Back to Journals » Clinical Ophthalmology » Volume 10
Multimodal imaging and diagnosis of myopic choroidal neovascularization in Caucasians
Authors Milani P , Massacesi A, Moschini S, Setaccioli M, Bulone E, Tremolada G, Ciaccia S, Mantovani E, Morale D, Bergamini F
Received 15 March 2016
Accepted for publication 25 May 2016
Published 12 September 2016 Volume 2016:10 Pages 1749—1757
DOI https://doi.org/10.2147/OPTH.S108509
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Paolo Milani,1 Amedeo Massacesi,1 Stefania Moschini,1 Marco Setaccioli,1 Ennio Bulone,1 Gemma Tremolada,1 Stefano Ciaccia,1 Elena Mantovani,1 Daniela Morale,2 Fulvio Bergamini1
1Ophthalmology Department, Istituto Auxologico, 2Institute of Mathematics, Universita’ degli Studi di Milano, Milan, Italy
Purpose: To investigate myopic choroidal neovascularization (mCNV) by fluorescein angiography (FA), spectral-domain optical coherence tomography (SD-OCT), near-infrared (NIR) reflectance, and autofluorescence (AF).
Methods: This retrospective study included 65 eyes of 62 Caucasian patients with a mean age of 66.72 years (95% confidence interval [CI] 63–70 years) and a mean refraction of -9.72 diopters (95% CI -8.74 to -10.70 diopters).
Results: Most of the mCNV cases were foveal-juxtafoveal (60/65, 92.3%), with thickening of the corresponding retina (62/65, 95.3%) and leakage on FA (44/65, 67.6%). No retinal fluid was detectable in 32 (49.2%) eyes and there was no hemorrhage in 25 (38.4%) eyes. Papillary chorioretinal atrophy was evident in 58 (89.2%), a shadowing effect in 48 (73.8%), and an epiretinal membrane in 38 (58.4%) eyes. If an area of macular chorioretinal atrophy was present, mCNV frequently developed adjacent to it and was hyperfluorescent rather than with leakage (P<0.001). In eyes with edema or hemorrhage, hyper-reflective foci were more frequent (P<0.005). NIR and AF features were indeterminable in 19 (29.2%) and 27 (41.5%) eyes, respectively. The predominant feature was black or grayish on NIR (34/65, 52.3%) and patchy (hypo- and hyperfluorescence was observed) on AF (25/65, 38.4%). FA and SD-OCT correctly detected mCNV in 49 (75.3%) and 48 (73.8%) eyes, respectively, whereas NIR and AF exhibited limited diagnostic sensitivity. Doubtful diagnosis was associated with hyperfluorescent mCNV (P<0.001), absence of retinal fluid and epiretinal membrane (P<0.05), and presence of macular chorioretinal atrophy (P<0.01).
Conclusion: Tomographic, angiographic, AF, and NIR features of mCNV are described in this study. Combination of SD-OCT and FA is recommendable for diagnosis.
Keywords: myopic neovascularization, pathologic myopia, fluorescein angiography, SD-OCT, imaging, CNV
Introduction
In the last few years, macular diseases have been studied better, thanks to the widespread use of instruments that can simultaneously combine spectral domain optical coherence tomography (SD-OCT) and fluorescein angiography (FA).
The Spectralis system (Heidelberg Engineering, Heidelberg, Germany) uses a solid-state laser diode to produce light at multiple wavelengths such as 830 nm near-infrared (NIR) and 488 nm blue autofluorescence (AF). Digital retinal imaging can be obtained by direct reflectance or by emitted autofluorescence through different barrier filters. The active eye tracking and confocal imaging system in the platform permits focused, clearly defined, high-contrast FA and tomography with 40,000 A-scans per second, resulting in higher resolution. This “multimodal approach” is now widely employed in highly specialized centers, especially to examine the retina when difficulties arise in diagnosis or for study purposes.1
Among macular pathologies, pathologic myopia (PM) is one of the main causes of vision loss in developed countries,2 with a prevalence of 0.9%–3.1%, and it has been recently reported that 5.2%–11.3% of myopic individuals develop myopic choroidal neovascularization (mCNV).3 It is therefore extremely important to diagnose the condition as early as possible, considering, too, that these lesions respond very well to anti-VEGF therapy.4
The details of mCNV have been studied from the tomographic viewpoint, but little has been done using AF5,6 and nothing at all with NIR. Moreover, the sensitivity of each of these methods is still debated, with reports of cases for FA varying from 60%7 up to 84.5%.8
The main aim of this study was to investigate and correlate the tomographic and angiographic findings in mCNV, and their NIR and AF features, in a retrospective series of patients. A secondary aim was to evaluate the specific contribution of each modality to the diagnosis.
