Back to Journals » Journal of Inflammation Research » Volume 12
Myelin basic protein charge isomers change macrophage polarization
Authors Tsitsilashvili E, Sepashvili M, Chikviladze M, Shanshiashvili L, Mikeladze D
Received 3 October 2018
Accepted for publication 14 December 2018
Published 23 January 2019 Volume 2019:12 Pages 25—33
DOI https://doi.org/10.2147/JIR.S189570
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Elene Tsitsilashvili,1 Maia Sepashvili,1,2 Marika Chikviladze,1 Lali Shanshiashvili,1,2,* David Mikeladze1,2,*
1Institute of Chemical Biology, Ilia State University, Tbilisi, Georgia; 2Department of Biochemistry, I. Beritashvili Center of Experimental Biomedicine, Tbilisi, Georgia
*These authors contributed equally to this work
Purpose: During a neuronal injury, a variety of immune cells infiltrate into the local microenvironment at the demyelination site. After the destruction of the intact myelin sheath, its major constituent myelin basic protein (MBP) dissociates from the plasma membrane and acts as a free ligand on the infiltrated immune cells. MBP exhibits charge microheterogeneity as a result of post-translational modifications, but the effect of various isomers of MBP on the activity of macrophages is not known.
Materials and methods: MBP was isolated and purified from bovine brain white matter. RAW 264.7 macrophages were cultured in DMEM supplemented with heat-inactivated fetal bovine serum. For evaluation of macrophage polarization following treatment of RAW 264.7 cells with MBP charge isomers, inducible nitric oxide synthase (iNOS) expression (M1 phenotype marker) and arginase-1 expression (M2 phenotype marker) were determined in cell lysates by ELISA. To assess Rac activity, G-LISA Rac Activation Assay system was used. The expression of receptor for advanced glycation end-products (RAGE) and high mobility group box 1 (HMGB1) protein were assayed by Western blot analysis.
Results: Our results have shown that minimally modified C1 component of MBP increases the expression of arginase-1 in cells, decreases the expression of iNOS, does not change the secretion of HMGB1 protein, but significantly elevates surface expression of RAGE, and in parallel, increases the activity of small GTPase Rac. On the other hand, highly modified deiminated isomer C8-MBP increases the secretion of HMGB1 protein but does not change the expression of arginase-1 or the content of RAGE.
Conclusion: These data indicate that deiminated C8 isomer of MBP tends to polarize RAW macrophages into M1 phenotypes, whereas C1 enhances the activity of M2 phenotype markers.
Keywords: Arginase-1, iNOS, HMGB1, RAGE
Introduction
Myelin basic protein (MBP), a major protein of the myelin sheath, is encoded by the Golli (genes of oligodendrocyte lineage)-MBP gene in myelinating glia and immune cells.1 Several size isoforms of classic MBP are formed by differential splicing of a single mRNA transcript. The major MBP isoform in the adult human and bovine CNS is 18.5 kDa protein that plays a structural role in maintaining myelin stability. MBP exhibits charge microheterogeneity as a result of post-translational modifications such as phosphorylation, deamidation, deimination, arginine methylation, and N-terminal acylation.2 The fractions of MBP isomers which are isolated by cation-exchange chromatography on a carboxymethyl cellulose-52 (CM-52) column3 have been named from C1 to C8. C1 is the least modified and most cationic, whereas C8 is the most modified and the least cationic.4 Among these modifications, deimination is the most significant that involves the conversion of MBP arginine into citrulline by the enzyme peptidylarginine deiminase. This modification reduces the cationicity of the protein.5 Citrullinated MBP is structurally less ordered and more susceptible to proteolytic attacks. Thus, the reduction in cationicity of citrullinated MBP impedes the membrane assembly and exposes an immunodominant epitope of the membrane-bound protein to proteases.3 It’s important that the degree of deimination limits MBP’s ability to maintain compact myelin and correlates with the severity of multiple sclerosis.6
