Back to Journals » Clinical Ophthalmology » Volume 14
Multimodal Chorioretinal Imaging in Erdheim-Chester Disease
Authors Sacconi R, Campochiaro C, Rabiolo A, Marchese A, Tomelleri A , Tomasso L, Cicinelli MV, Querques L, Bandello F , Dagna L , Querques G
Received 25 July 2019
Accepted for publication 19 September 2019
Published 28 February 2020 Volume 2020:14 Pages 581—588
DOI https://doi.org/10.2147/OPTH.S224672
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Riccardo Sacconi,1,* Corrado Campochiaro,2,* Alessandro Rabiolo,1,* Alessandro Marchese,1 Alessandro Tomelleri,2 Livia Tomasso,1 Maria Vittoria Cicinelli,1 Lea Querques,1 Francesco Bandello,1 Lorenzo Dagna,2,* Giuseppe Querques1,*
1Department of Ophthalmology, University Vita-Salute, IRCCS San Raffaele Scientific Institute, Milan, Italy; 2Unit of Immunology, Rheumatology, Allergy and Rare Diseases, IRCCS San Raffaele Scientific Institute, Milan, Italy
*These authors contributed equally to this work
Correspondence: Giuseppe Querques
Department of Ophthalmology, University Vita-Salute, IRCCS San Raffaele Scientific Institute, Via Olgettina 60, Milan 20132, Italy
Tel +39 0226432648
Fax +39 0226433643
Email [email protected]
Purpose: To analyze the subclinical intraocular involvement using multimodal imaging approach in patients affected by Erdheim-Chester disease (ECD) without ocular symptoms.
Patients and Methods: In this prospective cross-sectional study, 18 eyes of 9 consecutive patients with ECD were enrolled. Each patient underwent comprehensive ocular examination and extensive multimodal chorioretinal imaging.
Results: None of the patients presented any evidence of chorioretinal localization of disease using multimodal imaging. One patient exhibited a choroidal nevus complicated by active polypoidal choroidal neovascularization. Subretinal hyperreflective material was seen in three eyes, mainly resembling acquired vitelliform lesion. One patient had an isolated intraretinal hemorrhage. Most patients exhibited peripheral vascular abnormalities (ie, microaneurysms, peripheral vascular leakage). Fundus autofluorescence showed faint hyperautofluorescence in eleven eyes.
Conclusion: Intraocular involvement is an extremely rare event of an extremely rare disease. In patients affected by ECD without ocular symptoms, advance multimodal imaging examinations did not show signs of subclinical chorioretinal involvement related to the disease.
Keywords: Erdheim-Chester disease, histiocytosis, fluorescein angiography, indocyanine green angiography, optical coherence tomography
Introduction
Erdheim-Chester disease (ECD) is a rare, multisystemic disease caused by the accumulation and infiltration of non-Langerhans foamy histiocytes in different body sites; about 700 cases have been reported in the English literature.1 ECD mainly affects the skeletal apparatus, but it can potentially involve any other site, including the neurological, cardiovascular, respiratory, endocrine, and integumentary systems.1,2 Recently, mutations in mitogen-activated protein kinase (MAPK) pathways have been linked to the disease.2 Periocular involvement is common in ECD as the orbital and palpebral tissues represent one of the most typical presenting features of the disease. On the other hand, intraocular histiocytic infiltration occurs far less frequently. Indeed, only six cases of intraocular involvement have been described.3–6 Intraocular involvement ranged from subretinal to choroidal invasion associated with the presence of subretinal fluid.3,4,6,7 In all reported cases, the disease affected the posterior pole, and the patients complained of visual disturbances. On the other hand, extra-macular chorioretinal lesions may be asymptomatic, as reported in other diseases.8–10 Multimodal imaging evaluation represents a precious resource in these cases, as dilated fundus examination misses a substantial proportion of posterior segment lesions.11–13 Fluorescein angiography (FA) and indocyanine green angiography (ICGA), visualizing the retinal and choroidal vasculature, may play a significant role in the assessment of vascular changes and choroidal infiltrations.12,14 Furthermore, enhanced depth imaging (EDI) optical coherence tomography (OCT) may provide additional useful information on retinal and choroidal structures.14
The present study aims to prospectively analyze, using a multimodal imaging approach, the subclinical intraocular involvement in patients affected by ECD without ocular symptoms. This topic is of paramount relevance to assessing whether ECD patients should undergo periodic ophthalmic examinations.
