Back to Journals » Vascular Health and Risk Management » Volume 18
Myocardial Protecting Role of Glutamine in Patients with Low Ejection Fraction Undergoing Elective On-Pump Coronary Artery Bypass Graft Surgery
Authors Parmana IMA , Boom CE, Rachmadi L, Hanafy DA , Widyastuti Y , Mansyur M, Siswanto BB
Received 11 February 2022
Accepted for publication 25 March 2022
Published 5 April 2022 Volume 2022:18 Pages 219—231
DOI https://doi.org/10.2147/VHRM.S361298
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Harry Struijker-Boudier
I Made Adi Parmana,1 Cindy Elfira Boom,1 Lisnawati Rachmadi,2 Dudy Arman Hanafy,3 Yunita Widyastuti,4 Muchtaruddin Mansyur,5 Bambang Budi Siswanto6
1Department of Anesthesiology and Intensive Care, National Cardiovascular Center Harapan Kita, Jakarta, Indonesia; 2Department of Anatomical Pathology, Faculty of Medicine, Universitas Indonesia/Dr. Cipto Mangunkusumo Hospital, Jakarta, Indonesia; 3Department of Cardiothoracic and Vascular Surgery, Faculty of Medicine, Universitas Indonesia/National Cardiovascular Center Harapan Kita, Jakarta, Indonesia; 4Department of Anesthesiology and Intensive Care, Universitas Gadjah Mada/Dr. Sardjito Hospital, Yogyakarta, Indonesia; 5Department of Community Medicine, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia; 6Department of Cardiology and Vascular Medicine, Faculty of Medicine, Universitas Indonesia/National Cardiovascular Center Harapan Kita, Jakarta, Indonesia
Correspondence: I Made Adi Parmana, Department of Anesthesiology and Intensive Care, National Cardiovascular Center Harapan Kita, LetJen S. Parman St No. Kav. 87, West Jakarta, Jakarta, Indonesia, Tel +62 812-4601-212, Email [email protected]
Purpose: Myocardial injury due to on-pump coronary artery bypass grafting (CABG) in patients with low ejection fraction (EF) is associated with poor outcomes. This study determines whether intravenous glutamine could protect the myocardium during on-pump CABG in patients with low EF.
Materials and Methods: This was a double-blind, randomized controlled trial to assess glutamine as a myocardial protector during on-pump CABG in patients with left ventricle EF of 31– 50%, conducted from January to October 2021. Patients in the glutamine group (n = 30) received 0.5 g/kg of 20% glutamine solution diluted with 0.9% NaCl up to 500 mL in total volume over a period of 24 hours. Patients in the control group (n = 30) received 0.9% NaCl over the same period. The primary outcomes were plasma troponin I and plasma glutamine levels. Secondary outcomes included α-ketoglutarate (α-KG) levels and histopathology scoring of the right atrial appendage tissue, plasma lactate levels, hemodynamic measurement, and morbidity.
Results: Twenty-nine patients from each group (58 in total) were included in the analysis. Plasma troponin I levels at 6 and 24 hours after cardiopulmonary bypass (CPB) were significantly lower in the glutamine than the control group (mean 3.43 ± 1.51 ng/mL vs mean 4.41 ± 1.89 ng/mL; p = 0.034; median 3.08 ng/mL [min–max: 1.30– 6.59] vs median 3.77 ng/mL [min–max: 0.00– 36.53]; p = 0.038, respectively). Plasma glutamine levels at 24 hours after CPB were significantly higher in the glutamine than the control group (mean 935.42 ± 319.10 μmol/L vs mean 634.79 ± 243.89 μmol/L, p = 0.001). Plasma lactate levels at 6 and 24 hours after CPB were significantly lower in the glutamine than the control group (median 5.30 mmol/L [min-max: 1.20– 9.50] vs median 5.70 mmol/L [min-max: 2.80– 11.30], p = 0.042; mean 2.08 ± 0.67 mmol/L vs mean 2.46 ± 0.69 mmol/L, p = 0.044, respectively). Myocardial injury score was significantly lower in the glutamine than the control group (mean 1.30 ± 0.24 vs mean 1.48 ± 0.26, p = 0.011).
Conclusion: Perioperative administration of 0.5 g/kg intravenous glutamine solution over the period of 24 hours has myocardial protection effect in patients with low EF who undergo elective on-pump CABG.
