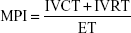Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 11 » Issue 1
Myocardial performance index correlates with the BODE index and affects quality of life in COPD patients
Authors Tannus-Silva D, Silva JB , Ribeiro L, Conde M, Rabahi M
Received 18 April 2016
Accepted for publication 1 August 2016
Published 16 September 2016 Volume 2016:11(1) Pages 2261—2268
DOI https://doi.org/10.2147/COPD.S110779
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Daniela Graner Schuwartz Tannus-Silva,1 João Batista Masson-Silva,1 Lays Silva Ribeiro,1 Marcus Barreto Conde,2,3 Marcelo Fouad Rabahi1
1Faculty of Medicine, Federal University of Goias, Goiânia, Goiás, 2Instituto de Doenças do Tórax da Universidade Federal do Rio de Janeiro, Rio de Janeiro, 3Faculdade de Medicina de Petrópolis, Petrópolis, Rio de Janeiro, Brazil
Background and objective: COPD, a systemic illness associated with the impairment of different organs, affects patient prognosis and quality of life. The aim of this study was to evaluate the association between right ventricle (RV) function, the BODE (body mass index, airflow obstruction, dyspnea, and exercise capacity) index (a multifunctional scale for the assessment of mortality risk), and quality of life in patients with COPD.
Methods: A cross-sectional study was carried out in 107 outpatients presenting with stable COPD who underwent clinical assessment, spirometry, arterial blood gas analyses, a 6-minute walk test, electrocardiography, and echocardiogram and who responded to the Saint George’s Respiratory Questionnaire (SGRQ).
Results: Among the study subjects, 53% (57/107) were males, and the mean age was 65.26±8.81 years. A positive correlation was observed between RV dysfunction measured by the myocardial performance index using tissue Doppler (MPIt) and the BODE index, even after adjustment for age and partial pressure of oxygen (r2=0.47; P<0.01). Patients with alterations in the MPIt had worse quality of life, and a statistically significant difference was found for different domains of the SGRQ. Patients with a normal MPIt had a mean total score of 46.2±18.6, whereas for those with MPIt alterations, the mean total score was 61.6±14.2 (P=0.005). These patients had a 1.49-fold increased risk of exhibiting SGRQ total score above the upper limit of the 95% CI (P=0.01).
Conclusion: The findings of this study suggest that RV dysfunction as measured by the MPIt was associated with impairment in quality of life and a worse BODE index in COPD patients, irrespective of age and hypoxemia status.
Keywords: COPD, right ventricle dysfunction, echocardiography, BODE score, quality of life
Introduction
Over the years, severity assessments and prognostic predictions of patients presenting with COPD have been changing. Since the term COPD was proposed for the first time during the ninth Aspen Emphysema Conference in 1965,1 assessments of disease severity and prognosis have become more complete and complex. In addition to classical variables such as the degree of airflow obstruction2 and presence of hypoxemia,3,4 other parameters have been proven to be important.
Celli et al5 identified certain variables that could predict a high risk of death in patients with COPD: body mass index (BMI), degree of airflow obstruction measured using postbronchodilator forced expiratory volume in 1 second (FEV1), degree of dyspnea evaluated using the modified Medical Research Council dyspnea scale (mMRC), and distance walked during the 6-minute walk test (6MWT). Based on these variables, a multidimensional scale ranging from 0 to 10 was created, the BODE index. The BODE index proved to be superior to FEV1 in predicting the risk of death from any cause in COPD patients. Since then, the importance of the presence of comorbidities in the prognoses of patients presenting with COPD has been proven time and again.6–8 The association between the presence of pulmonary hypertension and mortality in COPD patients has been known for a long time.9 Nonetheless, recent studies have shown that structural and functional alterations in the right heart may start before any increase in pulmonary arterial pressure to levels currently considered pathological. Hilde et al10 evaluated echocardiographic examinations and right-heart catheterizations in COPD patients with and without pulmonary hypertension as well as in healthy controls and found that impaired right ventricle (RV) function, hypertrophy, and dilation were present even with slight increases in mean pulmonary arterial pressure (mPAP; ≤25 mmHg), indicating an early impact on RV structure and function in patients with COPD. In another study using magnetic resonance imaging (MRI), greater RV concentric hypertrophy was observed in COPD patients without hypoxemia compared with that observed in controls without COPD.11
Although recent studies have proven the importance of MRI in RV structural and functional assessments,12 the scarce availability and high cost of this technique make the routine assessment of patients with COPD virtually impossible, which enhances the importance of carefully defining the parameters to be evaluated on echocardiograms. The American Society of Echocardiography guidelines suggest examining the right heart during every examination of adults and using multiple windows to assess qualitative and quantitative parameters. Moreover, the report should include at least one anatomic measure of RV, right atrium (RA), and pulmonary artery systolic pressure (PASP); RV systolic function (fractional area change [FAC], pulsed-wave tissue Doppler peak velocity at the annulus, and tricuspid annular plane systolic excursion [TAPSE] with or without the myocardial performance index [MPI]); and measures of RV diastolic function in some cases.13
Some studies have assessed the impact of echocardiographic alterations of RV on the prognosis of patients with COPD.14–17 Nonetheless, it is still unclear which parameter, among the available structural and functional ones, would be more suitable for this purpose.