A variety of immune cell types infiltrate into the local microenvironment at the demyelination site during a neuronal injury.7 After the destruction of the intact myelin sheath, MBP dissociates from the plasma membrane and acts in a free, membrane-unbound manner in the extracellular matrix.1 MBP is well known to be a nonspecific ligand forming weak contacts with many cellular proteins. This protein changes the shape of platelets,8 interrupts artificial membrane and acidic lipid vesicles,9,10 stimulates proliferation of astrocytes and Schwann cells,10–12 and depolarizes the neuronal membrane.13 Recent investigation has shown that MBP is a potent and specific ligand for the αMβ2-integrin (Mac 1, CD11b/CD18).14 This αMβ2-integrin is expressed predominantly in myeloid cells and mediates adhesive reactions of leukocytes during the inflammatory response.15 It is considered that αMβ2-integrin is involved in a wide range of cellular processes that are associated with cytoskeletal remodeling and with phagocytosis. In this case, signaling to the actin cytoskeleton includes either RhoA or Rac1.16 MBP through activating these signaling systems play a key role in inflammation and plasticity of macrophages.14
Classically activated pro-inflammatory (M1) and alternatively activated anti-inflammatory (M2) macrophages populate the local microenvironment after neuronal injury. It has been shown that poor axonal regeneration associated with decreased arginase-1 (M2 phenotype marker) and increased inducible nitric oxide synthase (iNOS) (M1 phenotype marker),17 suggesting that the pro-inflammatory M1 phenotype is neurotoxic while the M2 has positive effects on neuro-regeneration and is less toxic. In addition, while the M1 macrophage response is rapidly induced and sustained, M2 induction is transient. A promising strategy for the repair of neuronal injury and remyelination is to increase the fraction of M2 cells and prolong their residence time. Nevertheless, the effect of various forms of MBP on the plasticity of macrophages is not known. Thus, the purpose of this study was to elucidate the effects of various forms of charge isomers of MBP on the polarization of macrophages.
Materials and methods
Protein isolation and purification
MBP was isolated and purified from bovine brain white matter according to the method of Chou et al29 followed by high-performance liquid chromatography (HPLC) analysis. Briefly, the acid-soluble material was dissolved in urea-glycine buffer, pH 9.6, and applied on a CM-52 cellulose (Sigma-Aldrich Co., St Louis, MO, USA) cation-exchange column, equilibrated in the urea-glycine buffer, pH 10.5. The first unbound fraction was the least cationic isomer, MBP C8. The charge isomers C5, C4, C3, C2, and C1 were eluted from the column with a sodium chloride gradient (0–0.3M) in the glycine-urea buffer, pH 10.6. The most cationic and least modified charge isomer was C1. Further purification of C8 and C1 was achieved by HPLC (Agilent 1260 infinity; Agilent, Santa Clara, CA, USA) on a C-18 reverse phase column (Nova Pak, Waters, IL, USA) using trifluoroacetic acid (0.05%)-acetonitrile (0%–60%; Sigma-Aldrich Co.) solvent system.3 PAGE was used to verify the purity of the isomers. The purified proteins were lyophilized and stored until use at –20°C.
Cell culture
Mouse RAW 264.7 macrophages were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in plastic cell culture flasks (Greiner Bio One, Frickenhausen, Germany), at 37°C under 5% CO2/95% air in DMEM (ATCC) supplemented with 10% (v/v) heat-inactivated FBS (Sigma-Aldrich Co.), 100 U/mL penicillin (Thermo Fisher Scientific, Waltham, MA, USA), and 100 µg/mL streptomycin (Thermo Fisher Scientific). RAW 264.7 macrophages were used between 5 and 15 passage.
Macrophage polarization in the presence of MBP charge isomers
RAW 264.7 cells (5–105 cells per well) were incubated for 24 hours in the absence and presence of 0.5 µM MBP C8 or MBP C1 isomers. In other series of experiments, RAW 264.7 cells were treated with cytokines to induce either M1 or M2 cell state with or without 0.5 µM MBP charge isomers. For M1 polarization, cells were treated for 24 hours with and without 0.5 µM MBP charge isomers in the presence of 20 ng/mL of interferon-gamma (IFN-γ) (ab123747; Abcam, Cambridge, UK) and 100 ng/mL of lipopolysaccharide (LPS) (L2880-100MG; Sigma-Aldrich Co.); for M2 polarization, in the presence of 20 ng/mL of IL-4 (ab191628; Abcam) and 10 ng/mL of IL-10 (BMS347; eBioscience, Vienna, Austria). Cell viability was assessed by staining the cells with Trypan blue (#1450021; Bio-Rad, Hercules, CA, USA) using an automated Cell Counter TC 20TM (Bio-Rad). The cells were then harvested using Cell Scraper (C6106-100EA; Greiner Bio One, Frickenhausen Germany), and the harvested cells were maintained in growth medium and used for further analysis.