Materials and Methods
Nine consecutive patients affected by ECD followed-up at the Unit of Immunology, Rheumatology, Allergy and Rare Diseases, University Vita-Salute, San Raffaele Hospital, Milan, Italy were referred to the Medical Retina & Imaging Unit between May and June 2016 and prospectively enrolled in this cross-sectional study. The study was conducted in compliance with the Declaration of Helsinki and all patients signed a written consent, approved by the ethics committee of San Raffaele Hospital. Medical history and demographic data were recorded for each patient.
Inclusion criteria were: histologically-proven diagnosis of ECD; age ≥ 18 years; refractive status between −6 and +3 diopters, and ability to provide informed consent. Diagnostic delay was defined as the years between estimated disease onset and diagnosis.
Exclusion criteria were: significant media opacity limiting proper image quality; known allergy to fluorescein or indocyanine green. History of a severe allergic reaction to other drugs represented a relative contraindication; in these cases, the patients were pre-medicated with cortisone and anti-histamine prophylaxis before dye angiography, as per the standardized protocols of the institution. Both eyes were included in the analysis if they fulfilled inclusion and exclusion criteria.
Patients underwent a comprehensive systemic assessment and a complete ocular examination performed by a clinical immunologist and an ophthalmologist, respectively. The eye examination included the measurement of best-corrected visual acuity (BCVA) on Snellen charts and converted to the logarithm of the minimal angle of resolution (logMAR) for calculation purposes, intraocular pressure (IOP) with Goldmann applanation tonometry, anterior segment biomicroscopy, dilated fundus examination, central corneal thickness measurement using XR Avanti SD-OCT (Optovue, Inc, Fremont, CA), axial length (IOL master 500, Carl Zeiss Meditec, Inc., Dublin, CA, USA), infrared reflectance (IR), Multicolor® images, blue-light fundus autofluorescence (BAF), and spectral-domain (SD)-OCT (Spectralis HRA, Heidelberg Engineering, Heidelberg, Germany). Patients also underwent ultra-widefield (UWF) color fundus images, UWF FA and UWF ICGA (Optos PLC, Dunfermline, Scotland, UK) after pupil dilatation with tropicamide 1%.
The SD-OCT acquisition protocol included: 19 horizontal raster linear B-scans, each composed by 9 averaged OCT B-scans (1024 A-scans per line) at 240 µm intervals, covering an area of 20 degrees by 15 degrees; 6 radial linear B-scans, each composed of 25 averaged OCT B-scans (768 A-scans per line) at 30 degrees centered on the fovea; 49 horizontal raster dense linear B-scans, each produced by 16 averaged OCT B-scans (384 A-scans per line) at 30 µm intervals, covering an area of 15 degrees by 5 degrees. The latter two scan sequences were acquired in EDI mode.15 Central macular thickness (CMT) in the central 1-mm-diameter circle of the ETDRS thickness map was automatically recorded by the Spectralis software (Heidelberg Eye Explorer, Version 1.9.11.0, Heidelberg Engineering, Germany). Subfoveal choroidal thickness (SFCT) was manually measured as the vertical distance between the hyper-reflective line of Bruch’s membrane and the chorio-scleral interface.
UWF fundus photographs centered on the macula were performed before the dye-based imaging. After intravenous injection of the indocyanine green dye, UWF ICGA was acquired in both eyes in the early (<60 seconds), early-intermediate (1–3 mins), late-intermediate (3–15 mins), and late phases (>15 mins). Similarly, UWF FA was obtained after intravenous fluorescein dye injection in the early (<60 seconds), intermediate (2–3 mins), and late phases (>4 mins). All UWF ICGA and FA images were acquired centered in the macula and the 4 steering positions (ie, left, right, up, and down), to expose the maximum amount of peripheral retina.