Keywords: glutamine, myocardial protection, coronary artery bypass grafting, cardiopulmonary bypass, low ejection fraction, myocardial injury
Introduction
On-pump coronary artery bypass grafting (CABG) carries a risk of triggering myocardial injury due to ischemia, reperfusion, and inflammation. Myocardial injury disrupts normal cardiac myocyte membrane integrity and loss of intracellular content into the extracellular space, indicated by elevated cardiac enzymes (troponin) levels in the blood.1–3 Myocardial injury due to on-pump CABG procedure is generally tolerable in patients with good myocardial function, but it is controversial in patients with low ejection fraction (EF). Various studies have shown that patients with low EF have lower operating capacity and tolerance, hence the greater risk of injury and worse postoperative outcomes when compared to patients with normal EF.4–6
Glutamine has been reported to play a role as a myocardial protector throughout energy production during ischemic periods.7–10 Supplementation of glutamine leads to rapid glutamine uptake and increased levels of intracellular glutamate due to high levels of glutaminase activity in the heart. Glutamate dehydrogenase enzyme will convert glutamate into α-KG as the substrate of Krebs cycle. In the Krebs cycle, conversion of α-KG into succinate produces guanosine triphosphate (GTP) and nicotinamide adenine dinucleotide + hydrogen (NADH) as a source of energy. This process is anaerobic, and may serve to maintain the levels of NADH and Krebs cycle intermediates through an ischemic event, keeping the metabolic machinery primed to begin oxidative phosphorylation as soon as oxygen returns.7
Lomivorotov et al2 found that glutamine infusions given perioperatively during the first 24 hours in ischemic heart disease patients who underwent on-pump CABG procedure significantly reduced troponin I levels compared to controls and concluded improved outcomes. Fathi et al11 demonstrated that intravenous glutamine injection could improve cardiopulmonary bypass outcomes and found no significant differences in the results when glutamine administration was started 3 days prior to surgery compared to immediately after induction of anesthesia. Both studies did not investigate patients with an EF of <50%, thus, the effect of glutamine administration in patients with an EF of < 50% remained unknown.2,11
Patients with low EF are more vulnerable to myocardial injury and glutamine administration is expected to provide myocardial protection and mitigate the negative impact of on-pump CABG procedures and provide better clinical outcomes. This report assessed the role of glutamine as a myocardial protector in patients with low EF who underwent on-pump CABG.
Materials and Methods
This double-blind, randomized controlled trial was conducted from January to October 2021, approved by the Institutional Review Board of the National Cardiovascular Center Harapan Kita (LB.02.02/VII/466/KEP059/2020), the Ethics Committee of the Faculty of Medicine University of Indonesia (KET-965/UN2.F1/ETIK/PPM.002/2020), and has been registered at clinicaltrials.gov (NCT04560309). This study was conducted in accordance with the Declaration of Helsinki. In addition, written informed consent was obtained from all eligible patients.
Study Population
Inclusion criteria of this study were patients aged ≥ 18 years with coronary heart disease and who were indicated for elective CABG using cardiopulmonary bypass (CPB) machine, whose left ventricle EF was 31%–50% as confirmed by echocardiography or radionuclide imaging, never had a history of heart surgery and agreed to participate in the trial. Exclusion criteria include patients who underwent emergency CABG, had additional procedures other than CABG, had serum creatinine level more than 2 g/dL, ALT/AST level more than 1.5 times of normal value, use of intra-aortic balloon pump pre-operatively, had contraindications to pulmonary artery catheter insertion, and had positive history of stroke, myocardial infarction within the last three months, preoperative atrial fibrillation, and heart conduction problem or pacemaker use. Drop out criteria were stroke after surgery, perioperative myocardial infarction, surgery-related complications, patients who required re-operation, continuous veno-venous hemofiltration or hemodialysis after surgery, delayed sternal closure, aortic cross-clamp duration more than 120 minutes, or CPB time more than 180 minutes. A total of 60 patients who provided written informed consent and met the inclusion criteria were enrolled in the study.
Randomization was performed via sealed envelopes. Allocation of a participant to the treatment group was conducted through block randomization. The intervention drug was prepared by an independent pharmacist. Evaluating investigators, care providers, and participants were blinded to all study interventions.
