via Giustiniani n. 2, Padova 35128, Italy
Tel +39 0498212110
Fax +39 049 8755169
Email [email protected]
Abstract: Pathologic myopia (PM) is an ocular disorder characterized by a spherical equivalent (SE) of more than – 6.0 diopters (D) or by an axial length (AL) of more than 26.5 millimeters (mm). PM is associated with myopic maculopathy (MM). The ATN classification describes all the aspects of MM which regroups atrophic, tractional and neovascular consequences to the sclera, choroid and retina of highly myopic eyes. The advent of OCT allowed to define the ultrastructural characteristics of the tractional changes in MM, described by the term myopic traction maculopathy (MTM). They include foveoschisis/maculoschisis/retinoschisis (FS/MS/RS), retinal/foveal detachment (RD/FD), lamellar macular holes (LMH) and full-thickness macular holes (FTMH) with or without RD (MHRD). The MTM staging system (MSS) describes all foveal and retinal changes related to MTM and their natural history interpreting them as different stages of a single progressive disorder. The management of MTM can be just observation for the earliest cases with good vision or surgery for the severe stages with vision loss. There are two possible surgical approaches: ab externo, that acts on the alteration of the scleral shape and includes posterior scleral reinforcement and macular buckle. Ab interno, that targets the alteration of the foveal profile and consists in pars plana vitrectomy with removal of all the epiretinal tractions, maneuvers on the internal limiting membrane, and the use of intravitreal tamponade and laser. As they target two different sides of the same pathology, the two techniques have to be selected on the base of the MTM stage, single or combined.
Keywords: pathological myopia, posterior staphyloma, retinal detachment, full thickness macular hole, macular buckle, pars plana vitrectomy, myopic traction maculopathy
Introduction
Myopia is a common eye disorder that causes visual disability throughout the world. The severe form, called High Myopia (HM), is defined as a spherical equivalent (SE) of more than – 6.0 diopters (D) or as an axial lengths (AL) of more than 26.5 millimeters (mm).1,2 The cut-off of – 6.0 D is still controversial, with some authors that require a SE higher than –8 D.3 Pathologic myopia (PM) defines high myopia associated to degenerative changes in the sclera, choroid and retina.4 Lewis et al in 2014, by examining the scleral biomechanics of enucleated eyes of young chicks exposed to myopia-inducing and myopia-recovery conditions, found that there are two mechanisms that contribute to scleral deformation: elasticity and creep.5 The first is the reversible, instantaneous deformation of the sclera, due to an applied stress; the second is the time-dependent deformation, due to persistent mechanical stress. The dysregulation of the elongation of the eye in myopia seems to be related to the inhibition of collagen crosslinking, that normally influences, in the mammalian sclera, the regulation of the refractive development.6 This phenomenon can explain the reduced scleral rigidity in myopic eyes compared to emmetropic and hyperopic ones.7 The deformation determines an equatorial region expansion and an elongation and enlargement of the globe. The first process is directly related to the incidence of peripheral retinal degenerations like white without pressure, pigmentary degeneration, pavingstone degeneration and lattice degeneration.8 The second one induces an ectasia of the sclera, called staphyloma, which affects mainly the posterior pole (posterior staphyloma, PS) involving the optic nerve (ON) and/or the macular area. A staphyloma may also occur laterally.
Posterior Staphiloma
A feature that strongly suggests the presence of PM is the staphyloma, firstly described by Scarpa in 1801.9,10 Curtin, in 1977, sorted these lesions into 10 different types, on the base of their ophthalmoscopic appearance.11 The first five types were defined as primary staphylomas, the types from VI to X as combined ones. Posterior pole staphyloma (type I) was the most common type, involving macula and optic nerve, peripapillary (type III) and inferior staphylomas (type V) were the least common ones. Legal blindness was found to be more prevalent in eyes with staphyloma types III, VI and VII and in eyes with diffuse chorioretinal atrophy affecting the peripapillary area.11 The most recent definition of staphyloma was proposed by Spaide as “an outpouching of the wall of the eye that has a radius of curvature that is less than the surrounding curvature of the wall of the eye”.12 This definition does not completely cover all the possible types of staphyloma: the peripapillary and nasal ones do not show an abrupt change in terms of slope of the sclera, in comparison to the surrounding scleral profile, and rather create a nasal distortion of the globe.13 The staphyloma was originally considered a characteristic lesion of MM. More recently, several authors have reported an association between MTM, chorioretinal atrophy and staphyloma, supporting the hypothesis that the staphyloma itself could be considered one of the causes of MM inducing a mechanical damage to retina and ON.8,13–20 However, both MM and MTM can be present in absence of a staphyloma. A step forward in terms of staphyloma classification was made with the advent of modern imaging techniques, like three-dimensional magnetic resonance imaging (3D MRI), optical coherence tomography (OCT) and wide-field fundus imaging. These diagnostic instruments, overcoming the limits of traditional Ultrasonography (US), that does not allow to examine the area of a large staphyloma, permitted to visualize the shape of the entire