Variables included in the analysis were: age, sex, time interval between the diagnosis and enrollment in the study, time interval between the presumed disease onset and the enrollment, diagnostic delay, location of disease, therapy, systemic comorbidities, BRAF gene mutation, KRAS gene mutation, laterality, BCVA, anterior segment status, IOP, axial length, CMT, SFCT, presence and location of abnormalities at UWF imaging, and presence and location of abnormalities at SD-OCT.
Statistical analysis was performed using GraphPad Prism software 6.0 (GraphPad Software, Inc., San Diego, CA, USA) and SPSS software 22 (SPSS, Inc., Chicago, IL, USA). All data were expressed as mean ± standard deviation. All tests were 2-tailed and statistical significance was defined at p values <0.05.
Results
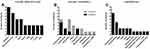
Eighteen eyes of 9 patients (6 males, 66.7%) were referred to our Department in the study period. All patients fulfilled the inclusion and exclusion criteria and were enrolled in the study. The mean age was 51.6±15.0 years and all patients were white Caucasians. The ophthalmic evaluation was performed 3.7±3.3 (range 0–11) and 8.4±3.9 (range 2–15) years after the established diagnosis and the presumed disease onset, respectively. The estimated diagnostic delay was 4.8 ± 5.0 (range 0–14) years. Mutation of BRAFV600E was found in 7 (78%) out of 9 patients. One of the remaining 2 subjects had a mutation in the KRAS gene. In the other case, the genetic analysis did not reveal any mutation. Figure 1 illustrates systemic manifestations, treatments, and comorbidities.
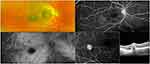
Table 1 summarizes the main ophthalmological findings. Dilated fundus examination and UWF color fundus images showed subclinical anomalies in three eyes (16.7%). One case presented a temporal extramacular choroidal nevus associated with an intraretinal hemorrhage and subretinal fluid (Figure 2). One eye showed one intraretinal hemorrhage in the temporal quadrant. Another eye had a paving-stone peripheral retinal degeneration.
 |
Table 1 Clinical Features of Study Population |
Dye angiography examination was performed in 16 out of 18 eyes (Table 2); one patient experienced a syncope after dye injection before FA and ICGA were recorded. UWF ICGA was unremarkable in all eyes except for the one with the choroidal nevus. Polypoidal choroidal neovascularization (CNV) with hyper-cyanescent vascular dilatation in the late phases was diagnosed on the nevus edge (Figure 2). On UWF FA, the lesion was hyper-fluorescent due to late leakage and there was a hypo-fluorescent lesion due to the intraretinal hemorrhage. The structural SD-OCT revealed a hyperreflective choroidal thickening corresponding to the nevus, with an irregular, dome-shaped, retinal pigment epithelium (RPE) detachment extending into the outer retinal layers. Sub-retinal exudation was present on the temporal side.
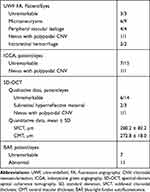 |
Table 2 Imaging Features |
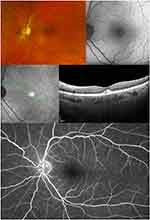
The UWF FAs were unremarkable only in 3 eyes. Besides the eyes with polypoidal CNV, other abnormalities were located in the extreme peripheral retina, including microaneurysms, peripheral vascular leakage, peripheral retinal hemorrhage, and paving-stone degeneration (Figure 3).
In both eyes of one patient, the structural SD-OCT revealed a small, well-defined, subretinal hyper-reflective material with preservation of outer retinal bands. The material, visible at fundus pseudocolor, appeared faintly hyper-autofluorescent at BAF images (Figure 4). Taking together the multimodal imaging features, the diagnosis of acquired vitelliform lesions was performed. Another eye showed unspecific subretinal material extending into the outer retinal layers. Eleven eyes exhibited nonspecific autofluorescence abnormalities. No choroidal abnormality in the macular area was detected in the study group.