Materials and methods
In this retrospective consecutive study, two authors (PM and AM), senior retina specialists, reviewed the charts and images of patients seen in our hospital (Istituto Auxologico Italiano) between 2010 and 2014. The authors advise that in Italy there is no need for ethical approval for retrospective study using imaging data or charts. In accordance with Italian law every patient signs a personal consent to allow the health institution they enter to manage their private medical information, and this type of consent was obtained by every patient included in the present study.
The inclusion criteria were as follows: recent (<30 days) vision deterioration with metamorphopsia and/or scotoma at the Amsler grid test, PM, refractive error <−6 diopters, staphyloma at SD-OCT, documentation or suspicion (based on the clinical history) of mCNV, or macular exudative pathologies on FA and SD-OCT.
Exclusion criteria were previous vitreoretinal surgery, diabetes or signs of age-related macular degeneration (AMD), such as drusen, or typical geographic atrophy. Similarly, we excluded patients with images of unsatisfactory quality or presenting with vitreoretinal interface-related pathologies such as macular hole, epiretinal membrane (ERM), or retinal detachment without mCNV.
The two investigators (PM and AM) considered only good-quality images, at first presentation, and stored them in the data base of the Spectralis multimodal imaging system which combines confocal scanning laser angiography, simultaneous SD-OCT, and multiple imaging using a solid-state laser diode that can produce excitation light at different wavelengths: NIR (excitation λ=830 nm), AF and FA after intravenous injection of 5 mL of fluorescein (excitation λ=488 nm, emission >500 nm). To increase the signal-to-noise ratio, the device is equipped with an eye tracker that averages multiple frames.
Only eyes for which the following images were evaluable were recruited:
- NIR and AF imaging of the macular area with the 30° module lens set
- FA documentation of initial (0–45 seconds), middle (1–2 minutes), and late (at least 3 minutes) phases of the macular area after intravenous injection of 2.5 mL of fluorescein
- Multiple horizontal tomographic linear scans, with the scanner positioned on the center of the macula in the volume mode and, additionally, in the center of the suspected lesion in the single linear mode, horizontal and vertical. The software has an “automatic real-time” (ART) function to reduce noise and improve image quality. With ART activated, multiple nine or 100 B-scan frames of the same scanning location were taken when using the volume or the single linear acquisition module, respectively.
The presence of mCNV was confirmed on the basis of the following features of the suspected lesion: initial dye uptake with hyperfluorescence and possibly late leakage on FA and the presence of a hyper-reflective lesion at SD-OCT.
The FA patterns of mCNV of each patient were rated as follows: “hyperfluorescence” (when there was only mild hyperfluorescence, possibly clearly circumscribed, without any leakage) or “mild leakage/leakage” (if there were typical signs of neovascular activity, ie, initial fluorescein uptake by the mCNV network and extension of dye beyond the boundaries delineated in the early fluorescein transit).Leakage was recorded as indistinguishable if the lesion could not be outlined clearly in the surrounding tissue.
The assessable neovascular complex inside the lesion in every volume and linear scan was also studied. The two investigators (PM and AM) had to establish the presence or absence of the following conditions:
- intraretinal edema (possibly with cystoid macular edema, intraretinal small and isolated cysts, and serous neuroretinal detachment)
- retinal thickening anterior to the mCNV
- ERM in correspondence (anterior and/or immediately adjacent) or not in correspondence with the lesion (ie, in other areas of the macular region)
- vitreomacular adhesion (defined as focal adhesion and/or traction of the posterior vitreous face within the macular region, as a sign of incomplete posterior vitreous detachment)
- hyper-reflective foci (HF).