Patient Management
Coronary Artery Bypass Graft with CPB was performed in all of the patients. Surgery was performed with standard anesthesia procedures and techniques. A dose of 0.05 mg/kg midazolam was given intravenously as premedication. Anesthesia was induced using intravenous 0.5–1 mcg/kg sufentanil, 1–2 mg/kg propofol, and 0.1 mg/kg vecuronium. Anesthesia was maintained using inhaled sevoflurane with Minimal Alveolar Concentration (MAC) of 1.0, continued with intravenous 0.1–0.3 mcg kg−1 min−1 sufentanil, and 0.04–0.06 mg kg−1 min−1 vecuronium. After inserting an endotracheal tube, a central venous catheter (Arrow®/Teleflex®, Wayne, USA) and a pulmonary artery catheterization with a Swan–Ganz catheter (Arrow®/Teleflex®, Wayne, USA) were performed. Transesophageal echocardiography (TEE) probe (Philips Ultrasound System Affiniti 50, USA) was subsequently placed. Patients in the glutamine group (n = 30) received 0.5 g/kg of 20% glutamine solution (Dipeptiven, Fresenius Kabi, Bad Homburg, Germany) diluted with 0.9% NaCl up to 500 mL in total volume via central venous catheter over a period of 24 hours. Patients in the control group (n = 30) received 0.9% NaCl via a central venous catheter over the same period. Administration of the intervention solution was started after a central venous catheter was placed. Full median sternotomy was performed in all patients, and systemic heparinization began before cannulation of the right atrium and ascending aorta. Heparin (300 IU/kg) was administered to sustain an activated clotting time of > 400 seconds. The normothermic (35.5°C–36.5°C) CPB flow rate was maintained at 2.5 L min−1 m−2, and mean arterial pressure was kept between 55 and 70 mmHg. All patients were admitted to the ICU following surgery. Patients were weaned off the ventilator when the patient was considered hemodynamically stable.
Data Collection and Blood Sampling
Data on sex, age, weight, height, preoperative EF, SYNTAX score II (SSII) CABG and systemic immune-inflammation index (SII) were collected through direct history-taking and preoperative medical records. Data on CPB time, aortic cross-clamping time, number of distal coronary artery grafts were collected from surgery reports. Arterial blood sampling for troponin I levels measurement were collected: (1) before induction, (2) at 5 minutes, (3) at 6 hours, (4) at 24 hours, and (5) at 48 hours after CPB. Plasma troponin I was measured using the troponin I (human) ELISA kit (Abnova, KA0233) with colorimetry method. Arterial blood samples for plasma glutamine levels measurement were collected before induction and 24 hours after the CPB. Plasma glutamine levels was measured using the Sigma–Aldrich GLN1 glutamine/glutamate determination kit (Sigma–Aldrich, GLN1-1KT) using colorimetry method. To obtain plasma samples, blood was centrifuged for 15 minutes at a speed of 3500 X G within a maximum period of 30 minutes after collection and was stored at −80°C.
A 5×5 mm sample of the right atrial appendage tissue was collected at 5 minutes after CPB and was immediately transported to the laboratory at 4°C within 30 minutes of collection. The tissue sample was divided for α-ketoglutarate (α-KG) measurement and paraffin block preparation for histopathological examinations. A total of 20 mg of tissue was homogenized and centrifuged to obtain the supernatant. A protease inhibitor was added to the supernatant and then stored at −80°C until cellular α-KG levels were examined. The examination of α-KG was performed using α-KG assay kit (Abcam, ab83431) with colorimetry method.
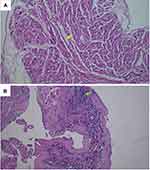
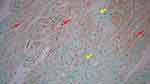
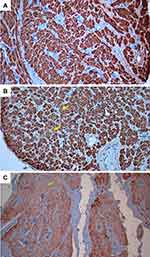
Myocardial injury score was measured on preparations stained with hematoxylin and eosin (H&E) with a scoring system from 0 to 3 as follows: 0 = no change; 1 = slight changes: focal myocyte damage or small multifocal degeneration with slight degree of inflammation; 2 = moderate changes: extensive myofibrillar degeneration and/or diffuse inflammatory process; 3 = severe changes: necrosis with diffuse inflammatory process.12 Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labelling (TUNEL) staining was performed using the TUNEL assay kit (Abcam, ab206386). Cell nuclei with positive TUNEL staining was colored brown. The apoptotic index was calculated based on the average number of cells with positive TUNEL staining.12 Expression of anti-cardiac troponin I was observed using anti-cardiac troponin I antibody (Abcam, ab47003). Measurement of anti-cardiac troponin I expression was performed on preparations stained with anti-cardiac troponin I antibody using a scoring system ranging from 0 to −3, as follows: 0 = no loss of staining; −1 = minimal decrease in staining, compared to normally stained tissue; −2 = clear decrease in staining with some positivity (brown color) remaining; −3 = no positive (brown color) staining.13 For quantitative analysis, histopathological examinations were made on as many as six fields of view per specimen under a light microscope (Olympus BX50, Tokyo, Japan) and photographed for further analysis. The histopathological specimens were assessed separately by two blinded examiners to get an average score.