eye. Ohno Matsui et al evaluated, by 3D MRI reconstruction of the ocular globe, the shape of highly myopic eyes, dividing them into symmetric and asymmetric globes. Symmetric globes were further subdivided in barrel and cylinder types, asymmetric ones in nasal and temporal distortion types.21 The same authors first defined four OCT patterns of curvature of the inner scleral surface of highly myopic eyes: a pattern with the curvature that slopes towards the ON, a pattern with symmetrical curvatures centered on the fovea, a pattern with asymmetrical curvatures and a pattern with irregular ones.22 A combination of 3D MRI, OCT and wide-field fundus imaging was finally exploited by Ohno Matsui et al, to develop a new classification, by examining a total of 105 Asiatic patients with PM.13 The authors classified 5 patterns of PS: wide macular, narrow macular, peripapillary, nasal, inferior and other staphylomas. A similar study was also conducted on Caucasian patients, combining A and B-scan US, 3D MRI, OCT, fundus autofluorescence (FAF), red free (RF) and color fundus photography, confirming that there is an association between the type of PS and MRI shape pattern of the eye, OCT macular profile pattern and chorioretinal atrophy.23 In 2017, Shinohara et al used widefield OCT (WF OCT) combined to 3D-MRI to examine 100 eyes of 57 patients affected by PS. The authors found that WF OCT could provide high-resolution images, potentially replacing 3D-MRI in assessing PS.24
Myopic Maculopathy
Curtin and Karlin, in 1970, described 5 fundus changes associated with PM: optic nerve crescent, chorioretinal atrophy, central pigment spot (Fuchs’s), lacquer cracks (Lc), and PS.25 These findings were further implemented by Tokoro et al, that classified the lesions of myopic maculopathy (MM) into four categories: tessellated fundus, diffuse chorioretinal atrophy, patchy chorioretinal atrophy and macular haemorrhage, the last one associated or not to myopic choroidal neovascularization (mCNV).26 In 1984, Avila et al proposed a classification on the basis of the increasing severity from a normal appearing posterior pole (M0) to choroidal pallor and tessellation (M1), with further addition of PS (M2), Lc (M3), focal areas of deep choroidal atrophy (M4) and large geographic areas of deep chorioretinal atrophy and bare sclera (M5).27 In 2010, Hayashi et al proposed a new classification based on the long-term progression pattern of MM by analysing 806 highly myopic eyes of 429 patients. Differently to Tokoro’s, Hayashi et al defined Lc as a stand-alone lesion, not included in the category of diffuse atrophy. Tokoro divided macular haemorrhage as associated or not to mCNV, whereas Hayashi included haemorrhages into the category of mCNV or Lc. Finally, Hayashi divided patchy atrophy into three subtypes: associated with Lc, diffuse atrophy or PS.28 Since none of these classification schemes became globally accepted, an international group of experts in HM developed, on the base of a meta-analysis of PM (META-PM), a systematic classification with 5 different categories of MM: no myopic retinal lesions (category 0), tessellated fundus only (category 1), diffuse chorioretinal atrophy (category 2), patchy chorioretinal atrophy (category 3) and macular atrophy (category 4). The presence of Lc, mCNV, and Fuch’s spot was added as ‘plus’ sign.29 A correlation between the presence of the atrophic lesions, the neovascular lesions and the tractional ones and the degree of PS had been previously hypothesized by several authors. Steidl and Pruett reported a correlation between PS and the presence of atrophy or Lc.30 Ohno Matsui et al reported a correlation between the width of PS and chorioretinal atrophy and tractional alterations.13 mCNV seems to be more common in eyes with less advanced PS, because of a better preserved choriocapillaris, but Ishida et al found, in cases of defect of Bruch’s membrane, a possible communication between mCNV and short posterior ciliary arteries.31 Recently, Medrano et al proposed a new definition of MM as macular alterations induced by PM, in which an excessive AL and/or PS is the main common factor but not the only factor.
These alterations included not only the atrophic lesions but also the tractional and neovascular ones. The new ATN classification of MM was based on three faces of the disease: atrophic MM (A0-no myopic retinal lesions; A1-tessellated fundus only; A2-diffuse chorioretinal atrophy; A3-patchy chorioretinal atrophy; A4-complete macular atrophy), neovascular MM (N0- no mCNV; N1-macular Lc; N2a-active CNV; N2b-scar or Fuch’s spot) and tractional MM (T0-no macular schisis; T1-inner or outer foveoschisis; T2-inner + outer foveoschisis; T3-foveal detachment; T4-full-thickness macular hole; T5-macular hole + retinal detachment).1
Myopic Traction Maculopathy
Definition
For years, the key factors defining MM were PS and the fundus changes. More recently, the focus was moved on all the tractional features of PM thanks to the development of OCT.
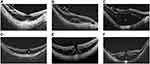
In 2014 Panozzo coined the term myopic traction maculopathy (MTM) to describe the spectrum of foveal tractional changes in highly myopic eyes.32 MTM included the following alterations: foveoschisis/maculoschisis/retinoschisis (FS/MS/RS), retinal/foveal detachment (RD/FD), lamellar macular holes (LMH) and full-thickness macular holes (FTMH) with (MHRD) or without RD.32 They analysed the OCT images of 125 eyes with HM, focusing on epiretinal tractions (epiretinal membrane, ERM, and vitreomacular traction, VMT) and retinal damage (retinal thickening, MS/RS, RD, LMH), finding a prevalence of epiretinal traction of 46.4% and retinal damage in 34.4% of eyes (Figure 1).