Considering that COPD is a systemic disease, all the impacts of other organ and system impairments,18 and the recently described presence of structural and functional alterations in the RVs of patients in the early stages of the disease and without hypoxemia,10,11 it is clear that the influence of impaired RV function on COPD patients’ prognoses and quality of life needs to be better understood.
This study aimed to assess the correlation between RV structural and functional variables and the BODE index (a multifunctional scale to evaluate mortality risk) and to check the association between these variables and quality of life in patients with COPD.
Methods
Patients and study design
Ethical approval for the study was obtained from the local research ethics committee of the Universidade Federal de Goiás (UFG, no 575696), and written informed consent was obtained from all subjects.
This cross-sectional study was carried out from May 6, 2014, to July 20, 2015, on outpatients followed up in the pneumology clinic of the Hospital das Clínicas of the Universidade Federal de Goiás, Goiânia, Goiás, Brazil, and those referred for pulmonary function tests. This pneumology clinic is located in a tertiary hospital, which generally follows up patients presenting with signs of severe COPD. The laboratory of pulmonary function in the same clinic accepts patients who are generally in the early stages of the disease and referred from the basic units of health to undergo spirometry. The inclusion criteria were: age ≥40 years, presence of the diagnostic criteria for COPD according to the Global initiative for chronic Obstructive Lung Disease (GOLD),19 and agreement to participate in the study by signing the informed consent form. The exclusion criteria were patients presenting with clinical instability in the 4 weeks before study inclusion, significant radiographic abnormalities not attributable to COPD, incapacity to complete or respond to the questionnaires presented or to perform the study procedures, arrhythmias, alterations greater than moderate in the mitral, pulmonary, and aortic valves or left ventricular function, ejection fraction <55% according to the Teichholz method, BMI >40 kg/m2, echocardiographic signals of myocardial ischemia, or severe renal failure (glomerular filtration rate <30 mL/min per 1.73 m2). The sample was composed of 107 patients (Figure 1).
  | Figure 1 Study sample. |
Procedures
Anthropometric, epidemiological, and clinical history data were collected, and physical examinations were carried out. Dyspnea was assessed by the mMRC (Table 1), and the patients were asked to respond to the validated version of the Saint George’s Respiratory Questionnaire (SGRQ) in Portuguese.20 Blood samples were collected for creatinine and arterial blood gas analyses. Additionally, spirometry, chest radiography, an electrocardiogram, 6MWT, and echocardiography were performed for all patients. Comorbidities were self-reported, and no confirmatory tests were carried out.
  | Table 1 Modified Medical Research Council Dyspnea Scale (mMRC) |
BODE index
The BODE index was calculated by adding the scores obtained from each of its components: BMI, postbronchodilator FEV1% predicted, mMRC, and distance walked during the 6MWT. The BODE index ranges from 0 to 10, and higher scores are associated with a greater death risk.5
Spirometry
Pre- and postbronchodilator spirometry analyses were performed using a MasterScope PC spirometer (Jaeger; VIASYS Healthcare Inc., Yorba Linda, CA, USA), and predicted values previously reported for the Brazilian population were employed.21
The 6-minute walk test
All patients underwent the 6MWT while supervised by trained physical therapists and following the protocol of the American Thoracic Society.22
Quality of life
Indices of health-related quality of life were obtained using the SGRQ. Three component indices were calculated using empirically derived weightings of the symptom, activity, and impact scores, from which a total score was computed. Scores range from 0 (no disability) to 100 (maximum disability).