Western blot analysis
Following incubation, cells were removed from the dishes, washed with PBS, and homogenized in ice-cold lysis buffer containing 100 mM NaCl, 1 mM EDTA, 0.5% Triton X100, 50 mM Tris-HCl, pH 7.4, and protease inhibitors 1 mM PMSF, 5 mg/mL aprotinin, 5 mg/mL pepstatin A, and 5 mg/mL leupeptin. Lysates were incubated at 4°C for 30 minutes followed by centrifugation at 13,000× g for 15 minutes. Fifty micrograms of proteins were denatured at 90°C for 5 minutes, separated by SDS-PAGE on 15% gels. After electrophoresis, the proteins were transferred onto a nitrocellulose membrane (UltraCruzTM Nitrocellulose Pure Transfer Membrane; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). After blocking with 5% BSA and 0.05% Tween 20 in Tris-HCl buffered saline, the membranes were incubated with the corresponding primary antibodies (anti-RAGE [ab172473; Abcam] and anti-HMGB1 [sc-26351; Santa-Cruz Biotechnology]), and immunoreactivity was detected by enhanced chemiluminescence autoradiography (ECL kit, sc-2048; Santa-Cruz Biotechnology). Protein concentrations were determined using a BCA protein assay kit (sc-202389; Santa Cruz Biotechnology).
Inducible nitric oxide synthase and arginase-1 expression assays
To determine M1 or M2 polarization in RAW 264.7 macrophages following treatment with IFN-γ/LPS and IL-4/IL-10, assays to assess increased iNOS expression, indicative of M1 polarization, and increased arginase-1 expression, indicative of M2 polarization, were performed.
To assess iNOS expression, an inducible Nitric Oxide Synthase kit (cat no MBS723353; MyBioSource, San Diego, CA, USA) was used. The analysis was carried out according to the manufacturer’s instructions.
For assessment of arginase-1 expression, an arginase-1 expression Sandwich ELISA Kit (cat no LS-F6864; LifeSpan BioSciences, Seattle, WA, USA) was used. The assay was performed according to the manufacturer’s instructions.
Rac activation assay
To assess Rac activation in cell lysates, G-LISA Rac 1/2/3 Activation Assay Kit (cat no BK125; Cytoskeleton, Denver, CO, USA,) was used according to manufacturer’s instructions.
Statistical analysis
All data are presented as mean ± SEM. Statistical analysis was performed by one-way ANOVA followed by Scheffe’s post hoc comparison test. P<0.05 was considered statistically significant.
Results
MBP was isolated from bovine brain white matter. For the separation of MBP isomers, the acid-soluble material was dissolved in 0.08 M urea-glycine buffer (pH 9.6) containing 6M urea and applied to a CM-52 cellulose cation-exchange column, equilibrated with similar urea-glycine buffer (pH 10.5). Following application of the sample, the passage of the pH 10.5 buffer continued until the first peak was completely eluted. This unbound fraction was the least cationic isomer, ie, citrullinated MBP C8. Other charge isomers were eluted from the column with a sodium chloride gradient (0–0.3M) in the glycine-urea buffer, pH 10.6. The most cationic and least modified charge isomer C1 was the last one to be eluted from the column. For verification of fraction purity, PAGE was used (Figure 1).
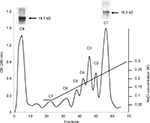  | Figure 1 Chromatography of MBP acid-soluble material on the CM-52 cellulose cation-exchange column and PAGE results. Abbreviations: CM-52, carboxymethyl cellulose-52; MBP, myelin basic protein. |
The effects of MBP charge isomers C1 and C8 on the expression of iNOS and arginase-1 in the RAW 264.7 macrophages
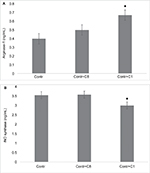
Two natural charge isomers of the classic 18.5 kDa MBP (C1 and C8) were used in these experiments. The most modified and least cationic C8 component corresponds to the protein preparation that does not bind to the CM-52 column. To determine the effects of MBP isomers on the polarization of macrophages, RAW 264.7 cells were incubated with most modified and least cationic (C8) and least modified and most cationic (C1) isomers of MBP. After 24 hours of incubation of RAW macrophages with MBP isomers, the arginase-1 and iNOS expressions were determined in cell lysates. We found that in the presence of C1 the expression of arginase-1 significantly increased (Figure 2A), whereas the expression of iNOS was slightly decreased (Figure 2B). C8 isomer does not change arginase-1 and iNOS expressions.