Arterial blood samples for plasma lactate levels measurement were collected: (1) before induction, (2) at 5 minutes, (3) at 6 hours, (4) at 24 hours, and (5) at 48 hours after CPB. Lactate examination was performed using the Nova Biomedical Stat Profile® pHOX Ultra analyzer (Nova Biomedical, Waltham, MA, USA) with basic enzymatic method.
Ejection fraction measurements were taken: (1) after induction and (2) at 5 minutes after CPB, through TEE examination using the modified Simpson method. Cardiac index (CI) measurements were taken: (1) after induction, (2) at 5 minutes, (3) at 2 hours, (4) at 6 hours, and (5) at 24 hours after CPB. The values were obtained from calculating cardiac output (CO) per body surface area (in m2). Cardiac output was measured via a pulmonary artery catheter using thermodilution method. The measurements were performed three times, averaged, and considered valid if the variation between the three values was less than 10%. Body surface area was calculated using the Mosteller formula. Morbidity variables consist of ventilator time, postoperative use of vasoactive and inotropic agents, and duration of intensive Care were collected from the medical record. Vasoactive and inotropic score (VIS) was calculated (VIS = dopamine dose [μg kg−1 min−1] + dobutamine [μg kg−1 min−1] + 100 × epinephrine dose [μg kg−1 min−1] + 10,000 × vasopressin [units kg−1 min−1] + 100 × norepinephrine dose [μg kg−1 min−1]) using the maximum dosing rates of vasoactive and inotropic medications (μg kg−1 min−1 or IU kg−1 min−1) during the first 24 hours after postoperative ICU admission.14
Statistics
Data analysis was performed using SPSS for Windows version 22.0 statistical software (SPSS Inc., Chicago, IL, USA). The significance level was 0.05 (two-tailed) with a pre-determined power of 80%. Normality test was performed using Kolmogorov‑Smirnov test. Normally distributed continuous variables were expressed in mean ± standard deviation (SD) and were analyzed using unpaired t‑test, and general linear model (GLM) was used for data with repeated measurements. Pillai’s trace was selected for GLM analysis when significance level (p < 0.05) was reached, followed by the Bonferroni post-hoc test. If the data were non-normally distributed, variables were expressed in median (minimum‑maximum) and were analyzed using the Mann‑Whitney U-test. Categorical variables were expressed in number and frequency and subsequently analyzed using Chi‑square test or Fisher’s exact test. A p-value of < 0.05 was considered statistically significant. The internal consistency of the histopathological examinations was assessed using the Cronbach’s alpha test, with an α ≥ 0.70 regarded as optimal for group comparison.15
Results
Sixty patients were enrolled and randomized into the glutamine group (n = 30), in which one patient dropped out of the study due to a perioperative myocardial infarction, and the control group (n = 30), in which one patient dropped out due to a bleeding complication requiring repeat surgery. The study flow chart is presented in Figure 1.
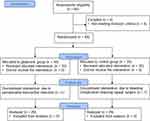 |
Figure 1 Study flow diagram. |
There were no significant differences (p > 0.05) in age, sex, body mass index, preoperative EF, number of distal coronary artery grafts, surgery time, CPB time, aortic cross-clamping time, SSII CABG and SII in both groups (Table 1).
 |
Table 1 Patients Characteristics |
The two primary outcomes of this study were plasma troponin I and glutamine levels. These are presented in Tables 2 and 3, respectively. There were no significant differences in plasma troponin I levels before induction, at 5 minutes, and at 48 hours after CPB between both groups. Plasma troponin I levels at 6 and 24 hours after CPB were significantly lower in the glutamine group than the control group. For the two groups, no significant difference was observed in terms of pre-induction plasma glutamine levels. On the other hand, significantly higher plasma glutamine levels were found 24 hours after CPB in the glutamine group than the control group.