Pathogenesis
The development of MTM was described to be caused by two different groups of forces that act on the retina: preretinal and subretinal factors.33 Preretinal factors included forces that cause centrifugal and tangential tractions to the retina, such as incomplete posterior vitreous detachment (PVD), VMT and ERM. Subretinal factors included forces that cause centrifugal tractions to the retina such as deformation of the scleral eyewall. Both can potentially damage the retina if they overcome its elasticity, which is limited by small retinal vessels, thickened or stiffened internal limiting membrane (ILM) or vitreous remnants.3,17,34,35 Additionally, the reduced retinal blood supply due to choroidal atrophy and retinal pigment epithelium (RPE) atrophy was hypothesized to contribute to reduce adhesions between the retinal nerve layers and between the photoreceptors and the RPE itself.33 A factor that accelerates the creation of tractions was reported to be the partial or complete PVD, that in myopic eyes occurs earlier and more frequently than in emmetropic eyes.36,37 In addition, in highly myopic eyes, the formation of a physiological lacuna was reported, between the vitreous cortex, in contact with the ILM, and vitreous. The lacuna was called posterior precortical vitreous pocket (PPVP) and could simulate a PVD.37
Morphological Changes in MTM
Foveoschisis
The earliest retinal alteration occurring in the progression of MTM is RS, defined as MS when involving the macula and FS when involving only the fovea. In 1959, this condition was described by Calbert Phillips, who noticed that localized posterior RD could occur over PS, without a detectable MH: he speculated that a condition such as FS might have explained the RD.38,39 The first who used the term ‘myopic foveoschisis’ were Takano and Kishi, in 1999, who described the splitting of the macular inner retinal layers, separating the retina into a thinner outer layer and a thicker inner layer.40 Baba et al, in 2003, found a correlation between the presence of FS and PS, assuming that FS could be due to scleral protrusion, overcoming the stretching capacity of the retina.15 Benhamou et al described FS as a result of thickening in the outer retinal layer and characterized by perpendicular columns of tissue bridging the outer and inner layers.14 The evolution was described as a progressive separation of retinal layers, which remained connected by Müller cells stretched in multiple columnar structures and appearing at OCT as “long, straight, highly reflective lines at the fovea and throughout the retinoschisis” area.41 FS was accompanied by other OCT features such as ERMs, detachment of ILM, retinal microfolds, ellipsoid zone (EZ) line defects, paravascular microholes, LMHs, FTMHs, chorioretinopathy.42
Several classifications have been proposed for FS. Shimada et al proposed 5 categories of outer FS based on the location and extension of it, ranging from no apparent FS (S0), extrafoveal FS (S1), only foveal FS (S2), foveal but not involving the entire macula FS (S3), FS with complete macular involvement (S4).3 The FS can involve different retinal layers: inner FS involves the inner plexiform layer (IPL), the ganglion cell layer (GCL), the retinal nerve fibre layer (RNFL); outer FS involves the outer plexiform (OPL) and the outer nuclear layers (ONL). Fujimoto et al and Ceklic et al classified the FS on the basis of the location of the splitting of the retina into inner, outer, inner and outer FS.41,43
Foveal/Retinal Detachment
FS can evolve into FD. The progression tends to be slow. In some cases, FS may remain stable or even reduce, if the tractions are spontaneously relieved.44 Shimada et al, by following 207 high myopic eyes for at least 24 months, described a decrease or resolution of the FS in 3.9% of eyes and a progression of MTM in 11.6% of eyes, noticing that eyes with a more extensive FS tended to progress more than eyes with a less extensive one.3 In another study, the same author described 4 different OCT stages of the progression from FS to RD: stage 1, an irregularity of the external retinal layer thickness with focal elevation of the retina; stage 2, an outer LMH (O-LMH) that developed foveally or extrafoveally, associated with a small RD; stage 3, the O-LMH increased vertically and separated horizontally the column-like structures within the RS layer, with an enlargement of RD; stage 4, the RD further enlarged and the RS resolved.45
Lamellar Macular Hole
LMH is characterized by an irregular foveal contour, an inner retinal defect with or without intraretinal splitting and the absence of full-thickness retinal foveal defect, defined by an intact foveal photoreceptor layer.46 In some cases, however, a disruption of outer retinal layers, external limiting membrane (ELM) and EZ has been described.47–53 LMHs in non-myopic eyes are associated with two types of ERM: conventional (commonly detected in macular pucker), that tomographically appears as a highly reflective line overlying the RNFL and with tractional properties; atypical, a thick membrane delimited by a highly reflective line and filled by moderately reflective material, without tractional properties.49 The same occurs in LMHs in myopic eyes.54 These two types of ERM show a different histological composition.49 Furthermore, there are two morphological types of LMH: LMH with intraretinal splitting (IR split LMH) and V-shaped LMH (V LMH).54,55 There is still debate whether myopic LMH is a stable condition or not: dell’Omo et al, in a retrospective observational case series of 44 myopic eyes with LMH, found that these entities are frequently associated with ERMs and LMH-associated epiretinal proliferation (LHEP) and reported a morphological and functional stability over years.56 By contrast, in a retrospective observational longitudinal study, Frisina et al studied 40 myopic eyes, affected by LMH and PS, and found that myopic LMHs, associated with atypical ERM, are a more severe entity than myopic LMH associated with conventional ERM and that myopic LMHs do not seem to be a stable condition54,55 and evolve in FTMH. LMH can be further divided into inner LMH (I-LMH), with splitting of the inner foveal layers, and O-LMH, with splitting of photoreceptors.57
Macular Hole and Macular Hole Retinal Detachment
Myopic FTMH is considered as one of the final stages of MTM and is associated with severe visual impairment. Ikuno et al observed in 44 eyes with myopic FS that the natural course of FS is from RS to MH to MHRD and that the foveal status is a prognostic factor for surgical success after vitrectomy and ILM peeling, with better outcomes in RS group and worse outcomes in MH group.58 According to Ikuno et al, there are two types of myopic MH: the “flat type” and the “schisis type.” The first one is morphologically similar to the idiopathic MH (iMH) and does not usually evolve to MHRD. The “schisis type” is characterized by a high and rectilinear wall with an acute angle to the RPE line, i.e. acute-angled edge at the top shorter than the base diameter. The “schisis type” is associated with an high risk of MHRD.59 Protective factors to MHRD are all those factors that reduce the subretinal forces: a reduced height of PS, dome-shaped macula (DSM) and greater choroidal and scleral thickness.60
Natural History and the MSS Classification
Parolini et al elaborated the new MTM Staging System (MSS) with the aim of offering information on the complete nomenclature, the pathogenesis, the natural history and the management of the disease.57 They collected OCT images of 281 eyes with MTM, found all the possible OCT types of retinal and foveal tractional changes, correlated it with best corrected visual acuity (BCVA) and age of the patients and studied the evolution of MTM by examining the changes in OCT of 126 eyes with MTM over 11 years. They avoided the term FS and choose to use MS because, in most cases, the schisis affected the whole macula and not only the fovea. The authors hypothesized that the myopic tractional changes in retina and fovea were not simply different types of MTM, but evolutive stages of the same disease. They explained the pathogenesis of MTM, describing different centrifugal forces that act on retina and fovea playing against the centripetal forces that hold the retina together, exerted by Müller cells, ELM and ILM. On the basis of these theories, Parolini et al described two evolution patterns, foveal and retinal ones. The retinal patterns, due to centrifugal forces perpendicular to the retinal plane, evolve from inner MS (I-MS) or inner and outer MS (IO-MS) in stage 1, to predominantly outer MS (O-MS) in stage 2, to MS with macular detachment (MS-MD) in stage 3, to macular detachment (MD) in stage 4. The foveal patterns, due to centrifugal forces tangential to the foveal plane, evolve from normal foveal profile in stage a, to I-LMH in stage b, to FTMH in stage c. The O-LMH and any epiretinal abnormalities are findings that can be associated with each pattern and are marked in the MSS, respectively, with “O” and “+”. Parolini et al found that the mean evolution time of MTM gradually decreased from stage 1 to 2 (20 months), stage 2 to 3 (12 months) and 3 to 4 (3 months), with BCVA decreasing with increasing stages.57 There is also a correlation between MTM stage and age.