Discussion
Ophthalmic involvement occurs in nearly one-third of patients affected by ECD.1,16 Palpebral and periocular xanthelasmas are the most common clinical manifestation. Orbital involvement occurs in almost a quarter of patients with symptoms related to a space-occupying lesion, including painless, progressive proptosis, optic neuropathy, ophthalmoplegia, and retina striae.16,17 Intraocular involvement is considered an exceptional event: only 6 cases have been reported in the current literature, after the first description by Biccas Neto and Zanetti.3,4,6,7 Recently, Tan and associated6 reported three patients with intraocular ECD complaining of visual disturbances. All cases were characterized by the presence of a choroidal mass at the posterior pole associated with chronic subretinal fluid. The diagnosis was performed between the diagnosis of the ECD and 2 years later and the mean age of patients was 60 years old. This data are in line with our series, in which the mean age was 51.6 years old and the ocular examination was performed between the diagnosis of ECD and 11 years later. In both the articles by Biccas Neto and Zanetti4 and by Tan et al.,6 a CNV was encountered during the follow-up. Notably, one eye underwent a biopsy of the choroidal mass revealing the typical CD45+CD68+CD1a− non-Langerhans lipid-laden histiocytes. In all the above cases, the patients were referred to the ophthalmologists due to visual symptoms. All lesions, indeed, were located in the posterior pole and had signs of activity, including macular subretinal fluid and CNV.
While lesions involving the posterior pole induce patients to seek medical attention for their visual symptoms, inactive choroidal masses sparing the macula can be asymptomatic for a long time before making a proper diagnosis. For instance, 25% of patients with uveal melanoma are asymptomatic at the time of the diagnosis.18 Similarly, 5–11% of patients with advanced breast cancer reveal silent choroidal metastasis at the ocular examination.19–21 The early identification of those tumors can change not only the visual but also the general prognosis of patients. For this reason, screening in high-risk population has been advocated.18,20 In ECD patients, we can speculate that an appropriate screening performed by an ophthalmologist may help in identifying a significant proportion of intraocular subclinical localizations of the disease. The early detection of posterior segment involvement would alert clinicians to initiate treatment before the disease invades the macular region, causing severe visual loss. In this study, we assessed the patients using a multimodal imaging approach, including color fundus photography, BAF, IR, Multicolor®, SD-OCT, UWF FA, and UWF ICG, with the purpose to analyze the intraocular involvement of ECD. Multimodal imaging has been proven to improve detection, guide therapy, and predict outcomes.22 Moreover, a multimodal approach provides a better characterization of chorioretinal lesions compared to ophthalmoscopic fundus examination.
None of the intraocular signs found in the enrolled subjects was suggestive of intraocular localization of the disease. One patient had an asymptomatic polypoidal CNV complicating a choroidal nevus. Choroidal nevi are found in 6.5% of the eyes and can be complicated by CNV in less than 1% of the cases.23 One patient suffering from both ECD and polycythemia vera had an intraretinal hemorrhage; it is reasonable to think that the sign was related to the hyperviscosity syndrome. The UWF FA revealed peripheral vascular abnormalities (ie, microaneurysms, vascular leakage) in six patients; however, these features have been extensively documented as para-physiologic changes in healthy subjects.24,25 One patient presented subretinal hyperreflectivity in both eyes; we can speculate it was related to the accumulation of lipofuscin at the level of the RPE, such as an acquired vitelliform lesion.26 However, acquired vitelliform lesions has been associated with a variety of retinal conditions, like retinal dystrophies, reticular pseudodrusen, cuticular drusen, pseudoxanthoma elasticum, central serous chorioretinopathy, macular telangiectasia type 2, RPE detachment, vitreomacular interface disease, immunogammapathies, drug toxicity, and paraneoplastic syndromes.26–33 We think that this may represent an incident finding in our patient, unrelated to ECD. Therefore, in our series, the most advanced multimodal chorioretinal imaging techniques have not shown any signs of subclinical intraocular involvement related to ECD in asymptomatic patients. However, we cannot completely conclude that there is no subclinical ocular involvement in these patients, as we may not yet be able to see it using the imaging techniques available today.