 |
Table 2 Plasma Troponin I Levels |
 |
Table 3 Plasma Glutamine Levels |
Right atrial appendage tissue α-KG levels, myocardial injury score, apoptotic index, and anti-cardiac troponin I expression are shown in Table 4. Levels of α-KG and anti-cardiac troponin I expression (Cronbach’s alpha index = 0.955) were significantly higher in the glutamine group than the control group. Myocardial injury score (Cronbach’s alpha index = 0.776) was significantly lower in the glutamine group than the control group. No significant differences were observed in apoptotic index between the groups, with Cronbach’s alpha index of 0.978. Histopathological examination images are shown in Figures 2–4.
 |
Table 4 Right Atrial Appendage Tissue Examination |
Significantly lower plasma lactate levels were found at 6 hours and 24 hours after CPB in the glutamine group compared to the control group (Table 5). The Pillai’s trace significance level of GLM was 0.024, which indicated significant differences in plasma lactate levels between the groups at each measurement time. The Bonferroni post-hoc test showed that the 1.30 mmol/L increase in lactate levels at 5 minutes to 6 hours was significantly lower in the glutamine group compared to the control group (Figure 5).
 |
Table 5 Plasma Lactate Levels |
Hemodynamic profile measurements consisted of EF and CI (Table 6). There were no significant differences in EF after induction and 5 minutes after CPB between both groups. No significant differences in CI after induction, at 5 minutes, and at 2 hours after CPB were seen in both groups. However, CI at 6 hours and 24 hours after CPB were significantly higher in the glutamine group than in the control group.
 |
Table 6 Hemodynamic Profiles |
Morbidity variables are listed in Table 7. There were no significant differences in all morbidity variables between the groups.
 |
Table 7 Morbidity |
Correlation between SII and plasma troponin I levels are showed in Table 8. No correlation was observed between SII and plasma troponin I levels at different time frames in both groups.
 |
Table 8 Correlation Between SII and Plasma Troponin I Levels |
Discussion
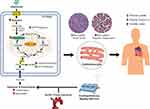
Patients who underwent on-pump CABG had higher troponin I concentrations due to the fact that it can precipitate myocardial injury.13,16 During the procedure, aortic cross-clamping was performed to stop cardiopulmonary circulation, and cardioplegic agents were given to stop the heartbeat. After the procedure, the aortic cross-clamp was released, and the heart was restarted to allow reperfusion.12,17 Ischemia and reperfusion will trigger myocardial injury (Figure 6) that can be reversible or irreversible (necrosis).1
The measurement of troponin I levels at 6 and 24 hours after the CPB was lower in the glutamine group (Figure 6), with the highest plasma troponin I concentration was found at 6 hours after CPB in both groups. Troponin I is generally found within 6–8 hours after the onset of myocardial infarction, reaching the highest level within 12–24 hours. Results from other studies also found the highest increase in troponin I level at 6 hours after the CPB in both treatment and control groups.2,11
Parenteral glutamine administration increased plasma glutamine levels above 800 μmol/L, indicating sufficient substrate concentration. A similar study also performed parenteral administration of glutamine (0.5 g/kg/day) and found a significant increase of glutamine levels in the glutamine group compared to the control group from the first post-operative day.8
The administration of intravenous glutamine has a role in increasing α-KG levels. Under nutrient-rich conditions, pyruvate as a major energy source would enter mitochondria through mitochondrial pyruvate carrier (MPC) for further metabolism to the Krebs cycle.18,19 On the other hand, under conditions of ischemia, glutamine enters the cytosol through SLC15/SLC7A5, and will be metabolized to glutamate catalyzed by glutaminase in the mitochondria18 (Figure 6). Glutamate will be converted into α-KG by glutamate dehydrogenase and release NH4 as a by-product of the process.18,20
Myocardial injury score was lower in the glutamine group. During ischemia, energy production is limited to glycolysis and substrate-level phosphorylation in the mitochondria.19 Once α-KG enters the Krebs cycle, it is converted to succinate, yielding one molecule each of GTP and NADH. This anaplerotic reaction of glutamine serves to maintain the Krebs cycle during ischemia,21 thus reducing cardiomyocytes injury during ischemia (Figure 6).