Cutoff values above the upper limit of the 95% CI of a previous study23 of COPD patients were taken into consideration for each domain and the total score to categorize the study subjects.
Echocardiography
Echocardiographic examinations were performed by an experienced echocardiographer using a MyLab™30 Gold Cardiovascular system (Esaote North America, Indianapolis, IN, USA), with images stored digitally. The measures obtained corresponded to the mean of three consecutive regular heart beats in sinus rhythm. The assessment of RV systolic function was both qualitative (subjective) and quantitative (Teichholz method). RV linear measures were obtained at the end of the diastole in the parasternal long axis (proximal RV), parasternal short axis (distal RV and pulmonary branches), and apical four chambers (basal, mid, and longitudinal cavity diameter). The measurement of RV wall thickness was obtained through the subcostal window. The linear measurements and the calculation of RA area were obtained through the apical four chamber plane at the end of the ventricular systole. Echocardiographic parameters of RV systolic function were measured in all patients. MPI was measured using pulsed-wave Doppler and the formula:
|
|
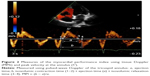
where IVCT, isovolumic contraction time; IVRT, isovolumic relaxation time; and ET, ejection time (Figure 2). FAC was obtained in the apical four chamber plane by tracing RV endocardium both in systole and diastole (FAC = [RV area in diastole - RV area in systole]/RV area in diastole). TAPSE was measured in apical four-chamber view. Peak velocity at the annulus (S′) was measured using tissue Doppler and apical four-chamber window with the sample volume placed at the basal level of the RV free wall. PASP was determined by measuring maximal tricuspid regurgitation velocity (continuous Doppler in the apical four chamber plane), applying the simplified Bernoulli equation, and adding to this value an estimated RA pressure. All the measurements were performed according to the criteria established by the American Society of Echocardiography.13
Statistical analysis
Data were stored in an electronic spreadsheet (Excel® Version 14.4.7; Microsoft, Redmond, WA, USA) and a unique database and analyzed using the IBM Statistical Package for the Social Sciences (SPSS) Version 23.0 (IBM Corporation, Armonk, NY, USA). Quantitative variables are presented as descriptive statistics: the mean, SD, median, minimum, and maximum, whereas qualitative variables of the studied group are presented as the absolute frequency and percentage.
A normality test of the quantitative data was performed using the Kolmogorov–Smirnov test. Parametric variables were analyzed using Student’s t-test and Pearson’s correlation; variables that did not present a normal distribution were analyzed using the Mann–Whitney test (two levels) and/or the Kruskal–Wallis test (more than two levels) and Spearman’s correlation. Furthermore, a multiple ordinal regression was carried out. The chi-squared test was performed to verify the strength of the association between the categorized MPI using tissue Doppler (MPIt) and quality of life. For all statistical analyses, a 5% (P<0.05) level was considered statistically significant.
Results
Table 2 shows that among the 107 study subjects included in the study, 53% were men. Over half of them (51%) presented at least one episode of exacerbation in the year before their inclusion in the study. Regarding comorbidities, 61% of the participants reported having at least one of the following: systemic arterial hypertension, 50%; diabetes, 14%; dyslipidemia, 11%; and osteoporosis, 8%. One patient reported being treated for depression. The functional parameters of the 107 study subjects are also shown in Table 2.
For the assessment of dyspnea, the mMRC was used to classify the patients according to GOLD criteria: 22.4% (24/107) were GOLD A, 18.7% (20/107) were GOLD B, 14% (15/107) were GOLD C, and 44.9% (48/107) were GOLD D. Their classifications according to the BODE index are presented in Figure 3. Median BODE index was 3±3.
  | Figure 3 Distribution of the 107 study subjects according to the BODE index. |
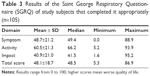
Quality of life was assessed using the SGRQ (Table 3) and valid scores were obtained for 105 patients. Table 4 shows the echocardiographic characteristics of the study subjects. Except for PSAP, the other RV structural and functional parameters were measured in the 107 study subjects. Among the 126 patients assessed in this study, only one was excluded due to the impossibility of performing echocardiographic measurements. Because of a lack of tricuspid insufficiency values, PASP estimates were possible for just 48% (61/107) of the study subjects. The mean LV ejection fraction was 66.8%±3%, and the RVSP was 37.4±20.73 mmHg. A positive correlation was observed between the BODE index and the diameter of the distal RV outflow tract, the MPI using pulsed-wave Doppler (MPIp), and the MPIt (Figure 4). For all other echocardiographic variables, the correlations were not significant (Table 4). Using an ordinal multiple regression model between the BODE index variable and all predictor variables obtained using echocardiography adjusted for age and partial pressure of oxygen, the MPIt maintained a significant value (r2=0.47; P=0.01).