The mCNV area was measured manually using the Spectralis software (Heidelberg Eye Explorer, version 1.9.10.0; Heidelberg Engineering, Heidelberg, Germany), which circumscribes the lesion and calculates the area in square mm. The boundaries were identified in the early phase of FA, and then overlaid with the mouse. The mCNV was considered foveal-juxtafoveal if positioned 1–199 μm from the foveal depression, identified in the SD-OCT scan, or extrafoveal if positioned 200 μm or more from the center. Focal or diffuse peripapillary areas of chorioretinal atrophy, contiguity of the lesion to an atrophic area (macular or papillary), and hemorrhage were noted.
A shadowing effect toward the choroid was considered when SD-OCT showed the typical cone of shadow, easily recognized when tomographic details on the outer part of the lesion are less evident than those around it due to the high reflectivity of the lesion.
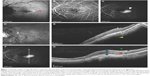
In our opinion, the NIR appearance of the mCNV is not easy to describe because of the typical tessellated appearance of the macular region in PM and frequent association with chorioretinal atrophy. We therefore compared the mCNV features with the diffuse reflectance of the surrounding area. The lesion was defined as “pale/white” if it appeared mainly lighter than the adjacent retinal tissue, and “grayish” or even “black” if it was mostly darker. Similarly, when possible, we assessed the hyper- or hypoautofluorescence, checking for any rings, and rated the lesion “indeterminable” if undistinguishable. Figure 1 shows an example of the characteristics investigated.
To establish the specific contribution of each of the four methods – NIR, AF, FA, and SD-OCT – to the diagnosis, the two investigators (PM and AM) showed the images of the selected patients to two other retina experts (SM and MS) on the monitor, who were then asked to what extent it was possible to identify mCNV on the basis of each of the single examinations, rating the diagnosis as “positive”, “negative”, or “doubtful”.
Statistical analysis
We performed a descriptive analysis of the characteristics through the estimates of either the mean value or the percentage of occurrence of a specific nominal value and their 95% confidence interval (CI). We used correlation analysis to first investigate the Spearman’s pairwise correlations among the characteristics and then between the characteristics and the diagnosis with the four methods (FA, SD-OCT, NIR, and AF). The value of P may range between 1, indicating a perfect correlation, and −1, indicating a perfect inverse (negative) correlation. Then, we performed a multivariate canonical correlation and a factorial analysis in order to highlight possible relationships among the characteristics.
We used Wilcoxon test to compare the average behavior of every variable (eg, presence/absence of HF) between the two subgroups (eg, presence/absence of hemorrhage). Finally, we compared the performance of each of the four diagnostic methods. The proportion of agreement for pairs was assessed using the Cohen’s kappa (κ) statistic and the overall agreement using the κm statistic (k<0 [poor agreement]; k=0–0.20 [slight agreement], 0.21–0.40 [fair], 0.41–0.60 [moderate], 0.61–0.8 [substantial]; and k>0.81 [almost perfect agreement]). All analyses were done with SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Sixty-five eyes of 62 patients (44 females and 21 males), all Caucasians, met the criteria for inclusion in the study. Mean age was 66.72 years (95% CI 63−70), range 18 to 89. Mean refraction was −9.72 diopters, range −6 to −22 (95% CI −8.74 to −10.70). Table 1 lists the FA and OCT characteristics.
Most of the mCNV cases were foveal-juxtafoveal (60/65, 92.3%); small (mean area 1.51 mm2); and presented with hemorrhage (40/65, 61.6%), thickening of the corresponding retina (62/65, 95.3%) at OCT, and leakage (44/65, 67.6%) on FA. In 32 eyes (49.2%), no retinal fluid of any type was detectable. Papillary chorioretinal atrophy was evident in 58 (89.2%) and ERM in 38 (58.4) eyes. Neovascularization developed on the edge of a chorioretinal atrophic lesion (macular or papillary) in 18/65 eyes (27.6%) and in one eye presented with two distinct but contiguous areas of leakage.