Surgical Management
The history of the ab externo approach for MTM begins long before the definition of MTM itself, in 1930. The idea was to reinforce the posterior sclera limiting the progressive myopic elongation of the eye. Different types of material, such as fascia lata,45 donor sclera,61,62 and Lyodura, derived from processed cadaver dura mater,63 were used for this this purpose. Fifty years later, a modified posterior scleral reinforcement (PSR) technique was proposed by Snyder and Thompson and further developed by several authors.64–67 Finally, the macular buckle (MB) technique was firstly described by Schepens, Okamura and Brockhurst in 195768 and for long time considered as the best surgical approach for the treatment of myopic MHRD.68–77 The rationale of MB was to overcome the pathologic posterior bulging and stretching of the sclera, especially in eyes with a pronounced PS, which is an important risk factor for the development of MTM.78,79 The aim of it is to relieve the extern traction due to PS. On the other hand, considering DSM as a protective factor for the occurrence of MTM, the MB provides, in fact, an iatrogenic DSM.
After a long time-length, in which the ab externo approach was overwhelmed by the ab interno approach, the MB, with the use of a sponge or a solid silicone exoplant, returned back in vogue in the 2000s,80–82 showing a very high reattachment rate, but still technically challenging. Attention was pointed to facilitate this surgery, with the design of different types of buckle. A T-shaped semirigid rod-exoplant, made with silicone and reinforced with titanium and with an indenting head was published by Tanaka, Ando and Usui in 2005.83 An L-shaped buckle, made with a silicone sleeve to embrace a titanium stent (MRI compatible), that allowed a macular indentation by a soft silicone sponge and an anterior suture, was developed in 2009 by Parolini et al. MB was reported to assure an effective axial myopia control in 2009 by Ward,84 to have better visual outcomes compared to PPV,85 and, in comparison with ab interno approach, the gold standard to treat MHMD, according to a 16-year review on MB for MTM of Alkabes et al.86
Pars plana vitrectomy (PPV) was introduced in the 1980s for the first time as treatment for MHRD.87–89 Different types of intravitreal tamponades combined to PPV were proposed, like gas and silicone oil (SO). A higher anatomical success rate was reached with SO in comparison to gas, but an unsatisfactory functional success was reported by several authors.90–93 The introduction of surgical maneuvers such as ILM peeling94–96 and laser treatment around the edges of MH had encouraging anatomical results, reaching among 90% of success rate.97,98 The laser treatment, however, limited the functional recovery. Subsequently, PPV began to be used also for the treatment of RS, MS and RD without MH. The limits of PPV are many. The power of the tamponade is restricted by the configuration of the PS, ILM peeling could weaken an already weak retina and is not easy to see with the atrophic changes in the myopic choroid. The success rate was extremely variable between different studies. Therefore, the use PPV in MTM remained debatable.99–104
Summary
After several studies, MTM has been recognized not just as a group of different foveal and retinal changes in a tractional myopic environment, but as a single evolutive pathology with different stages. The same happened previously to MM as a whole, when the different aspects were found to be different stages of the same disease, only years after its first description.
An important role was played by the OCT, that allowed, on one hand, to precisely define the ultrastructural characteristics of the various features of MTM and permitted, on the other hand, to follow eyes over time, discovering the progressive nature of the disease. MTM is a diagnostic challenge because, in early stages, is often asymptomatic and difficult to identify biomicroscopically. OCT allows to detect minimal macular changes in an asymptomatic stage. In light of these observations, the Authors of the current review recommend following eyes with PM over time using OCT, in order to identify early stages of MTM, and emphasize the need to standardise the follow-up time. The proposed MSS staging system, based on OCT findings, lead to a better understanding of MTM as a whole and offered precise indications on prognosis and therefore management.
The treatment of MTM is a complex and debated topic for different reasons. In the early stages of MTM, such as FS, the surgical approach is not considered by everyone to be the right choice. Some authors, assuming that MTM is a progressive pathology, surgically treat the FS. Others, based on the slow morphological and functional progression of MTM, prefer to wait and follow the patient over time. Even in the management of advanced MTM stages, i.e. the presence of a MH, a RD or the association of both in MHRD, controversies exist. The main concern is the type of surgical approach: ab externo or ab interno. In the last decade, several MB techniques have been proposed with encouraging results. On the other hand, the ab interno approach, PPV, allows to perform a series of maneuvers facilitating the treatment of RD and MH. The choice between macular buckle or PPV for the treatment of MTM, is not obvious. For the treatment choice, the key is the understanding of the pathogenesis, i.e. the combination of centrifugal forces which are either tangential and\or perpendicular to the macula. The tangential forces are mainly counteracted by PPV and maneuvers on the ILM, while the perpendicular forces are mainly counteracted by the MB. Therefore, the two techniques have to be selected on the base of the MTM stage, single or combined.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Ruiz-Medrano J, Montero JA, Flores-Moreno I, Arias L, García-Layana A, Ruiz-Moreno JM. Myopic maculopathy: current status and proposal for a new classification and grading system (ATN). Prog Retin Eye Res. 2019;69(October2018):80–115. doi:10.1016/j.preteyeres.2018.10.005
2. Norton TT, Metlapally R, Young TL. Myopia. Garner Klintworths Pathobiol Ocul Dis. 2008;324(May):537–556. doi:10.5005/jp/books/12584_5
3. Shimada N, Tanaka Y, Tokoro T, Ohno-Matsui K. Natural course of myopic traction maculopathy and factors associated with progression or resolution. Am J Ophthalmol. 2013;156(5):948–957.e1. doi:10.1016/j.ajo.2013.06.031
4. Wolf S, Balciuniene VJ, Laganovska G, et al. RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology. 2014;121(3):682–692.e2. doi:10.1016/j.ophtha.2013.10.023
5. Lewis JA, Garcia MB, Rani L, Wildsoet CF. Intact globe inflation testing of changes in scleral mechanics in myopia and recovery. Exp Eye Res. 2014;127(1):42–48. doi:10.1038/jid.2014.371
6. McBrien NA, Norton TT. Prevention of collagen crosslinking increases form-deprivation myopia in tree shrew. Exp Eye Res. 1994;59(4):475–486. doi:10.1006/exer.1994.1133
7. Sergienko NM, Shargorogska I. The scleral rigidity of eyes with different refractions. Graefes Arch Clin Exp Ophthalmol. 2012;250(7):1009–1012. doi:10.1007/s00417-012-1973-0
8. Karlin DB, Curtin BJ. Peripheral chorioretinal lesions and axial length of the myopic eye. Am J Ophthalmol. 1976;81(5):625–635. doi:10.1016/0002-9394(76)90129-x
9. Ohno-Matsui K. What is the fundamental nature of pathologic myopia? Retina. 2017;37(6):1043–1048. doi:10.1097/IAE.0000000000001348