Anti-cardiac troponin I staining the surface of cardiomyocytes indicates the presence of troponin I on the cell surface.22 Decreased staining compared to positive control tissue indicates troponin loss in myocardial tissue.12 Myocardial tissue necrosis caused by myocardial infarction results in a troponin I burst into blood circulation.23 Glutamine administration can reduce the loss of troponin I due to ischemia by providing intracellular energy supply for cardiomyocytes (Figure 6).
Glutamine provides citrate and maintains the Krebs cycle during ischemia and the metabolic machinery once oxygen returns and re-enter aerobic metabolism.19,21 In ischemia and reperfusion injury cases, glutamine improve the myocardial adenosine triphosphate (ATP)/adenosine diphosphate (ADP) ratio and decrease myocardial lactate accumulation.24
General linear model test result showed a significantly lower increase of lactate levels at 5 minutes to 6 hours in the glutamine group. Therefore, perioperative glutamine administration has a role in reducing the increase of lactate levels in patients undergoing CABG at 5 minutes to 6 hours after CPB. An increase of lactate levels due to the CABG procedure has a two-peak distribution pattern: immediately after the initiation of CPB (early-onset hyperlactatemia) and after 4 to 24 hours post-operatively, during intensive care (late-onset hyperlactatemia).25 Mechanism of late-onset hyperlactatemia remains uncertain.26 The highest increase in lactate levels found in this study was 6 hours after the release of the CPB machine, which was considered late-onset hyperlactatemia. The finding also supported glutamine’s role in reducing increased lactate levels in late-onset hyperlactatemia.
Pyruvate levels in the isolated cardiomyocytes decrease by 50% during ischemia, and lactate levels increase 27-fold after 20 minutes of ischemia.7 Lactate causes a decrease in intracellular pH and interferes with myofibril function.27,28 Therefore, glutamine can maintain substrate supply for the Krebs cycle during ischemia.7,19 It can improve cardiac contractility and CI after CPB (Figure 6). This is also supported by a previous study reporting that perioperative glutamine infusion during the first 24 hours significantly improved the cardiac index value at 4 hours after CPB.2
SSII measures the four year mortality in patients. A pilot study by Hayiroglu et al29 demonstrated SSII as an independent prognostic indicator for prediction of in-Hospital mortality and major adverse cardiac events (MACE). In our study, SSII CABG in both groups showed no significant difference.
Another method of assessing prognoses include SII. Systemic inflammation plays a central role in cardiovascular disease, and SII along with several blood parameters have been used as a prognostic inflammatory marker. High SII had previously been shown to indicate poor prognoses in patients with coronary artery disease, myocardial infarction and chronic heart disease.30 In our study, we observed no significant difference in SII. Furthermore, no correlation was found between SII and different time frames of plasma troponin I levels in both groups. A study by Alam et al31 stated peak high-sensitivity cardiac troponin I release at 6 hours following CABG appears to be related to the surgical process and non-specific myocardial injury, while a continuing increase at 24 hours suggests myocardial infarction.
Limitations and Implications
This study has some limitations. Limitations includes the sampling time of right atrial appendage tissue which was only at 5 minutes after CPB. Right atrial appendage tissue was used for apoptotic index assessment using TUNEL staining. According to a study conducted by Krijnen et al32 timing of myocardial apoptosis in human were between 6 hours up to 120 hours after myocardial infarction onset. Right atrial appendage tissue could not be sampled at 6 hours after CPB because sternal closure was performed prior to 6 hours. Another limitation, this study evaluated SII only once before surgery and did not dynamically assess SII perioperatively. Measurement at adjacent time frames (5 minutes, 6 hours, 24 hours and 48 hours after CPB) are advised to find further correlation between SII and troponin I levels.
Clinical implications of the present study should be noted. Regarding the myocardial protection effects of glutamine, we revealed that perioperative intravenous glutamine improves hemodynamic profile after CPB through measurement of CI. Improvement of CI could indirectly contribute to decreased rate of morbidity and mortality. Future studies with larger number of patients are needed to evaluate the clinical effect and advantages of glutamine.