Despite being treated with potentially sight-threatening systemic drugs, no one of our patients showed signs of ocular toxicity. Vemurafenib, a specific inhibitor of BRAFV600E implicated in the mitogen-activated protein kinase pathway, is usually associated with iridocyclitis, intermediate uveitis with macular edema, conjunctivitis, and dry eye disease.34 A case of acute exudative paraneoplastic polymorphous vitelliform maculopathy during vemurafenib therapy has been reported.33 On the other hand, Interferon-α may cause retinal toxicity, which usually manifests as retinal hemorrhages, cotton wool spots and, rarely, central retinal vein occlusion.35 None of the aforementioned side effects was observed in our cohort.
The strength of the study is the number of eyes, whose posterior segment has been investigated through multimodal imaging. As ECD is an extremely rare disease, it is difficult to reach significant numbers in a monocentric study. Heterogeneity of the patients as far as age, the onset of disease, previous and ongoing treatments, represents an obvious limitation. Moreover, the cross-sectional nature of the study and the lack of follow up may affect the interpretation of the results.
Conclusions
In summary, we confirm that intraocular involvement is extremely rare in ECD. In patients affected by ECD without ocular symptoms, advance multimodal imaging examinations did not show signs of subclinical chorioretinal involvement related to the disease. Larger, multicenter, observational studies are needed to generate any recommendation about ophthalmic screening in these patients.
Acknowledgment
The authors thank Karl Anders Knutsson (IRCCS Ospedale San Raffaele, University Vita-Salute San Raffaele) for the English language editing.
Disclosure
Alessandro Rabiolo, Corrado Campochiaro, Riccardo Sacconi, Alessandro Marchese, Alessandro Tomelleri, Livia Tomasso, Maria Vittoria Cicinelli, Lea Querques, and Lorenzo Dagna report no conflicts of interest in this work. Francesco Bandello is a consultant for: Alcon (Fort Worth, Texas, USA), Alimera Sciences (Alpharetta, Georgia, USA), Allergan Inc (Irvine, California, USA), Farmila-Thea (Clermont-Ferrand, France), Bayer Shering-Pharma (Berlin, Germany), Bausch And Lomb (Rochester, New York, USA), Genentech (San Francisco, California, USA), Hoffmann-La-Roche (Basel, Switzerland), Novagali Pharma (Évry, France), Novartis (Basel, Switzerland), Sanofi-Aventis (Paris, France), Thrombogenics (Heverlee, Belgium), Zeiss (Dublin, USA). Giuseppe Querques is a consultant for: Alimera Sciences (Alpharetta, Georgia, USA), Allergan Inc (Irvine, California, USA), Amgen (Thousand Oaks, USA), Bayer Shering-Pharma (Berlin, Germany), Heidelberg (Germany), KBH (Chengdu; China), LEH Pharma (London, UK), Lumithera (Poulsbo; USA), Novartis (Basel, Switzerland), Sandoz (Berlin, Germany), Sifi (Catania, Italy), Sooft-Fidea (Abano, Italy), Zeiss (Dublin, USA). The authors report no other conflicts of interest in this work.