Moreover, after separating the study subjects into two groups according to normal or altered MPIt values, a significant difference was observed in the mean BODE index between the two groups: 5.6±1.8 in patients with an altered MPIt and 2.5±1.8 in those with a normal MPI (P<0.001).
Patients with altered MPIt had the worst quality of life, with significant statistical differences in the activity and impact domains and the total score. The mean total score was 46.2±18.6 for participants with normal MPIt and 61.6±14.2 for those with altered MPIt (P=0.005; Figure 5). Patients presenting with altered MPIt had a 1.67-fold increased risk of exhibiting impact domain values above the upper limit of the 95% CI (P=0.004) and a 1.49-fold increased risk regarding the total score (P=0.01). For the symptom and activity domains, the associations were not significant.
Discussion
The findings of the present study showed a correlation between impaired RV function according to the MPI and the BODE index score. Furthermore, it showed that patients with impaired RV function had worse quality of life than those presenting with normal parameters.
The MPI is an easily measurable parameter, feasible in most patients, and reproducible.13,14 It provides an index of both diastolic and systolic global RV function. It is defined as the relationship between isovolumic time (isovolumic contraction time + isovolumic relaxation time) and ejection time (Figure 2).
The prognostic value of echocardiographic findings regarding RV structure and function in COPD patients has been focused on in some studies. In a longitudinal study, Burgess et al14 evaluated 87 patients with chronic pulmonary diseases (including 18 with COPD) and reported that combined systolic and diastolic function measurements as well as the MPI presented significant prognostic value. The MPI could be measured in almost all study subjects (86/87), similar to our study. In a retrospective study including 49 patients with COPD, a significant correlation was observed between the MPI and mMRC, one of the components of the BODE index. In the same study, the MPI presented correlations with the general survival rate and hospitalization.15
In two other studies, the authors searched for confirmations of associations between echocardiographic alterations and the BODE index. In the first, Cuttica et al16 found an association between RA area, RV wall thickness, and distance walked during the 6MWT, regardless of pulmonary function, sex, age, race, or BMI (P=0.003 and P=0.04, respectively). Each 1 mm change in RV wall thickness was associated with a 43.8 m decline in the distance walked during the 6MWT. In the present study, no significant correlation was found between structural measures of the RV and the 6MWT (data not shown) or the BODE index. Similarly, Cuttica et al16 did not find correlations between variables of RV function (TAPSE and FAC) and the 6MWT or the BODE index. However, different from the present study, they did not assess the MPI. The authors included patients presenting a higher mean age and, despite having included only subjects with an FEV1 >50%, they reported a smaller mean value for the 6MWT compared with the present study, which could account for the differences between the findings of the studies.
In the second study conducted by Gökdeniz et al,17 which included 135 patients with COPD and 37 controls not presenting with any respiratory disease, the authors observed impairment of RV functional parameters in patients presenting with higher BODE index scores. They also found significant differences in TAPSE, systolic myocardial velocity, FAC, MPI, and RV-free wall strain between groups divided into quartiles according to the BODE index, as well as significant correlations between two of the variables that compose the BODE index (FEV1 and 6MWT) and all the RV functional variables. In our study, the MPI was the only functional parameter that presented a significant correlation with the BODE index and its components. Most subjects in the study conducted by Gökdeniz et al17 were male (92.5%), different from our study, and it also included a higher number of patients presenting a higher BODE index, which could imply they were sicker and explain the differences in the results of the studies.
In a broad recent prospective study, which encompassed 777 patients presenting other forms of pulmonary hypertension, the higher prognostic value of the MPI was confirmed among all assessed echocardiographic RV function parameters (hazard ratio, 3.42; 95% CI, 1.777–6.584; P<0.001).24
MPI evaluates global cardiac function (systolic + diastolic), different from the other assessed parameters that measure systolic function (FAC, TAPSE, S′). RV concentric hypertrophy precedes systolic dysfunction.11 In some recent studies, the correlation between diastolic function and exercise capacity, measured by 6mWT, one of the components of the BODE index, has been demonstrated.25,26 These findings may explain why only MPI and not the other functional variables were correlated with the BODE index in our study, which included patients presenting with all stages of COPD.