10. Scarpa A Essay on Observations and Experiences with the Main Eye Diseases. 1801.
11. Curtin BJ. The posterior staphyloma of pathologic myopia. Trans Am Ophthalmol Soc. 1978;75:67–86.
12. Spaide RF. Staphyloma: part 1. In: Spaide RF, Ohno-Matsui K, Yannuzzi LA, editors. Pathologic Myopia. New York: Springer New York; 2014:167–176. doi:10.1007/978-1-4614-8338-0_12
13. Ohno-Matsui K. Proposed classification of posterior staphylomas based on analyses of eye shape by three-dimensional magnetic resonance imaging and wide-field fundus imaging. Ophthalmology. 2014;121(9):1798–1809. doi:10.1016/j.ophtha.2014.03.035
14. Benhamou N, Massin P, Haouchine B, Erginay A, Gaudric A. Macular retinoschisis in highly myopic eyes. Am J Ophthalmol. 2002;133(6):794–800. doi:10.1016/S0002-9394(02)01394-6
15. Baba T, Ohno-Matsui K, Futagami S, et al. Prevalence and characteristics of foveal retinal detachment without macular hole in high myopia. Am J Ophthalmol. 2003;135(3):338–342. doi:10.1016/s0002-9394(02)01937-2
16. Hirakata A, Hida T. Vitrectomy for myopic posterior retinoschisis or foveal detachment. Jpn J Ophthalmol. 2006;50(1):53–61. doi:10.1007/s10384-005-0270-4
17. Oie Y, Ikuno Y, Fujikado T, Tano Y. Relation of posterior staphyloma in highly myopic eyes with macular hole and retinal detachment. Jpn J Ophthalmol. 2005;49(6):530–532. doi:10.1007/s10384-005-0249-1
18. Rahimy E, Beardsley RM, Gomez J, Hung C, Sarraf D. Grading of posterior staphyloma with spectral-domain optical coherence tomography and correlation with macular disease. Can J Ophthalmol. 2013;48(6):539–545. doi:10.1016/j.jcjo.2013.07.006
19. Wu PC, Chen YJ, Chen YH, et al. Factors associated with foveoschisis and foveal detachment without macular hole in high myopia. Eye. 2009;23(2):356–361. doi:10.1038/sj.eye.6703038
20. Ohno-Matsui K, Lai TYY, Lai CC, Cheung CMG. Updates of pathologic myopia. Prog Retin Eye Res. 2016;52:156–187. doi:10.1016/j.preteyeres.2015.12.001
21. Moriyama M, Ohno-Matsui K, Modegi T, et al. Quantitative analyses of high-resolution 3D MR images of highly myopic eyes to determine their shapes. Investig Ophthalmol Vis Sci. 2012;53(8):4510–4518. doi:10.1167/iovs.12-9426
22. Ohno-Matsui K, Akiba M, Modegi T, et al. Association between shape of sclera and myopic retinochoroidal lesions in patients with pathologic myopia. Investig Ophthalmol Vis Sci. 2012;53(10):6046–6061. doi:10.1167/iovs.12-10161
23. Frisina R, Baldi A, Cesana BM, Semeraro F, Parolini B. Morphological and clinical characteristics of myopic posterior staphyloma in Caucasians. Graefes Arch Clin Exp Ophthalmol. 2016;254(11):2119–2129. doi:10.1007/s00417-016-3359-1
24. Shinohara K, Shimada N, Moriyama M, et al. Posterior staphylomas in pathologic myopia imaged by widefield optical coherence tomography. Investig Ophthalmol Vis Sci. 2017;58(9):3750–3758. doi:10.1167/iovs.17-22319
25. Curtin BJ, Karlin DB. Axial length measurements and fundus changes of the myopic eye. Am J Ophthalmol. 1971;71(1):42–53. doi:10.1016/0002-9394(71)91092-0
26. Tokoro T. Types of fundus changes in the posterior pole BT - atlas of posterior fundus changes in pathologic myopia. In: Atlas of Posterior Fundus Changes in Pathologic Myopia. 1998:5–22.