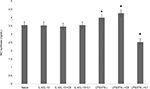
To study the effects of MBP isomers on the polarization of macrophages, in the next series of experiments, the isomers were co-incubated with various types of cytokines: IFN-γ/LPS (predominantly M1 phenotype polarization) or IL-4/IL-10 (predominantly M2 phenotype polarization). We have found that C1 decreased the expression of arginase-1 in M1 phenotype as well as in M2 phenotype (Figure 3). C1 significantly decreased the expression of iNOS in M1 phenotypes, but did not affect the expression of iNOS in M2 macrophages (Figure 4). MBP C8 isomer did not significantly change the arginase-1 expression in the differentiated macrophages (Figure 3), but iNOS expression was decreased in M2 phenotype (Figure 4).
The effects of MBP charge isomers C1 and C8 on the expression of HMGB1 and RAGE in control non-polarized RAW 264.7 macrophages
High mobility group box 1 is an important inflammatory factor involved in cell differentiation, migration, tumor metastasis, and autoimmune diseases.18 Myeloid cells secrete HMGB1 once they are activated by the presence of pathogens, by the detection of injured cells, or by cytokines. On the other hand, HMGB1 itself through TLR4-PI3Kγ-Erk1/2 pathway facilitates macrophage reprogramming toward a pro-inflammatory M1-like phenotype.19 To study the effects of MBP isomers on the synthesis of HMGB1 proteins, we determined the amount of these proteins in cell lysates by Western blotting. We have found that only C8 isomer increases the expression of HMGB1 (Figure 5A, B). Elevation in the amount of this protein may indicate that the MBP C8 can differentiate macrophages into M1 phenotype.
Receptor for advanced glycation end-products (RAGE) is the major target for the pro-inflammatory activity of HMGB1 in macrophages.20 This receptor enhances macrophage differentiation into pro-inflammatory M1 phenotype at least partly via NF-kB pathway.21 Thus, in the next series of experiments, the expression of RAGE in the macrophages was determined after treatment of cells with C8 and C1. Surprisingly, only C1 isomer of MBP increased the expression of RAGE, while C8 isomer had no effect (Figure 5C, D). According to these data, probably the effect of C8 does not involve alterations in the expression of RAGE and only C1 could act by a RAGE-dependent mechanism.22
The effects of MBP charge isomers C1 and C8 on Rac activation in control non-polarized RAW 264.7 macrophages
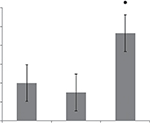
There is some evidence that small GTPase Rac controls the polarization of M2 macrophages.23,24 Signals transmitted from the extracellular matrix via the α4β1-integrin lead to the activation of Rac which regulates alternative macrophage differentiation into M2 phenotype. To study the role of Rac proteins in the effects of MBP charge isomers, the levels of active Rac were determined in the lysates of RAW 264.7 macrophages. We have found that the content of active Rac was higher in the RAW 264.7 macrophages after treatment of cells with MBP C1 isomer (Figure 6).