Conclusion
Three markers were significantly lower in the glutamine group: plasma troponin I levels at 6 and 24 hours after CPB (mean 3.43 ± 1.51 ng/mL, p = 0.034; median 3.08 ng/mL [min–max: 1.30–6.59], p = 0.038), plasma lactate levels at 6 and 24 hours after CPB (median 5.30 mmol/L [min-max: 1.20–9.50], p = 0.042; mean 2.08 ± 0.67 mmol/L, p = 0.044), and the myocardial injury score measured at 5 minutes after CPB (mean 1.30 ± 0.24, p = 0.011). Plasma glutamine levels at 24 hours after CPB, α-KG levels, anti-cardiac troponin I expression at 5 minutes after CPB, and CI at 6 and 24 hours after CPB were significantly higher in the glutamine group. Overall, perioperative administration of 0.5 g/kg intravenous glutamine solution over the period of 24 hours has a protective effect towards the myocardium in patients with low EF undergoing elective on-pump CABG.
Data Sharing Statement
Individual deidentified participant data reported in this study will be made available on request after publication and ending 36 months following article publication. Researchers needs to state their aims of analyses and provide a methodologically sound proposal. Proposals should be directed to the corresponding author.
Disclosure
The authors declare no conflict of interests for this work.
References
1. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138(20). doi:10.1161/CIR.0000000000000617
2. Lomivorotov VV, Efremov SM, Shmirev VA, Ponomarev DN, Lomivorotov VN, Karaskov AM. Glutamine is cardioprotective in patients with ischemic heart disease following cardiopulmonary bypass. Heart Surg Forum. 2011;14(6):E384–388. doi:10.1532/HSF98.20111074
3. Warltier DC, Laffey JG, Boylan JF, Cheng DCH. The systemic inflammatory response to cardiac surgery implications for the anesthesiologist. Anesthesiology. 2002;97(1):215–252. doi:10.1097/00000542-200207000-00030
4. Heusch G. Molecular basis of cardioprotection. Circ Res. 2015;116(4):674–699. doi:10.1161/CIRCRESAHA.116.305348
5. Scott T, Swanevelder J. Perioperative myocardial protection. Crit Care Pain. 2009;9(3):97–101. doi:10.1093/bjaceaccp/mkp011
6. Van Allen NR, Krafft PR, Leitzke AS, Applegate RL, Tang J, Zhang JH. The role of volatile anesthetics in cardioprotection: a systematic review. Med Gas Res. 2012;2:22. doi:10.1186/2045-9912-2-22
7. Drake KJ, Sidorov VY, McGuinness OP, Wasserman DH, Wikswo JP. Amino acids as metabolic substrates during cardiac ischemia. Exp Biol Med. 2012;237(12):1369–1378. doi:10.1258/ebm.2012.012025
8. Engel JM, Mühling J, Kwapisz M, Heidt M. Glutamine administration in patients undergoing cardiac surgery and the influence on blood glutathione levels. Acta Anaesthesiol Scand. 2009;53(10):1317–1323. doi:10.1111/j.1399-6576.2009.02084.x
9. Hamiel CR, Pinto S, Hau A, Wischmeyer PE. Glutamine enhances heat shock protein 70 expression via increased hexosamine biosynthetic pathway activity. Am J Physiol Cell Physiol. 2009;297(6):C1509–C1519. doi:10.1152/ajpcell.00240.2009
10. Hayashi Y, Sawa Y, Fukuyama N, Nakazawa H, Matsuda H. Preoperative glutamine administration induces heat-shock protein 70 expression and attenuates cardiopulmonary bypass–induced inflammatory response by regulating nitric oxide synthase activity. Circulation. 2002;106(20):2601–2607. doi:10.1161/01.CIR.0000035651.72240.07
11. Fathi H, Mowafy S, Helmy K. Evaluation of the effectiveness of glutamine in different times of administration in patients undergoing cardiopulmonary bypass during elective cardiac surgeries: randomized controlled study. Egypt J Cardiothorac Anesth. 2018;12(1):4. doi:10.4103/ejca.ejca_4_16
12. Kocak E, Kocak C, Aksoy A, et al. High-sensitivity cardiac troponin T is more helpful in detecting peri-operative myocardial injury and apoptosis during coronary artery bypass graft surgery. Cardiovasc J Afr. 2015;26(6):234–241. doi:10.5830/CVJA-2015-052
