Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Multiple Lesions at Different Stages of Pyoderma Gangrenosum in a Crohn’s Disease Patient
Authors Zhang H , Sun Y, Li K, Zhang J, Chen X
Received 21 May 2022
Accepted for publication 29 July 2022
Published 9 August 2022 Volume 2022:15 Pages 1593—1596
DOI https://doi.org/10.2147/CCID.S374973
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Heng Zhang, Yifang Sun, Kun Li, Jianzhong Zhang, Xue Chen
Department of Dermatology, Peking University People’s Hospital, Beijing, 100044, People’s Republic of China
Correspondence: Xue Chen, Department of Dermatology, Peking University People’s Hospital, # 11. Xizhimen South St, Beijing, 100044, People’s Republic of China, Tel +86 10 88325472, Fax +86 10 88325474, Email [email protected]
Abstract: Pyoderma gangrenosum (PG) is an inflammatory dermatosis characterized by the rapid progression of a painful, necrolytic ulcer with an irregular and undermined border. The prevalence of PG in patients with Crohn’s disease (CD) has been estimated to be 0.7%. Here, we report a case that presented various painful skin lesions, including erythema, vesicles, plaques, and ulcers, one week before the fourth infliximab infusion for CD. PG was diagnosed and the lesions subsided after a 390-mg ustekinumab infusion for one month. It suggests that different lesions of PG may occur concomitantly in CD patients, and the therapy should be re-evaluated on time.
Keywords: pyoderma gangrenosum, Crohn’s disease, cutaneous manifestation
Introduction
Pyoderma gangrenosum (PG) was first described in 1908 as a “geometric phagedena” (phagédénisme géométrique) and was later redefined as PG in 1930.1 It is an inflammatory dermatosis characterized by the rapid progression of a painful, necrolytic ulcer with an irregular, undermined border. The early stages of pyoderma gangrenosum may present as pustules and nodules that may progress to ulcers. The pathogenesis of PG remains unclear. Pathophysiology is believed to be related to the exaggerated recruitment of neutrophils to the dermis, which is a typical pathological feature of PG. PG is usually a diagnosis of exclusion; however, recently diagnostic criteria for PG have been proposed.1
PG is associated with systemic disorders, such as rheumatoid arthritis, hematological malignancies, and inflammatory bowel disease (IBD) in about half of the cases.2,3 Almost one-third of patients with PG have an underlying IBD and the prevalence of PG in patients with Crohn’s disease (CD) has been estimated to be 0.7%.4 Here, we report a patient with CD presented with various stages of lesions of PG.
Case Report
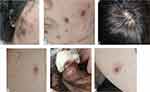
A 29-year-old man presented with various painful skin lesions, including erythema, vesicle, plaques, and ulcers, one week before the fourth infliximab infusion for moderately active CD. Physical examination showed multiple erythematous papules/plaques covered with crust on the scalp and face ranging from 0.5 to 1.5 cm. An erythematous papulovesicle was on the abdomen, and an oval, 0.5cm-sized, erythematous ulcer was on the calf. In addition, a single oval, 1cm-sized, sharply demarcated ulcer is located on the penis (Figure 1). Skin biopsies revealed epidermal acanthosis and a lichenoid infiltration of lymphocytes in the superficial dermis with dermal edema (the papule on the abdomen) and a dense polymorphic inflammatory dermal infiltrate, very rich in neutrophils, compatible with PG (the ulcer on the calf) (Figure 2). Laboratory and radiologic tests were negative for infection, vasculitis, and malignancy. Bone marrow biopsy was normal. Tissue cultures grew neither bacteria nor fungi. The patient was diagnosed with PG, and because of inadequate response to infliximab, a ustekinumab infusion was given. The lesions gradually subsided after a 390-mg ustekinumab infusion for one month.