Discussion
The major adult classic 18.5 kDa MBP isoform is positively charged and exists as some charge components due to various post-translational modifications which reduce their net positive charge, including deamidation, Ser/Thr phosphorylation, and deimination of arginine to citrulline.25,26 The charge components are named C1 to C8 according to their elution pattern from a cation-exchange column. In this work, we compared the effect of two charge isomers, C1 and C8 of the classic 18.5 kDa MBP, on the expression of phenotype markers of RAW 264.7 macrophages. Component C1 is the most cationic and the least modified, whereas C8 is the least charged and most modified variant. C8 is a major deiminated isomer of MBP that differs from C1 by deimination of 6–11 arginines to citrulline.3 Deimination disrupts the secondary structure of MBP, as shown by circular dichroism spectroscopy and molecular dynamics simulation.6 Deiminated MBP is structurally less ordered and more susceptible to proteolytic attack than the native form.27 Importantly, C8/C1 ratio increases several-fold in individuals with chronic multiple sclerosis, suggesting that deimination of MBP is linked to the demyelinating diseases.6,26
MBP can interact with a variety of intracellular and surface exposed binding partners including MARCKS, KRas, 4B, Src, tubulin, clathrin, calmodulin, actin, etc.1 Recent investigation has shown that MBP is a potent and specific ligand for the αMβ2-integrin (Mac 1, CD11b/CD18).14 Mac 1 is expressed predominantly in myeloid cells and mediates adhesive reactions of leukocytes during the inflammatory response.15 It has been found that the least folded regions of MBP have the highest affinity to this integrin.14 Since the affinity of the MBP to the Mac 1 depends on the secondary/tertiary structure of the MBP, we hypothesized that various charge isomers of this protein might act differentially on the macrophage activity.
We have found that minimally modified C1 component of MBP increases the expression of arginase-1, decreases the expression of iNOS, does not change the expression of HMGB1 protein, but significantly elevates the expression of RAGE and increases the activity of Rac1. On the other hand, highly modified deiminated isomer (C8) increases the content of HMGB1 proteins but does not change the expression of arginase-1 or the content of RAGE. These data indicate that C8 and C1 isomers act differently with the corresponding binding partners (presumably with the αMβ2- integrin) of RAW 264.7 macrophages. MBP C8 isomer tends to polarize RAW 264.7 macrophages into M1 phenotypes, whereas C1 isomer of MBP does not have such activity, and vice versa, enhances the expression of M2 phenotype markers. Besides, C1 isomer induces Rac activation which can also be mediated by αMβ2-integrin receptor.23 This small GTPase controls the differentiation of macrophage M1 to M2 and the metastatic phenotype formation in vivo. Thus, MBP isomers, depending on the intensity of the post-translational modification, in particular from deimination, can participate both in the induction of inflammatory potential of macrophages and in the differentiation of the immunosuppressive phenotype.
Conclusion
A variety of immune cell types infiltrate into the local microenvironment at the demyelination site during a neuronal injury.7 Active demyelination lesions are characterized by infiltration of MBP-positive macrophages28 and by the release of MBP in the demyelinating area. After the destruction of the intact myelin sheath, MBP dissociates from the plasma membrane and acts in a free, membrane-unbound manner in the extracellular matrix.1 We conclude that the prevalence of the highly deiminated phenotype of MBP, like C8 in the microenvironment at the demyelination site, can accelerate the process of myelin destruction, and conversely, an increase in the amount of C1 can favorably act on the processes of repair and remyelination.
Acknowledgment
This research was supported by the SRNSF Georgia RF17_534 grant.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Boggs JM. Myelin basic protein: a multifunctional protein. Cell Mol Life Sci. 2006;63(17):1945–1961. | ||
Zand R, Jin X, Kim J, Wall DB, Gould R, Lubman DM. Studies of posttranslational modifications in spiny dogfish myelin basic protein. Neurochem Res. 2001;26(5):539–547. | ||
Wood DD, Moscarello MA. The isolation, characterization, and lipid-aggregating properties of a citrulline containing myelin basic protein. J. Biol Chem. 1989;264:5121–5127. | ||