References
1. Campochiaro C, Tomelleri A, Cavalli G, et al. Erdheim-Chester disease. Eur J Intern Med. 2015;26:223–229. doi:10.1016/j.ejim.2015.03.004
2. Cavalli G, Guglielmi B, Berti A, et al. The multifaceted clinical presentations and manifestations of Erdheim-Chester disease: comprehensive review of the literature and of 10 new cases. Ann Rheum Dis. 2013;72:1691–1695. doi:10.1136/annrheumdis-2012-202542
3. Abdellatief A, Mason CM, Ytterberg SR, et al. Choroidal involvement in Erdheim-Chester disease. Ophthalmic Surg Lasers Imaging Retina. 2015;46:674–676. doi:10.3928/23258160-20150610-13
4. Biccas Neto L, Zanetti F. Acometimento intra-ocular na doença de Erdheim-Chester - primeiro relato na literatura: relato de caso [Intraocular involvement in Erdheim-Chester disease–first report in the literature: case report]. Arq Bras Oftalmol. 2007;70:862–867. Portuguese. doi:10.1590/S0004-27492007000500025
5. Diamond EL, Dagna L, Hyman DM, et al. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood. 2014;124:483–492. doi:10.1182/blood-2014-03-561381
6. Tan AC, Yzer S, Atebara N, et al. Three cases of Erdheim-Chester disease with intraocular manifestations: imaging and histopathology findings of a rare entity. Am J Ophthalmol. 2017;176:141–147. doi:10.1016/j.ajo.2017.01.017
7. Huang LC, Topping KL, Gratzinger D, et al. Orbital and chorioretinal manifestations of Erdheim-Chester disease treated with vemurafenib. Am J Ophthalmol Case Rep. 2018;11:158–163. doi:10.1016/j.ajoc.2018.07.005
8. Arepalli S, Kaliki S, Shields CL. Choroidal metastases: origin, features, and therapy. Indian J Ophthalmol. 2015;63:122–127. doi:10.4103/0301-4738.154380
9. Shields CL, Honavar SG, Shields JA, et al. Circumscribed choroidal hemangioma: clinical manifestations and factors predictive of visual outcome in 200 consecutive cases. Ophthalmology. 2001;108:2237–2248. doi:10.1016/S0161-6420(01)00812-0
10. Soysal HG. Metastatic tumors of the uvea in 38 eyes. Can J Ophthalmol. 2007;42:832–835. doi:10.3129/i07-155
11. Invernizzi A, Mapelli C, Viola F, et al. Choroidal granulomas visualized by enhanced depth imaging optical coherence tomography. Retina. 2015;35:525–531. doi:10.1097/IAE.0000000000000312
12. Herbort CP, Papadia M, Mantovani A. Classification of choroiditis based on inflammatory lesion process rather than fundus appearance: enhanced comprehension through the ICGA concepts of the iceberg and jellyfish effects. Klin Monbl Augenheilkd. 2012;229:306–313. doi:10.1055/s-0031-1299394
13. Papadia M, Herbort CP. Unilateral papillitis, the tip of the iceberg of bilateral ICGA-detected tuberculous choroiditis. Ocul Immunol Inflamm. 2011;19:124–126. doi:10.3109/09273948.2010.530872
14. Chhablani J, Barteselli G. Clinical applications of choroidal imaging technologies. Indian J Ophthalmol. 2015;63:384–390. doi:10.4103/0301-4738.159861
15. Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811–815. doi:10.1016/j.ajo.2008.12.008
16. Estrada-Veras JI, O’Brien KJ, Boyd LC, et al. The clinical spectrum of Erdheim-Chester disease: an observational cohort study. Blood Adv. 2017;1:357–366. doi:10.1182/bloodadvances.2016001784
17. Alper MG, Zimmerman LE, Piana FG. Orbital manifestations of Erdheim-Chester disease. Trans Am Ophthalmol Soc. 1983;81:64–85.