27. Avila MP, Weiter JJ, Jalkh AE, Trempe CL, Pruett RC, Schepens CL. Natural history of choroidal neovascularization in degenerative myopia. Ophthalmology. 1984;91(12):1573–1581. doi:10.1016/s0161-6420(84)34116-1
28. Hayashi K, Ohno-Matsui K, Shimada N, et al. Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology. 2010;117(8):1595–1611.e4. doi:10.1016/j.ophtha.2009.11.003
29. Ohno-Matsui K, Kawasaki R, Jonas JB, et al. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015;159(5):877–883.e7. doi:10.1016/j.ajo.2015.01.022
30. Steidl SM, Pruett RC. Macular complications associated with posterior staphyloma. Am J Ophthalmol. 1997;123(2):181–187. doi:10.1016/s0002-9394(14)71034-7
31. Ishida T, Watanabe T, Yokoi T, Shinohara K, Ohno-Matsui K. Possible connection of short posterior ciliary arteries to choroidal neovascularisations in eyes with pathologic myopia. Br J Ophthalmol. 2019;103(4):457–462. doi:10.1136/bjophthalmol-2018-312015
32. Panozzo G, Mercanti A. Optical coherence tomography findings in myopic traction maculopathy. Arch Ophthalmol. 2004;122(10):1455–1460. doi:10.1001/archopht.122.10.1455
33. Ouyang PB, Duan XC, Zhu XH. Diagnosis and treatment of myopic traction maculopathy. Int J Ophthalmol. 2012;5(6):754–758. doi:10.3980/j.issn.2222-3959.2012.06.19
34. VanderBeek BL, Johnson MW. The diversity of traction mechanisms in myopic traction maculopathy. Am J Ophthalmol. 2012;153(1):93–102. doi:10.1016/j.ajo.2011.06.016
35. Sayanagi K, Ikuno Y, Tano Y. Tractional internal limiting membrane detachment in highly myopic eyes. Am J Ophthalmol. 2006;142(5):850–852. doi:10.1016/j.ajo.2006.05.031
36. Akiba J. Prevalence of posterior vitreous detachment in high myopia. Ophthalmology. 1993;100(9):1384–1388. doi:10.1016/s0161-6420(93)31471-5
37. Itakura H, Kishi S, Li D, Nitta K, Akiyama H. Vitreous changes in high myopia observed by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55(3):1447–1452. doi:10.1167/iovs.13-13496
38. Phillips C. Retinal detachment at the posterior pole. Br J Ophthalmol. 1958;42(12):749–753. doi:10.1136/bjo.42.12.749
39. Phillips C, Dobbie J. Posterior staphyloma and retinal detachment. Am J Ophthalmol. 1963;45:332–335. doi:10.1016/0002-9394(63)92692-8
40. Takano M, Kishi S. Foveal retinoschisis and retinal detachment in severely myopic eyes with posterior staphyloma. Am J Ophthalmol. 1999;128(4):472–476. doi:10.1016/s0002-9394(99)00186-5
41. Fujimoto M, Hangai M, Suda K, Yoshimura N. Features associated with foveal retinal detachment in myopic macular retinoschisis. Am J Ophthalmol. 2010;150(6):863–870.e1. doi:10.1016/j.ajo.2010.06.023
42. Sayanagi K, Morimoto Y, Ikuno Y, Tano Y. Spectral-domain optical coherence tomographic findings in myopic foveoschisis. Retina. 2010;30(4):623–628. doi:10.1097/IAE.0b013e3181ca4e7c
43. Ceklic L, Munk MR, Wolf-Schnurrbusch U, Gekkieva M, Wolf S. Visual acuity outcomes of ranibizumab treatment in pathologic myopic eyes with macular retinoschisis and choroidal neovascularization. Retina. 2017;37(4):687–693. doi:10.1097/IAE.0000000000001236
44. Hoang QV, Chen C-L, Garcia-Arumi J, Sherwood PR, Chang S. Radius of curvature changes in spontaneous improvement of foveoschisis in highly myopic eyes. Br J Ophthalmol. 2016;100(2):222–226. doi:10.1136/bjophthalmol-2015-306628
45. Shimada N, Ohno-Matsui K, Yoshida T, Sugamoto Y, Tokoro T, Mochizuki M. Progression from macular retinoschisis to retinal detachment in highly myopic eyes is associated with outer lamellar hole formation. Br J Ophthalmol. 2008;92(6):762–764. doi:10.1136/bjo.2007.131359
46. Witkin AJ, Ko TH, Fujimoto JG, et al. Redefining lamellar holes and the vitreomacular interface: an ultrahigh-resolution optical coherence tomography study. Ophthalmology. 2006;113(3):388–397. doi:10.1016/j.freeradbiomed.2008.10.025.The
47. Reibaldi M, Parravano M, Varano M, et al. Foveal microstructure and functional parameters in lamellar macular hole. Am J Ophthalmol. 2012;154(6):974–980.e1. doi:10.1016/j.ajo.2012.06.008
48. Bottoni F, Deiro AP, Giani A, Orini C, Cigada M, Staurenghi G. The natural history of lamellar macular holes: a spectral domain optical coherence tomography study. Graefes Arch Clin Exp Ophthalmol. 2013;251(2):467–475. doi:10.1007/s00417-012-2044-2
49. Parolini B, Schumann RG, Cereda MG, Haritoglou C, Pertile G. Lamellar macular hole: a clinicopathologic correlation of surgically excised epiretinal membranes. Invest Ophthalmol Vis Sci. 2011;52(12):9074–9083. doi:10.1167/iovs.11-8227
50. Frisina R, Pilotto E, Midena E. Lamellar macular hole: state of the art. Ophthalmic Res. 2019;61(2):73–82. doi:10.1159/000494687
51. Zampedri E, Romanelli F, Semeraro F, Parolini B, Frisina R. Spectral-domain optical coherence tomography findings in idiopathic lamellar macular hole. Graefes Arch Clin Exp Ophthalmol. 2017;255(4):699–707. doi:10.1007/s00417-016-3545-1
52. Frisina R, Parrozzani R, Pilotto E, Midena E, Double Inverted A. Flap surgical technique for the treatment of idiopathic lamellar macular hole associated with atypical epiretinal membrane. Ophthalmologica. 2019;242(1):49–58. doi:10.1159/000496297
53. Frisina R, Tessarolo F, Marchesoni I, et al. Microscopic observation of proliferative membranes in fibrocontractive retinal disorders. J Ophthalmol. 2019;2019:1–10. doi:10.1155/2019/9647947
54. Rino F, Elena Z, Ivan M, Paolo B, Barbara P, Federica R. Lamellar macular hole in high myopic eyes with posterior staphyloma: morphological and functional characteristics. Graefes Arch Clin Exp Ophthalmol. 2016;254(11):2141–2150. doi:10.1007/s00417-016-3371-5
55. Frisina R, Zampedri E, Marchesoni I, Bosio P, Parolini B, Romanelli F. Erratum to: lamellar macular hole in high myopic eyes with posterior staphyloma: morphological and functional characteristics. Graefes Arch Clin Exp Ophthalmol. 2016;254(11):2289. doi:10.1007/s00417-016-3383-1
56. dell’Omo R, Virgili G, Bottoni F, et al. Lamellar macular holes in the eyes with pathological myopia. Graefes Arch Clin Exp Ophthalmol. 2018;256(7):1281–1290. doi:10.1007/s00417-018-3995-8
57. Parolini B, Palmieri M, Finzi A, et al. The new myopic traction maculopathy staging system. Eur J Ophthalmol. 2020;112067212093059. doi:10.1177/1120672120930590.