Discussion
PG is a rare neutrophilic dermatosis with an estimated incidence of 3–10 cases per million per year.2 It occurs most commonly in individuals 20–60 years old, with the average age of onset at 44 years old.3,5 Approximately 50% of patients have other associated conditions such as IBD, arthritis, and hematological malignancies (acute myelogenous leukemia, myelodysplasia, and IgA monoclonal gammopathies).5–8 PG has five widely recognized subtypes-ulcerative PG, bullous PG, vegetative PG, pustular PG, and peristomal PG.9 The most common morphologic variant is ulcerative PG.10
Ulcerative PG typically presents with an inflammatory and tender nodule or pustule that rapidly expands into an ulcer. The ulcer was always demonstrated with an undermined violaceous border and a purulent base. The disease recovers slowly, and the healing of lesions may result in disfiguring cribriform scar formation. PG is a diagnosis of exclusion. Clinical, histological, and laboratory findings are nonspecific and cannot be used alone for a definitive diagnosis. Although widespread validated diagnostic criteria are lacking for PG, practical criteria have been suggested.11 We finally excluded vasculitis, thrombophilic conditions, malignancies, infections, and other inflammatory diseases through a series of laboratory tests and radiological examinations.
CD is an inflammatory condition of the gastrointestinal tract and can affect other organs, including the skin, eyes, liver, and joints.12 Cutaneous extraintestinal manifestations are currently divided into four categories: (1) disease-specific lesions, (2) reactive lesions, (3) associated conditions, and (4) therapy-related lesions.4,12 Disease-specific lesions that are seen only in CD,13 including perianal fissures/fistulae and metastatic CD, are caused by the direct involvement of the skin by a pathologic mechanism identical to the underlying CD.4 Erythema nodosum (EN) and pyoderma gangrenosum are common cutaneous manifestations of reactive lesions, which are induced by cross antigenicity between the integument and gut mucosa without exhibiting the exact pathologic features of the IBD.13 As therapeutic options for IBD expand, some investigators have proposed a fourth category of cutaneous extraintestinal manifestations-therapy-related lesions.12 Associated conditions and therapy-related lesions, including psoriasis, vitiligo, epidermolysis bullosa acquisita, eczema, and alopecia areata, sometimes cannot be distinguished. In our case, the pathology was not consistent with the CD and EN, and the lesions also did not meet the diagnostic criterion of associated lesions and therapy-related lesions reported.
The histopathologic findings of PG are not specific, and the primary objective in obtaining a biopsy specimen is to rule out other causes of ulceration. Skin biopsy specimens taken from the erythematous regions adjacent to ulcers may show the disruption or necrosis of dermal or pannicular blood vessels (with lymphocytes as the primary inflammatory cells).11 Classical histopathological findings demonstrated mixed cellular inflammation with neutrophil predominance in skin biopsy specimens of necrotic, undermined ulcer border. In this case, the skin biopsy of the ulcer showed typical features of neutrophil infiltration. In contrast, that of the papulovesicle demonstrated a lichenoid lymphocyte infiltrate in the superficial dermis with dermal edema, indicating that different stages of PG lesions revealed different histopathological manifestations.
Systemic corticosteroids and immunosuppressive agents are the main treatment for PG.14,15 Biologics and small-molecule drugs are emerging. In addition, treating the underlying disease may be helpful.16 Infliximab-Tumor Necrosis Factor (TNF) antagonists have shown a more remarkable improvement for PG.17,18 Other biologics, such as Tocilizumab,19 Ustekinumab,20 Vedolizumab,21 anakinra, canakinumab,22 and small-molecule drugs, including tofacitinib23 and apremilast,24 have been reported to be effective in some small sample cases.
Conclusion
Here, we present an unusual case of various stages of lesions of PG concomitantly in a Crohn’s disease patient one week before the fourth infliximab infusion and the lesions gradually subsided after taking ustekinumab for one month. It suggests that various painful skin lesions, including erythema, vesicles, plaques, and ulcers, might be the manifestation of PG and the therapy should be re-evaluated.