The appearance on NIR and AF varied widely and was indeterminable in 19 (29.2%) and 27 cases (41.5%), respectively. It was predominantly black or grayish on NIR (34/65, 52.3%) and hypo-hyperautofluorescent on AF (“patchy”, 25/65, 38.4%). The lesion was surrounded by a ring either on NIR or on AF in 37 cases (28.4%) (Table 2).
  | Table 2 Characteristics of mCNV assessed by near-infrared (NIR) reflectance and autofluorescence (AF); (65 eyes) |
In eyes with edema or hemorrhage, the prevalence of HF was more frequent than in eyes without (64.2% vs 25.4%, P<0.05 and 72.0% vs 32.5%, P<0.05).
If macular chorioretinal atrophy was present, hemorrhages were much less frequent (P<0.05); mCNV often developed adjacent to it or to an area of peripapillary atrophy (ρ=0.6, P<0.0001) and was more frequently hyperfluorescent on FA rather than with leakage (P<0.0001).
Doubtful diagnosis on FA was significantly related to a higher percentage of eyes with macular choroidal atrophy (P<0.001), with mCNV close to choroidal atrophy (P<0.001) and with hyperfluorescent mCNV (P<0.001).
In the group with doubtful diagnosis at SD-OCT, there was a higher percentage of eyes without any kind of retinal fluid (P<0.05) and a smaller percentage of eyes with HF (P<0.05), hemorrhage (P<0.05), ERM (P<0.05), and leakage (P<0.01). We also observed a higher percentage of lesions close to the region of choroidal atrophy (P<0.01). Table 3 presents the majority of significant comparisons.
Diagnostic difficulties of SD-OCT and FA were associated (ρ=0.47, P<0.0001) as were positive cases diagnosed by NIR and AF (ρ=0.77, P<0.0001), which also resulted in discrete correlation with the presence of hemorrhage, HF, and larger mCNV (R2>0.4, P>0.4). In fact, AF and NIR were of some diagnostic utility (ρ ∈[0.4, 0.53] P<0.0001) only in eyes with hemorrhage, cystoid macular edema, or edema.
FA and SD-OCT offered similar diagnostic sensitivity (k=0.5), as mCNV was properly detected in 49 (75.3%) and 48 (73.8%) eyes, respectively, on the basis of single modality (Table 4).
There was no significant difference in the dimension of the lesion with respect to age and refraction. Leakage on FA was more prominent in patients younger than 66 years (P<0.05) and did not differ statistically between the eyes with or without edema (P=0.35). Hyperfluorescence on FA, ERM, and the shadowing effect seemed to be more frequent in older patients (P<0.05).
Discussion
Numerous papers have been published on the multimodal description of retinal lesions, but not on mCNV, especially in Caucasians. The present study, though only covering a fairly small series of patients, shows up many important features of mCNV, some known and others unknown, such as the frequent presence of ERM (38/65, 58.4%), especially in larger lesions and in older patients. In 6/65 (9.2%) cases, ERM was present only at the site of mCNV, in 19 (29.2%) it involved the entire macular area, and in 13 (19.9%) it was outside the mCNV area. The prevalence of ERM varies across studies, and comparison is problematic. Similar to the nonmyopic population, the findings in myopic eyes also vary widely: from 23% in Chinese9 to 11%–40% in Caucasians.10,11 The relationship between ERM and CNV has not been widely investigated, but many authors found lower rates of posterior vitreous detachment in eyes with exudative AMD than in eyes with dry AMD or no disease.12–14 Since statistically relevant association was also observed between CNV and VMA (located just anterior to the CNV area) in 19%–28% of patients with exudative AMD,15,16 it has been suggested that persistent attachment of the posterior vitreous cortex to the macula may be a risk factor for the development of CNV. The early vitreous fluidification and detachment, which typically occur prematurely in high myopia,17 and the relative older mean age of patients may also explain the larger proportion of ERM in our study.