58. Ikuno Y, Sayanagi K, Soga K, Oshima Y, Ohji M, Tano Y. Foveal anatomical status and surgical results in vitrectomy for myopic foveoschisis. Jpn J Ophthalmol. 2008;52(4):269–276. doi:10.1007/s10384-008-0544-8
59. Jo Y, Ikuno Y, Nishida K. Retinoschisis: a predictive factor in vitrectomy for macular holes without retinal detachment in highly myopic eyes. Br J Ophthalmol. 2012;96(2):197–200. doi:10.1136/bjo.2011.203232
60. Ohsugi H, Ikuno Y, Matsuba S, et al. Morphologic characteristics of macular hole and macular hole retinal detachment associated with extreme myopia. Retina. 2019;39(7):1312–1318. doi:10.1097/IAE.0000000000002155
61. Borley W, Snyder A. Surgical treatment of degenerative myopia; the combined lamellar scleral resection with scleral reinforcement using donor eye. Trans Pac Coast Otoophthalmol Soc Annu Meet. 1958;39:275–291.
62. Curtin B. Surgical support of the posterior sclera: part II. Clinical results. Am J Ophthalmol. 1961;52:253. doi:10.1016/0002-9394(61)90328-2
63. Momose A. Surgical treatment of myopia–with special references to posterior scleral support operation and radial keratotomy. Indian J Ophthalmol. 1983;31(6):759–767.
64. Snyder AA, Thompson FB. A simplified technique for surgical treatment of degenerative myopia. Am J Ophthalmol. 1972;74(2):273–277. doi:10.1016/0002-9394(72)90544-2
65. Qi Y, Duan A, You Q, Jonas JB, Wang N. Posterior scleral rein- forcement and vitrectomy for myopic foveoschisis in extreme myopia. Retina. 2015;35:351–357. doi:10.1097/IAE.0000000000000313
66. Peng C, Xu J, Ding X. Effects of posterior scleral reinforcement in pathological myopia: a 3-year follow-up study. Graefes Arch Clin Exp Ophthalmol. 2019;257:607–617. doi:10.1007/s00417-018-04212-y
67. Frisina R. A customized posterior scleral reinforcement for myopic macular hole with retinal detachment and posterior staphyloma: a case report. Eur J Ophthalmol. 2020;112067212092726. doi:10.1177/1120672120927268
68. Schepens C, Okamura I. The scleral buckling procedures. I. Surgical techniques and management. AMA Arch Ophthalmol. 1957;58:797–811. doi:10.1001/archopht.1957.00940010819003
69. Siam A. Management of central retinal detachment due to a macular hole. Br J Ophthalmol. 1973;57:351–354. doi:10.1136/bjo.57.5.351
70. Calabria G, Pruett R, Refojo M, Schepens C. Sutureless scleral buckling. An experimental technique. Arch Ophthalmol. 1970;83:613–618. doi:10.1001/archopht.1970.00990030613017
71. Theodossiadis GP, Theodossiadis P. Optical coherence tomography in optic disk pit maculopathy treated by the macular buckling procedure. Am J Ophthalmol. 2001;132:184–190. doi:10.1016/S0002-9394(01)00997-7
72. Theodossiadis G, Sasoh M. Macular buckling for retinal detachment due to macular hole in highly myopic eyes with posterior staphyloma. Retina. 2002;22:129. doi:10.1097/00006982-200202000-00030
73. Rosengren B. The silver plomb method in amotio retinae: clinical experience and results. Bibl Ophthalmol. 1966;70:253–256.
74. Klöti R. Silver clip for central retinal detachments with macular hole. Mod Probl Ophthalmol. 1974;12:330–336.
75. Maćkowiak A. Retinal detachment surgery for giant tear or macular hole. The oblique encircling silastic band without evacuation of subretinal fluid. Japan J Ophthalmol. 1970;18:245.