18. Shields CL, Kels JG, Shields JA. Melanoma of the eye: revealing hidden secrets, one at a time. Clin Dermatol. 2015;33:183–196. doi:10.1016/j.clindermatol.2014.10.010
19. Fenton S, Kemp EG, Harnett AN. Screening for ophthalmic involvement in asymptomatic patients with metastatic breast carcinoma. Eye (Lond). 2004;18:38–40. doi:10.1038/sj.eye.6700535
20. Wiegel T, Kreusel KM, Bornfeld N, et al. Frequency of asymptomatic choroidal metastasis in patients with disseminated breast cancer: results of a prospective screening programme. Br J Ophthalmol. 1998;82:1159–1161. doi:10.1136/bjo.82.10.1159
21. Mewis L, Young SE. Breast carcinoma metastatic to the choroid. Analysis of 67 patients. Ophthalmology. 1982;89:147–151. doi:10.1016/S0161-6420(82)34838-1
22. Novais EA, Baumal CR, Sarraf D, et al. Multimodal imaging in retinal disease: a consensus definition. Ophthalmic Surg Lasers Imaging Retina. 2016;47:201–205. doi:10.3928/23258160-20160229-01
23. Sumich P, Mitchell P, Wang JJ. Choroidal nevi in a white population: the blue mountains eye study. Arch Ophthalmol. 1998;116:645–650. doi:10.1001/archopht.116.5.645
24. Lu J, Mai G, Luo Y, et al. Appearance of far peripheral retina in normal eyes by ultra-widefield fluorescein angiography. Am J Ophthalmol. 2017;173:84–90. doi:10.1016/j.ajo.2016.09.024
25. Seo EJ, Kim JG. Analysis of the normal peripheral retinal vascular pattern and its correlation with microvascular abnormalities using ultra-widefield fluorescein angiography. Retina. 2019;39:530–536. doi:10.1097/IAE.0000000000001984
26. Freund KB, Laud K, Lima LH, et al. Acquired vitelliform lesions: correlation of clinical findings and multiple imaging analyses. Retina. 2011;31:13–25. doi:10.1097/IAE.0b013e3181ea48ba
27. Pang CE, Shields CL, Jumper JM, Yannuzzi LA. Paraneoplastic cloudy vitelliform submaculopathy in primary vitreoretinal lymphoma. Am J Ophthalmol. 2014;158:1253–1261 e2. doi:10.1016/j.ajo.2014.08.031
28. Saito M, Iida T, Freund KB, et al. Clinical findings of acquired vitelliform lesions associated with retinal pigment epithelial detachments. Am J Ophthalmol. 2014;157:355–365 e2. doi:10.1016/j.ajo.2013.10.009
29. Zweifel SA, Spaide RF, Yannuzzi LA. Acquired vitelliform detachment in patients with subretinal drusenoid deposits (reticular pseudodrusen). Retina. 2011;31:229–234. doi:10.1097/IAE.0b013e3181f049bd
30. Govetto A, Bhavsar KV, Virgili G, et al. Tractional abnormalities of the central foveal bouquet in epiretinal membranes: clinical spectrum and pathophysiological perspectives. Am J Ophthalmol. 2017;184:167–180. doi:10.1016/j.ajo.2017.10.011
31. Rusu IM, Mrejen S, Engelbert M, et al. Immunogammopathies and acquired vitelliform detachments: a report of four cases. Am J Ophthalmol. 2014;157:648–657 e1. doi:10.1016/j.ajo.2013.11.020
32. Viola F, Barteselli G, Dell’Arti L, et al. Multimodal imaging in deferoxamine retinopathy. Retina. 2014;34:1428–1438. doi:10.1097/IAE.0000000000000073
33. Sandhu HS, Kolomeyer AM, Lau MK, et al. Acute exudative paraneoplastic polymorphous vitelliform maculopathy during vemurafenib and pembrolizumab treatment for metastatic melanoma. Retin Cases Brief Rep. 2019;13:103–107. doi:10.1097/ICB.0000000000000604
34. Choe CH, McArthur GA, Caro I, et al. Ocular toxicity in BRAF mutant cutaneous melanoma patients treated with vemurafenib. Am J Ophthalmol. 2014;158:831–837 e2. doi:10.1016/j.ajo.2014.07.003
35. Liu CY, Francis JH, Brodie SE, et al. Retinal toxicities of cancer therapy drugs: biologics, small molecule inhibitors, and chemotherapies. Retina. 2014;34:1261–1280. doi:10.1097/IAE.0000000000000242
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.