Another interesting finding is the occurrence of very high frequency of peripapillary chorioretinal atrophy (89.2%), slightly more prevalent than reported in myopic patients without mCNV (81.2%).18 This finding is very common in PM and was recently reported to be negatively related to visual acuity improvement after intravitreal injection19 and to be the only relevant factor associated with the need for retreatment.20 Peripapillary atrophy may be secondary to the degree of choroidal ischemia in PM, as choroidal circulatory disturbances may contribute to crescent development and enlargement over the years21 and to mCNV onset. As was found in other studies, macular chorioretinal atrophy in our series was also less prevalent than papillary atrophy, and this was probably because of differences in circulation. Overall, it is not surprising that 47/65 mCNVs developed on the edge of an area of chorioretinal atrophy, macular or papillary, presumably in response to an ischemic cause. Increased thinning of the retina in the border of areas of chorioretinal atrophy may account for higher prevalence of hyperfluorescence than leakage on FA and to fewer hemorrhages (P<0.05).
We have no explanations for the low prevalence of serous neuroretinal fluid at SD-OCT (27% compared to 60% in CNV secondary to AMD22), although we can speculate that it might be secondary to the lower activity of mCNV. The HF observed in the neovascular complex have also been found to be commonly associated with CNV in AMD, with retinal vein occlusion and diabetic macular edema. HF are thought to be migrating retinal pigment epithelium (RPE) cells or leucocytes, or very initial intraretinal lipoprotein exudates, and therefore their presence is a sign of lesion activity.23,24 In our study, HF appeared to be clearly related to the presence of retinal edema, serous neuroretinal detachment, and hemorrhage (P<0.05, Table 3), thus confirming the association with exudative retinal changes.
The shadowing effect toward the choroid is intuitively due to the hyper-reflectivity of the thickened retina–mCNV complex, and its results were unaccountably associated with the presence of ERM (84.2%, P<0.005).
The NIR features of macular neovascular lesions and AF characteristics of mCNV have been investigated but not as thoroughly.5,6,25–27 Using NIR and AF imaging, we were unable to define the mCNV features in 29.2% and 41.5% of cases, respectively – these are substantial proportions. Differences in the patterns may be caused by the typical RPE thinning and/or by the RPE cells “coating” the neovascular complex. NIR mainly correlates with melanin inside the RPE27 and, similar to the findings reported in a small sample with AMD,26 nearly half of the lesions (49.2%) presented a gray or black appearance, surrounded by a whitish or black-grayish ring in 13 and seven cases, respectively.
The presence of a reactive, hyperautofluorescent ring is partially confirmed by AF imaging, where the main finding, unlike to a recent report by Parodi et al,28 was a hypo-hyperautofluorescent (“patchy”) rather than a hyperautofluorescent lesion. Autofluorescence is related to the presence of lipofuscin in the RPE, and is probably due to the displacement of macular pigments and subsequent increased exposure of lipofuscin. In 15/65 (23%) eyes, a hyperautofluorescent ring was found around the mCNV. Previously, it has been considered secondary to fibrin accumulation,26 but we believe that a sort of RPE reactive hyperplasia develops secondary to mCNV formation, as confirmed by some studies.29,30
The type of NIR reflectance or AF by the ring or even by the lesion itself may be indicative of the different stages of RPE hyperplasia or the portion and shape of the neovascular membrane complex.
Angiographic visualization of the mCNV has improved greatly since the scanning laser system came into clinical practice. Despite Avila’s findings in 1984,31 it is now clear that most of the lesions commonly present with at least mild leakage. This was seen in 76% of the eyes in our series, especially in patients younger than 66 years, whereas in the others we detected only some hyperfluorescence and no leakage, making diagnosis impossible on the basis of FA alone in eleven of these cases.