76. Mateo C, Burés-Jelstrup A, Navarro R, Corcóstegui B. Macular buckling for eyes with myopic foveoschisis secondary to posterior staphyloma. Retina. 2012;32:1121–1128. doi:10.1097/IAE.0b013e31822e5c32
77. Parolini B, Frisina R, Pinackatt S, Mete M. A new L-shaped design of macular buckle to support a posterior staphyloma in high myopia. Retina. 2013;33:1466–1470. doi:10.1097/IAE.0b013e31828e69ea
78. Shinohara K, Tanaka N, Jonas JB, et al. Ultrawide-field OCT to investigate relationships between myopic macular retinoschisis and posterior staphyloma. Ophthalmology. 2018;125(10):1575–1586. doi:10.1016/j.ophtha.2018.03.053
79. Zheng F, Wong C, Sabanayagam C. Prevalence, risk factors and impact of posterior staphyloma diagnosed from wide-field optical coherence tomography in Singapore adults with high myopia. Acta Ophthalmol. 2020. doi:10.1111/aos.14527
80. Sasoh M, Yoshida S, Ito Y, Matsui K, Osawa S, Uji Y. Macular buckling for retinal detachment due to macular hole in highly myopic eyes with posterior staphyloma. Retina. 2000;20(5):445–449. doi:10.1097/00006982-200009000-00003
81. Ripandelli G, Coppé AM, Fedeli R, Parisi V, D’Amico DJ, Stirpe M. Evaluation of primary surgical procedures for retinal detachment with macular hole in highly myopic eyes: a comparison [corrected] of vitrectomy versus posterior episcleral buckling surgery. Ophthalmology. 2001;108(12):
82. Theodossiadis GP, Theodossiadis PG. The macular buckling procedure in the treatment of retinal detachment in highly myopic eyes with macular hole and posterior staphyloma: mean follow-up of 15 years. Retina. 2005;25(3):285–289. doi:10.1097/00006982-200504000-00006
83. Tanaka T, Ando FU, Usui M. Episcleral macular buckling by semirigid shaped-rod exoplant for recurrent retinal detachment with macular hole in highly myopic eyes. Retina. 2005;25:147–151. doi:10.1097/00006982-200502000-00005
84. Ward B, Tarutta E, Mayer J. The efficacy and safety of posterior pole buckles in the control of progressive high myopia. Eye. 2009;23:2169–2174. doi:10.1038/eye.2008.433
85. Liu B, Chen S, Li Y, et al. Comparison of macular buckling and vitrectomy for the treatment of macular schisis and associated macular detachment in high myopia: a randomized clinical trial. Acta Ophthalmol. 2020;98:266–272. doi:10.1111/aos.14260
86. Alkabes M, Mateo C. Macular buckle technique in myopic traction maculopathy: a 16-year review of the literature and a comparison with vitreous surgery. Graefes Arch Clin Exp Ophthalmol. 2018;256(5):863–877. doi:10.1007/s00417-018-3947-3
87. Gonvers M, Machemer R. A new approach to treating retinal detachment with macular hole. Am J Ophthalmol. 1982;94:468–472. doi:10.1016/0002-9394(82)90240-9
88. Vallat M. Surgical treatment of retinal detachment from macular hole. Graefes Arch Clin Exp Ophthalmol. 1986;224:238–239. doi:10.1007/BF02143062
89. Oshima Y, Ikuno Y, Motokura M, Nakae K, Tano Y. Complete epiretinal membrane separation in highly myopic eyes with retinal detachment resulting from a macular hole. Am J Ophthalmol. 1998;126(5):669–676. doi:10.1016/S0002-9394(98)00180-9
90. Kokame GT, Yamamoto I. Silicone oil versus gas tamponade. Ophthalmology. 2004;111(4):851–852. doi:10.1016/j.ophtha.2004.01.015
91. Rizzo S, Belting C, Genovesi-Ebert F, Cresti F, Vento A, Martini R. Successful treatment of persistent macular holes using “heavy silicone oil” as intraocular tamponade. Retina. 2006;26(8):905–908. doi:10.1097/01.iae.0000250006.76155.3d
92. Cheung B, Lai TYY, Yuen CYF, Lai WWK, Tsang C-W, Lam DSC. Results of high-density silicone oil as a tamponade agent in macular hole retinal detachment in patients with high myopia. Br J Ophthalmol. 2007;91:719–721. doi:10.1136/bjo.2006.111526
93. Mester V, Kuhn F. Internal limiting membrane removal in the management of full-thickness macular holes ´. Am J Ophthalmol Case Rep. 2000;129(6):769–777. doi:10.1016/s0002-9394(00)00358-5
94. Sasaki H, Shiono A, Kogo J, et al. Inverted internal limiting membrane flap technique as a useful procedure for macular hole-associated retinal detachment in highly myopic eyes. Eye (Lond). 2017;31(4):545–550. doi:10.1038/eye.2016.263
95. Yuan J, Zhang -L-L, Lu Y-J, Han M-Y, Yu A-H, Cai X-J. Vitrectomy with internal limiting membrane peeling versus inverted internal limiting membrane flap technique for macular hole-induced retinal detachment: a systematic review of literature and meta-analysis. BMC Ophthalmol. 2017;17(1):219. doi:10.1186/s12886-017-0619-8
96. Kinoshita T, Onoda Y, Maeno T. Long-term surgical outcomes of the inverted internal limiting membrane flap technique in highly myopic macular hole retinal detachment. Graefes Arch Clin Exp Ophthalmol. 2017;255:1101–1106. doi:10.1007/s00417-017-3614-0
97. Kadonosono K, Yazama F, Itoh N, et al. Treatment of retinal detachment resulting from myopic macular hole with internal limiting membrane removal. Am J Ophthalmol. 2001;131(2):203–207. doi:10.1016/S0002-9394(00)00728-5
98. Lu L, Li Y, Cai S, Yang J. Vitreous surgery in highly myopic retinal detachment resulting from a macular hole. Clin Experiment Ophthalmol. 2002;30(4):261–265. doi:10.1046/j.1442-9071.2002.00530.x
99. Kanda S, Uemura A, Sakamoto Y, Kita H. Vitrectomy with internal limiting membrane peeling for macular retinoschisis and retinal detachment without macular hole in highly myopic eyes. Am J Ophthalmol. 2003;136:177–180. doi:10.1016/S0002-9394(03)00243-5
100. Ikuno Y, Sayanagi K, Ohji M. Vitrectomy and internal limiting membrane peeling for myopic foveoschisis. Am J Ophthalmol. 2004;137:719–724. doi:10.1016/j.ajo.2003.10.019
101. Scholda C, Wirtitsch M, Biowski R, Stur M. Primary silicone oil tamponade without retinopexy in highly myopic eyes with central macular hole detachments. Retina. 2005;25(2):141–146. doi:10.1097/00006982-200502000-00004
102. Chen Y, Chen T-L, Yang K-R. Treatment of retinal detachment resulting from posterior staphyloma-associated macular hole in highly myopic eyes. Retina. 2006;26:25–31. doi:10.1097/00006982-200601000-00005
103. Panozzo G, Mercanti A. Vitrectomy for myopic traction maculopathy. Arch Ophthalmol. 2007;125:767–772. doi:10.1001/archopht.125.6.767
104. Mete M, Parolini B, Maggio E, Pertile G. 1000 cSt silicone oil vs heavy silicone oil as intraocular tamponade in retinal detachment associated to myopic macular hole. Graefes Arch Clin Exp Ophthalmol. 2011;249(6):821–826.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.