Thus far, to the best of our knowledge, no study has been conducted to evaluate the impact of changes in RV structure and function on the quality of life of COPD patients as assessed using specific questionnaires. It has already been shown that even patients with mild airflow obstruction can have worse quality of life scores than normal individuals27,28 and that the presence of comorbidities can be related.28,29
Other variables such as obstruction severity,30 degree of dyspnea,31 and exacerbations32 have been reported as independent factors associated with quality of life. In this study, the SGRQ was used, and statistically significant differences were found in the total score and other domains when comparing groups of patients with or without altered MPIs, which is the same variable that was correlated with the BODE index. Study subjects with altered MPIs had higher scores, which indicate a more compromised quality of life compared with those with normal MPIs. In this study, a significant association was observed between the MPI and quality of life regarding the impact domain and the total score, which mostly reflect cardiac conditions, since they assess activities affected by the disease as well as its psychosocial impact. The lack of association with the symptom domain was expected, because the questions are intended to mainly evaluate bronchial symptoms. For the activity domain, the small number of patients presenting altered values together with the small number of questions in this domain might not have been sufficient to show statistical significance. This finding suggests an impaired quality of life in patients presenting with altered RV function.
Limitations
Although the total number of patients included in this study was relatively small compared with the number of all patients with COPD, since they were consecutive patients scheduled for appointments or spirometry, this might have resulted in good representativeness of the patient population of both a tertiary hospital and of a primary health care unit. Nevertheless, the small proportion of patients presenting with BODE index scores >5 may have hindered a stronger correlation between this index and echocardiographic variables. The lack of comparisons using MRI, considered the gold standard for assessing the RV ejection fraction, may also be considered a limitation of this study. However, the objective of the present study was to assess the correlation between RV structural and functional variables and the BODE index in a feasible and easily reproducible way, even in small municipalities with scarce resources.
Conclusion
RV functional alteration, appropriately measured in most patients using the MPI, was a predictor of a worse BODE index in COPD patients, regardless of age and degree of hypoxemia, and a compromised quality of life. These findings corroborate the importance of a better understanding of echocardiographic parameters that reflect RV function, and especially the MPI, to better treat patients with COPD.
Acknowledgment
This research was supported by the Fundação de Amparo à Pesquisa do Estado de Goiás (FAPEG, process number: 201210267001130).
Author contributions
Daniela GS Tannus-Silva had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, and contributed to the study conception, data gathering, analysis and interpretation of the data, and revision of the manuscript. João BM Silva performed the echocardiograms and contributed to data gathering and revision of the manuscript. Lays S Ribeiro contributed to data gathering, interpretation of the data, and revision of the manuscript. Marcus B Conde contributed to the study conception, interpretation of the data, and revision of the manuscript. Marcelo F Rabahi contributed to the study conception, analysis, and interpretation of the data, and revision of the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Petty TL. The history of COPD. Int J Chron Obstruct Pulmon Dis. 2006;1(1):3–14. | ||
Renzetti AD Jr, McClement JH, Litt BD. The Veterans Administration cooperative study of pulmonary function: III. Mortality in relation to respiratory function in chronic obstructive pulmonary disease. Am J Med. 1966;41(1):115–129. | ||
Boushy SF, Thompson HK Jr, North LB, Beale AR, Snow TR. Prognosis in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1973;108(6):1373–1383. | ||
Kim V, Benditt JO, Wise RA, Sharafkhaneh A. Oxygen therapy in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):513–518. | ||
Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. | ||
Cilli A, Uslu A, Oğüş C, Ozdemir T. KOAH’da Komorbiditenin Prognoza Etkisi. [The effect of comorbidity on prognosis in patients with COPD]. Tuberk Toraks. 2004;52(1):52–55. [Turkish]. | ||