In contrast with previous findings7 and with NIR and AF, FA and SD-OCT, when used separately, offered overall similar, discrete good diagnostic sensitivity, although 16 (24.6%) and 17 (26.1%) patients, respectively, would not have been diagnosed correctly and consequently not properly treated. In all these cases, it was direct comparison of the two imaging modalities that permitted a correct diagnosis. The presence of macular chorioretinal atrophy in 12/16 and 11/17 cases was significantly related to a doubtful evaluation either on FA or on SD-OCT. Intuitively, the absence of any kind of retinal fluid, hemorrhage, and HF also resulted in diagnostic difficulties on SD-OCT (P<0.05).
This study has some limitations, such as the small number of patients and the lack of masked evaluation of the images by several examiners. Interpretation of the NIR and AF images was problematic, highly variable, and subjective. We must also consider that it may take several weeks for some older patients to become aware of their visual loss. This partly explains the variability in the size and appearance of lesions at diagnosis. Another limitation is that the tomographic evaluation may be influenced by the number of sections in the macular volume module. However, this is only the first paper to describe multimodal imaging features in mCNV.
Conclusion
This study reported a description and correlation of the mCNV characteristics on FA, SD-OCT, NIR, and AF. The angiographic and tomographic diagnostic sensitivity appear to be similar, but the combination of both modalities is recommendable to achieve the diagnosis in all the cases.
Disclosure
The authors report no conflicts of interest in this work.
References
Hassenstein A, Meyer CH. Clinical use and research applications of Heidelberg retinal angiography and spectral-domain optical coherence tomography – a review. Clin Experiment Ophthalmol. 2009;37(1):130–143. | ||
Hayashi K, Ohno-Matsui K, Shimada N, et al. Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology. 2010;117(8):1595–1611. | ||
Wong TY, Ferreira A, Hughes R, Carter G, Mitchell P. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol. 2014;157(1):9–25. | ||
Wang E, Chen Y. Intravitreal anti-vascular endothelial growth factor for choroidal neovascularization secondary to pathologic myopia: systematic review and meta-analysis. Retina. 2013;33(7):1375–1392. | ||
Sawa M, Gomi F, Tsujikawa M, et al. Abnormal fundus autofluorescence patterns in myopic choroidal neovascularisation. Br J Ophthalmol. 2008;92(9):1236–1240. | ||
Parodi MB, Iacono P, Ravalico G. Fundus autofluorescence in subfoveal choroidal neovascularisation secondary to pathological myopia. Br J Ophthalmol. 2009;93(6):771–774. | ||
Leveziel N, Caillaux V, Bastuji-Garin S, Zmuda M, Souied EH. Angiographic and optical coherence tomography characteristics of recent myopic choroidal neovascularization. Am J Ophthalmol. 2013;155(5):913–919. | ||
Milani P, Massacesi A, Setaccioli M, et al. Sensitivity of fluorescein angiography alone or with SD-OCT for the diagnosis of myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2013;251(8):1891–1900. | ||
You QS, Peng XY, Xu L, Chen CX, Wang YX, Jonas JB. Myopic maculopathy imaged by optical coherence tomography: the beijing eye study. Ophthalmology. 2014;121(1):220–224. | ||
Henaine-Berra A, Zand-Hadas IM, Fromow-Guerra J, García-Aguirre G. Prevalence of macular anatomic abnormalities in high myopia. Ophthalmic Surg Lasers Imaging Retina. 2013;44(2):140–144. | ||
Milani P, Pece A, Pierro L, Bergamini F. Imaging of naive myopic choroidal neovascularization by spectral-domain optical coherence tomography. Ophthalmologica. 2014;232(1):28–36. | ||
Simpson AR, Petrarca R, Jackson TL. Vitreomacular adhesion and neovascular age-related macular degeneration. Surv Ophthalmol. 2012;57(6):498–509. | ||