Ethics and Consent Statements
Written informed consent was provided by the patient to have the case details and any accompanying images published. Institutional approval was not required to publish the case details.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international Experts. JAMA Dermatol. 2018;154(4):461–466. doi:10.1001/jamadermatol.2017.5980
2. Ruocco E, Sangiuliano S, Gravina A, Miranda A, Nicoletti G. Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol. 2009;23(9):1008–1017. doi:10.1111/j.1468-3083.2009.03199.x
3. Binus A, Qureshi A, Li V, Winterfield L. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol. 2011;165(6):1244–1250. doi:10.1111/j.1365-2133.2011.10565.x
4. Thrash B, Patel M, Shah K, Boland C, Menter A. Cutaneous manifestations of gastrointestinal disease: part II. J Am Acad Dermatol. 2013;68(2):
5. Bennett M, Jackson J, Jorizzo J, Fleischer A, White W, Callen J. Pyoderma gangrenosum. A comparison of typical and atypical forms with an emphasis on time to remission. Case review of 86 patients from 2 institutions. Medicine. 2000;79(1):37–46. doi:10.1097/00005792-200001000-00004
6. Crowson A, Mihm M, Magro C. Pyoderma gangrenosum: a review. J Cutan Pathol. 2003;30(2):97–107. doi:10.1034/j.1600-0560.2003.00024.x
7. Platzer K, Kostner L, Vujic I, et al. Clinical characteristics and treatment outcomes of 36 pyoderma gangrenosum patients - a retrospective, single institution observation. J Eur Acad Dermatol Venereol. 2019;33(12):e474–e475. doi:10.1111/jdv.15803
8. Kridin K, Cohen AD, Amber KT. Underlying systemic diseases in pyoderma gangrenosum: a systematic review and meta-analysis. Am J Clin Dermatol. 2018;19(4):479–487. doi:10.1007/s40257-018-0356-7
9. Ashchyan HJ, Nelson CA, Stephen S, James WD, Micheletti RG, Rosenbach M. Neutrophilic dermatoses: pyoderma gangrenosum and other bowel- and arthritis-associated neutrophilic dermatoses. J Am Acad Dermatol. 2018;79(6):1009–1022. doi:10.1016/j.jaad.2017.11.063
10. Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13(3):191–211. doi:10.2165/11595240-000000000-00000
11. Su W, Davis M, Weenig R, Powell F, Perry H. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43(11):790–800. doi:10.1111/j.1365-4632.2004.02128.x
12. Hagen JW, Swoger JM, Grandinetti LM. Cutaneous manifestations of Crohn disease. Dermatol Clin. 2015;33(3):417–431. doi:10.1016/j.det.2015.03.007
13. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–434. doi:10.1038/nature06005
14. Alavi A, French L, Davis M, Brassard A, Kirsner R. Pyoderma gangrenosum: an update on pathophysiology, diagnosis and treatment. Am J Clin Dermatol. 2017;18(3):355–372. doi:10.1007/s40257-017-0251-7
15. Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14(3):225–233. doi:10.1080/1744666X.2018.1438269
16. Wenzel J, Gerdsen R, Phillipp-Dormston W, Bieber T, Uerlich M. Topical treatment of pyoderma gangraenosum. Dermatology. 2002;205(3):221–223. doi:10.1159/000065843
17. Brooklyn T, Dunnill M, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55(4):505–509. doi:10.1136/gut.2005.074815
18. Ben Abdallah H, Fogh K, Bech R. Pyoderma gangrenosum and tumour necrosis factor alpha inhibitors: a semi-systematic review. Int Wound J. 2019;16(2):511–521. doi:10.1111/iwj.13067
19. Lee W, Choi Y, Yoo W. Use of tocilizumab in a patient with pyoderma gangrenosum and rheumatoid arthritis. J Eur Acad Dermatol Venereol. 2017;31(2):e75–e77. doi:10.1111/jdv.13736
20. Nunes G, Patita M, Fernandes V. Refractory Pyoderma gangrenosum in a patient with Crohn’s disease: complete response to ustekinumab. J Crohns Colitis. 2019;13(6):812–813. doi:10.1093/ecco-jcc/jjy200
21. Dubinsky M, Cross R, Sandborn W, et al. Extraintestinal manifestations in vedolizumab and anti-TNF-treated patients with inflammatory bowel disease. Inflamm Bowel Dis. 2018;24(9):1876–1882. doi:10.1093/ibd/izy065
22. Ben Abdallah H, Fogh K, Vestergaard C, Bech R. Pyoderma gangrenosum and interleukin inhibitors: a semi-systematic review. Dermatology. 2022;238(4):785–792. doi:10.1159/000519320
23. Kochar B, Herfarth N, Mamie C, Navarini A, Scharl M, Herfarth H. Tofacitinib for the treatment of Pyoderma gangrenosum. Clin Gastroenterol Hepatol. 2019;17(5):991–993. doi:10.1016/j.cgh.2018.10.047
24. Vernero M, Ribaldone D, Cariti C, et al. Dual-targeted therapy with apremilast and vedolizumab in pyoderma gangrenosum associated with Crohn’s disease. J Dermatol. 2020;47(6):e216–e217. doi:10.1111/1346-8138.15283
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.