Marti S, Muñoz X, Rios J, Morell F, Ferrer J. Body weight and comorbidity predict mortality in COPD patients treated with oxygen therapy. Eur Respir J. 2006;27(4):689–696. | ||
Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962–969. | ||
Weitzenblum E, Hirth C, Ducolone A, Mirhom R, Rasaholinjanahary J, Ehrhart M. Prognostic value of pulmonary artery pressure in chronic obstructive pulmonary disease. Thorax. 1981;36(10):752–758. | ||
Hilde JM, Skjørten I, Grøtta OJ, et al. Right ventricular dysfunction and remodeling in chronic obstructive pulmonary disease without pulmonary hypertension. J Am Coll Cardiol. 2013;62(12):1103–1111. | ||
Vonk-Noordegraaf A, Marcus JT, Holverda S, Roseboom B, Postmus PE. Early changes of cardiac structure and function in COPD patients with mild hypoxemia. Chest. 2005;127(6):1898–1903. | ||
Tadic M. Multimodality evaluation of the right ventricle: an updated review. Clin Cardiol. 2015;38(12):770–776. | ||
Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. | ||
Burgess MI, Mogulkoc N, Bright-Thomas RJ, Bishop P, Egan JJ, Ray SG. Comparison of echocardiographic markers of right ventricular function in determining prognosis in chronic pulmonary disease. J Am Soc Echocardiogr. 2002;15(6):633–639. | ||
Tanaka Y, Hino M, Mizuno K, Gemma A. Evaluation of right ventricular function in patients with COPD. Respir Care. 2013;58(5):816–823. | ||
Cuttica MJ, Shah SJ, Rosenberg SR, et al. Right heart structural changes are independently associated with exercise capacity in non-severe COPD. PLoS One. 2011;6(12):e29069. | ||
Gökdeniz T, Kalaycioğlu E, Boyaci F, et al. The BODE index, a multidimensional grading system, reflects impairment of right ventricle functions in patients with chronic obstructive pulmonary disease: a speckle-tracking study. Respiration. 2014;88(3):223–233. | ||
Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33(5):1165–1185. | ||
Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016. Available from: http://goldcopd.org/. Accessed February 25, 2016. | ||
Sousa TC, Jardim JR, Jones P. Validação do Questionário do Hospital Saint George na Doença Respiratória (SGRQ) em pacientes portadores de doença pulmonar obstrutiva crônica no Brasil [Validation of the Saint George’s Respiratory Questionnaire in patients with chronic obstructive pulmonary disease in Brazil]. J Pneumologia. 2000;26(3):119–128. [Portuguese]. | ||
Pereira CAC, Sato T, Rodrigues SC. Novos valores de referência para espirometria forçada em brasileiros adultos de raça branca [New reference values for forced spirometry in white adults in Brazil]. J Bras Pneumol. 2007;33(4):397–406. [Portuguese]. | ||
ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS Statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. | ||
Leiva-Fernández J, Leiva-Fernández F, García-Ruiz A, Prados-Torres D, Barnestein-Fonseca P. Efficacy of a multifactorial intervention on therapeutic adherence in patients with chronic obstructive pulmonary disease (COPD): a randomized controlled trial. BMC Pulm Med. 2014;14:70. | ||
Grapsa J, Pereira Nunes MC, Tan TC, et al. Echocardiographic and hemodynamic predictors of survival in precapillary pulmonary hypertension seven-year follow-up. Circ Cardiovasc Imaging. 2015;8(6):e002107. | ||
Faludi R, Hajdu M, Vértes V, et al. Diastolic dysfunction is a contributing factor to exercise intolerance in COPD. COPD. 2016;13(3):345–351. | ||
Fenster BE, Holm KE, Weinberger HD, et al. Right ventricular diastolic function and exercise capacity in COPD. Respir Med. 2015;109(10):1287–1292. | ||
Nonato NL, Díaz O, Nascimento OA, Dreyse J, Jardim JR, Lisboa C. Behavior of quality of life (SGRQ) in COPD patients according to BODE scores. Arch Bronconeumol. 2015;51(7):315–321. [English, Spanish]. | ||
Ferrer M, Alonso J, Morera J, et al. Chronic obstructive pulmonary disease stage and health-related quality of life. The Quality of Life of Chronic Obstructive Pulmonary Disease Study Group. Ann Intern Med. 1997;127(12):1072–1079. | ||
Huber MB, Wacker ME, Vogelmeier CF, Leidl R. Comorbid influences on generic health-related quality of life in COPD: a systematic review. PLoS One. 2015;10(7):e0132670. | ||
Ståhl E, Lindberg A, Jansson SA, et al. Health-related quality of life is related to COPD disease severity. Health Qual Life Outcomes. 2005;3:56. | ||
Hajiro T, Nishimura K, Tsukino M, Ikeda A, Oga T, Izumi T. A comparison of the level of dyspnea vs disease severity in indicating the health-related quality of life of patients with COPD. Chest. 1999;116(6):1632–1637. | ||
Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 pt 1):1418–1422. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.