Fannon AM, Moscarello MA. Characterization of myelin basic protein charge isomers from adult mouse brain. Neuro report. 1991;2(3):135–138. | ||
Musse AA, Li Z, Ackerley CA, et al. Peptidylarginine deiminase 2 (PAD2) overexpression in transgenic mice leads to myelin loss in the central nervous system. Dis Model Mech. 2008;1(4–5):229–240. | ||
Harauz G, Musse AA. A tale of two citrullines – structural and functional aspects of myelin basic protein deimination in health and disease. Neurochem Res. 2007;32(2):137–158. | ||
Plemel JR, Wee Yong V, Stirling DP. Immune modulatory therapies for spinal cord injury – past, present and future. Exp Neurol. 2014;258:91–104. | ||
Laubscher A, Pletscher A, Honegger CG, Richards JG, Colombo V. Shape change of blood platelets induced by myelin basic protein. Experientia. 1979;35(8):1081–1083. | ||
Jo E, Boggs JM. Aggregation of acidic lipid vesicles by myelin basic protein: dependence on potassium concentration. Biochemistry. 1995;34(41):13705–13716. | ||
Shanshiashvili LV, Suknidze Nch, Machaidze GG, Mikeladze DG, Ramsden JJ. Adhesion and clustering of charge isomers of myelin basic protein at model myelin membranes. Arch Biochem Biophys. 2003;419(2):170–177. | ||
South SA, Deibler GE, Tzeng SF, et al. Myelin basic protein (MBP) and MBP peptides are mitogens for cultured astrocytes. Glia. 2000;29(1):81–90. | ||
Tzeng SF, Deibler GE, Devries GH. Myelin basic protein and myelin basic protein peptides induce the proliferation of Schwann cells via ganglioside GM1 and the FGF receptor. Neurochem Res. 1999;24(2):255–260. | ||
Gähwiler BH, Honegger CG. Myelin basic protein depolarizes neuronal membranes. Neurosci Lett. 1979;11(3):317–321. | ||
Stapulionis R, Oliveira CL, Gjelstrup MC, et al. Structural insight into the function of myelin basic protein as a ligand for integrin alpha M beta 2. J Immunol. 2008;180(6):3946–3956. | ||
Prince JE, Brayton CF, Fossett MC, et al. The differential roles of LFA-1 and Mac-1 in host defense against systemic infection with Streptococcus pneumoniae. J Immunol. 2001;166(12):7362–7369. | ||
Dupuy AG, Caron E. Integrin-dependent phagocytosis: spreading from microadhesion to new concepts. J Cell Sci. 2008;121(11):1773–1783. | ||
Lloyd AF, Miron VE. Cellular and molecular mechanisms underpinning macrophage activation during remyelination. Front Cell Dev Biol. 2016;4(Pt 2):60. | ||
Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;8(4):195–202. | ||
Su Z, Zhang P, Yu Y, et al. HMGB1 facilitated macrophage reprogramming towards a proinflammatory M1-like phenotype in experimental autoimmune myocarditis development. Sci Rep. 2016;6(1):21884. | ||
Kokkola R, Andersson A, Mullins G, et al. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol. 2005;61(1):1–9. | ||
Jin X, Yao T, Zhou Zhong’e, Tongqing Y, Zhong’e Z. Advanced glycation end products enhance macrophages polarization into M1 phenotype through activating RAGE/NF-κB pathway. Bio Med Research International. 2015;2015(5, article 579):1–12. | ||
Rojas A, Delgado-López F, Perez-Castro R, et al. HMGB1 enhances the protumoral activities of M2 macrophages by a RAGE-dependent mechanism. Tumour Biol. 2016;37(3):3321–3329. | ||
Joshi S, Singh AR, Zulcic M, et al. Rac2 controls tumor growth, metastasis and M1-M2 macrophage differentiation in vivo. PLoS One. 2014;9(4):e95893. | ||
Joshi S, Singh AR, Wong SS, et al. Rac2 is required for alternative macrophage activation and bleomycin induced pulmonary fibrosis; a macrophage autonomous phenotype. Plos One. 2017;12(8):e0182851. | ||
Harauz G, Ishiyama N, Hill CMD, Bates IR, Libich DS, Farès C. Myelin basic protein –diverse conformational states of an intrinsically unstructured protein and its roles in myelin assembly and multiple sclerosis. Micron. 2004;35(7):503–542. | ||
Harauz G, Libich DS. The classic basic protein of myelin – conserved structural motifs and the dynamic molecular barcode involved in membrane adhesion and protein-protein interactions. Curr Protein Pept Sci. 2009;10(3):196–215. | ||
Pritzker LB, Joshi S, Gowan JJ, Harauz G, Moscarello MA. Deimination of myelin basic protein. 1. Effect of deimination of arginyl residues of myelin basic protein on its structure and susceptibility to digestion by cathepsin D. Biochemistry. 2000;39(18):5374–5381. | ||
Kuhlmann T, Lingfeld G, Bitsch A, Schuchardt J, Bruck W. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain. 2002;125(10):2202–2212. | ||
Chou FC, Chou CH, Shapira R, Kibler RF. Basis of microheterogeneity of myelin basic protein. J Biol Chem. 1976;251:2671–2679. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.