13. Fishbein MC, Wang T, Matijasevic M, Hong L, Apple FS. Myocardial tissue troponins T and I: an immunohistochemical study in experimental models of myocardial ischemia. Cardiovasc Pathol. 2003;12(2):65–71. doi:10.1016/S1054-8807(02)00188-6
14. Koponen T, Karttunen J, Musialowicz T, Pietiläinen L, Uusaro A, Lahtinen P. Vasoactive-inotropic score and the prediction of morbidity and mortality after cardiac surgery. Br J Anaesth. 2019;122(4):428–436. doi:10.1016/j.bja.2018.12.019
15. Arraras JI, Greimel E, Sezer O, et al. An international validation study of the EORTC QLQ-INFO25 questionnaire: an instrument to assess the information given to cancer patients. Eur J Cancer. 2010;46(15):2726–2738. doi:10.1016/j.ejca.2010.06.118
16. Januzzi JL
17. Babuin L, Jaffe AS. Troponin: the biomarker of choice for the detection of cardiac injury. Can Med Assoc J. 2005;173(10):1191–1202. doi:10.1503/cmaj.050141
18. Shen Y, Zhang Y, Li W, Chen K, Xiang M, Ma H. Glutamine metabolism: from proliferating cells to cardiomyocytes. Metabolism. 2021;121:154778. doi:10.1016/j.metabol.2021.154778
19. Yang C, Ko B, Hensley CT, et al. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol Cell. 2014;56(3):414–424. doi:10.1016/j.molcel.2014.09.025
20. Rajendram R, Preedy VR, Patel VB, eds. Glutamine Clin Nutr. Humana Press; 2015.
21. Drake KJ, Shotwell MS, Wikswo JP, Sidorov VY. Glutamine and glutamate limit the shortening of action potential duration in anoxia-challenged rabbit hearts. Physiol Rep. 2015;3(9):e12535. doi:10.14814/phy2.12535
22. Vilela EM, Bettencourt-Silva R, da Costa JT, et al. Anti-cardiac troponin antibodies in clinical human disease: a systematic review. Ann Transl Med. 2017;5(15):307. doi:10.21037/atm.2017.07.40
23. Ricchiuti V, Sharkey SW, Murakami MM, Voss EM, Apple FS. Cardiac troponin I and T alterations in dog hearts with myocardial infarction: correlation with infarct size. Am J Clin Pathol. 1998;110(2):241–247. doi:10.1093/ajcp/110.2.241
24. Wischmeyer PE, Jayakar D, Williams U, et al. Single dose of glutamine enhances myocardial tissue metabolism, glutathione content, and improves myocardial function after ischemia-reperfusion injury. J Parenter Enteral Nutr. 2003;27(6):396–403. doi:10.1177/0148607103027006396
25. O’Connor E, Fraser JF. The interpretation of perioperative lactate abnormalities in patients undergoing cardiac surgery. Anaesth Intensive Care. 2012;40(4):598–603. doi:10.1177/0310057X1204000404
26. Minton J, Sidebotham DA. Hyperlactatemia and cardiac surgery. J Extra Corpor Technol. 2017;49(1):9.
27. Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123(1):92–100. doi:10.1172/JCI62874
28. Xia Z, Li H, Irwin MG. Myocardial ischaemia reperfusion injury: the challenge of translating ischaemic and anaesthetic protection from animal models to humans. Br J Anaesth. 2016;117:ii44–ii62. doi:10.1093/bja/aew267
29. Hayiroglu MI, Keskin M, Uzun AO, et al. Predictive value of SYNTAX score II for clinical outcomes in cardiogenic shock underwent primary percutaneous coronary intervention; a pilot study. Int J Cardiovasc Imaging. 2018;34:329–336. doi:10.1007/s10554-017-1241-9
30. Yoon J, Jung J, Ahn Y, Oh J. Systemic immune-inflammation index predicted short-term outcomes in patients undergoing isolated tricuspid valve surgery. J Clin Med. 2021;10(18):4147. doi:10.3390/jcm10184147
31. Alam SR, Stirrat C, Spath N, et al. Myocardial inflammation, injury and infarction during on-pump coronary artery bypass graft surgery. J Cardiothorac Surg. 2017;12(1):115. doi:10.1186/s13019-017-0681-6
32. Krijnen PAJ. Apoptosis in myocardial ischaemia and infarction. J Clin Pathol. 2002;55(11):801–811. doi:10.1136/jcp.55.11.801
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.