Robison CD, Krebs I, Binder S, et al. Vitreomacular adhesion in active and end-stage age-related macular degeneration. Am J Ophthalmol. 2009;148(1):79–82. | ||
Krebs I, Brannath W, Glittenberg C, Zeiler F, Sebag J, Binder S. Posterior vitreomacular adhesion: a potential risk factor for exudative age-related macular degeneration? Am J Ophthalmol. 2007;144(5):741–746. | ||
Lee SJ, Lee CS, Koh HJ. Posterior vitreomacular adhesion and risk of exudative age-related macular degeneration: paired eye study. Am J Ophthalmol. 2009;147(4):621–626. | ||
Mojana F, Cheng L, Bartsch DU, et al. The role of abnormal vitreomacular adhesion in age-related macular degeneration: spectral optical coherence tomography and surgical results. Am J Ophthalmol. 2008;146(2):218–227. | ||
Akiba J. Prevalence of posterior vitreous detachment in high myopia. Ophthalmology. 1993;100(9):1384–1388. | ||
Chang L, Pan CW, Ohno-Matsui K, et al. Myopia-related fundus changes in Singapore adults with high myopia. Am J Ophthalmol. 2013;155(6):991–999. | ||
Yoon JU, Kim YM, Lee SJ, Byun YJ, Koh HJ. Prognostic factors for visual outcome after intravitreal anti-VEGF injection for naive myopic choroidal neovascularization. Retina. 2012;32(5):949–955. | ||
Gharbiya M, Cruciani F, Parisi F, Cuozzo G, Altimari S, Abdolrahimzadeh S. Long-term results of intravitreal bevacizumab for choroidal neovascularisation in pathological myopia. Br J Ophthalmol. 2012;96(8):1068–1072. | ||
Yasuzumi K, Ohno-Matsui K, Yoshida T, et al. Peripapillary crescent enlargement in highly myopic eyes evaluated by fluorescein and indocyanine green angiography. Br J Ophthalmol. 2003;87(9):1088–1090. | ||
Major JC Jr, Wykoff CC, Mariani AF, Chen E, Croft DE, Brown DM. Comparison of spectral-domain and time-domain optical coherence tomography in the detection of neovascular age-related macular degeneration activity. Retina. 2014;34(1):48–54. | ||
Bolz M, Schmidt-Erfurth U, Deak G, Mylonas G, Kriechbaum K, Scholda C; Diabetic Retinopathy Research Group Vienna. Optical coherence tomographic hyperreflective foci: a morphologic sign of lipid extravasation in diabetic macular edema. Ophthalmology. 2009;116(5):914–920. | ||
Framme C, Wolf S, Wolf-Schnurrbusch U. Small dense particles in the retina observable by spectral-domain optical coherence tomography in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51(11):5965–5969. | ||
Hartnett ME, Elsner AE. Characteristics of exudative age-related macular degeneration determined in vivo with confocal and indirect infrared imaging. Ophthalmology. 1996;103(1):58–71. | ||
Semoun O, Guigui B, Tick S, Coscas G, Soubrane G, Souied EH. Infrared features of classic choroidal neovascularisation in exudative age-related macular degeneration. Br J Ophthalmol. 2009;93(2):182–185. | ||
Elsner AE, Burns SA, Weiter JJ, Delori FC. Infrared imaging of sub-retinal structures in the human ocular fundus. Vision Res. 1996;36(1):191–205. | ||
Parodi MB, Iacono P, Sacconi R, Iuliano L, Bandello F. Fundus autofluorescence changes after ranibizumab treatment for subfoveal choroidal neovascularization secondary to pathologic myopia. Am J Ophthalmol. 2015;160(2):322–327. | ||
Baba T, Ohno-Matsui K, Yoshida T, et al. Optical coherence tomography of choroidal neovascularization in high myopia. Acta Ophthalmol Scand. 2002;80(1):82–87. | ||
Parodi MB, Da Pozzo S, Ravalico G. Retinal pigment epithelium changes after photodynamic therapy for choroidal neovascularization in pathological myopia. Acta Ophthalmol Scand. 2007;85(1):50–54. | ||
Avila MP, Weiter JJ, Jalkh AE, Trempe CL, Pruett RC, Schepens CL. Natural history of choroidal neovascularization in degenerative myopia. Ophthalmology. 1984;91(12):1573–1581. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.