Back to Journals » Clinical Ophthalmology » Volume 14
Multifocal ERG and Microperimetry Changes in Response to Ranibizumab Treatment of Neovascular AMD: Randomized Phase 2 Open-Label Study
Authors Asahi MG , Wallsh J , Onishi SM, Kuroyama S, Gallemore RP
Received 30 July 2020
Accepted for publication 5 October 2020
Published 29 October 2020 Volume 2020:14 Pages 3599—3610
DOI https://doi.org/10.2147/OPTH.S270243
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Masumi G Asahi, Josh Wallsh, Spencer M Onishi, Shari Kuroyama, Ron P Gallemore
Clinical Research Department, Retina Macula Institute, Torrance, CA, USA
Correspondence: Ron P Gallemore Tel +1 (310) 944-9393
Fax +1 (310) 944-3393
Email [email protected]
Purpose: To compare monthly versus pro re nata (PRN) ranibizumab injections in the treatment of exudative macular degeneration (AMD) while assessing the utility of microperimetry (MP) and multifocal electroretinography (mfERG) testing when monitoring response to treatment.
Methods: A randomized exploratory trial comparing the efficacy of monthly versus PRN dosing of ranibizumab (0.5 mg or 2.0 mg) for patients with exudative AMD over 12 months. High-resolution optical coherence tomography (HR-OCT) studies were used to guide PRN treatment and any cystic spaces or subretinal fluid prompted retreatment. Macular function was assessed using mean sensitivity on MP and N1-P1 response density on mfERG. Best-corrected visual acuity (BCVA) was measured with Early Treatment Diabetic Retinopathy Study (ETDRS) letters and anatomic response assessed with central foveal thickness (CFT) using HR-OCT studies.
Results: The 12-month study was completed by 43 patients in the PRN cohort and 33 patients in the monthly cohort. Mean BCVA improved by 6.0 ± 1.3 ETDRS letters in the PRN cohort compared to 7.3 ± 2.8 ETDRS letters in the monthly cohort (p=0.68). A reduction in mean CFT of 64.5 ± 13.3 and 96.3 ± 22.0 μm occurred in the PRN and monthly cohorts, respectively (p=0.22). Macular function assessed with mfERG decreased comparably in both the PRN and monthly cohorts (p=0.33). For all patients, average mean sensitivity significantly improved by 1.7 ± 0.5 dB (p< 0.05) and N1-P1 response density significantly decreased by 0.52 ± 0.21 nV/deg2 (p< 0.05).
Conclusion: Both PRN and monthly treatment of exudative AMD with ranibizumab improve visual function as assessed by BCVA and MP. Macular thickening also improved as demonstrated by HR-OCT findings. However, the decreased retinal function noted by mfERG suggests that some loss of retinal function still occurs despite effective treatment. These measures of visual function may be useful in assessing retinal health and response to treatment in future clinical trials.
Keywords: age-related macular degeneration, microperimetry, multifocal ERG, ranibizumab
Introduction
Age-related macular degeneration (AMD) remains the leading cause of blindness in adults over 50 years of age in developed nations throughout the world and the third leading cause globally.1,2 Approximately 11 million individuals are affected with AMD in the United States with a global prevalence of 170 million.1 The disease is categorized into nonexudative and exudative AMD with the latter referring to the advanced neovascular stage of the disease. The current mainstay of treatment for neovascular AMD (nAMD) is intravitreal anti-vascular endothelial growth factor (anti-VEGF) injections delivered utilizing various treatment protocols.
Multiple anti-VEGF drugs are available to treat nAMD, but the four most commonly utilized include bevacizumab (Avastin; Genentech, Inc., South San Francisco, CA, USA), ranibizumab (Lucentis; Genentech; South San Francisco, CA), aflibercept (Eylea; Regeneron; Tarrytown, NY), and most recently, brolucizumab (Beovu; Novartis; Basel, Switzerland). Ranibizumab was the first drug approved by the FDA for the treatment of nAMD. The initial clinical trials of this agent utilized a monthly injection protocol for one to two years with further studies utilizing an as needed (PRN) dosing.3–9 As needed dosing uses multiple parameters, including the presence of fluid on optical coherence tomography (OCT), to determine whether treatment is required at each visit. Other protocols have also been utilized, including quarterly injections and treat and extend protocol.10,11 Repeat injections incur a financial burden to patients and can carry risks such as atrophy.12
The majority of studies investigating ranibizumab have used best-corrected visual acuity (BCVA) as the primary measure of improvement.4–10,13 Although a valid measure, there are cases where BCVA does not accurately reflect the health of the retina. Underlying pathology of the retina or potential for vision to decline further are situations that are not addressed using BCVA alone. High-resolution OCT (HR-OCT) has the ability to locate fluid in the macula and can provide an indication to treat with PRN therapy based on anatomical indications, but fails to provide information regarding retinal function. Multifocal electroretinography (mfERG) and microperimetry (MP) represent diagnostic modalities that have been utilized for various retinal pathologies to evaluate retinal function.14,15 In the present study, we assess the safety and efficacy of PRN versus monthly ranibizumab therapy for nAMD using the multiple endpoints of BCVA, HR-OCT, MP and mfERG.
Methods
Study Design
This randomized exploratory trial was performed in a single-center conducted from January 2009 to November 2011. All patients gave written informed consent. Institutional Review Board/Ethics Committee approval was obtained for the study protocol by Western Institutional Review Board (Protocol #20,081,560) and the study was registered on clinicaltrials.gov as NCT00764738. The trial was conducted in accordance with the Declaration of Helsinki. Support for this investigator sponsored trial was provided by Genentech, Inc.
Patients
Patients diagnosed with active nAMD were enrolled in this 12-month study. Major exclusion criteria included presence of concurrent eye disease in the study eye (eg, diabetic retinopathy), previous treatment with photodynamic therapy (PDT), intravitreal steroid therapy within the past 3 months, and anti-VEGF therapy within a month of study enrollment.
After screening, patients were stratified into either treatment-naïve or previously treated groups. Patients were then randomized into 1) 0.5 mg ranibizumab monthly or 2) 0.5 mg ranibizumab PRN. After group 1 and 2 enrollment was complete, patients subsequently enrolling were randomized into 3) 2.0 mg ranibizumab monthly or 4) 2.0 mg ranibizumab PRN. Treatment in the PRN cohorts was initially monthly for 4 months followed by PRN thereafter with monthly visits and treatment determined based on the presence of fluid on HR-OCT testing.
Assessments
Monthly evaluations included a full ophthalmic examination, BCVA in Early Treatment Diabetic Retinopathy Study (ETDRS) letters, and HR-OCT (Cirrus HD-OCT; Carl Zeiss Meditec, Dublin, CA) with central foveal thickness (CFT) measurement. If segmentation errors of the ILM-RPE were encountered during the scan, these were corrected manually prior to analysis. Assessment with mfERG (UTAS-E300; LKC Technologies, Gaithersburg, MD) and MP (MP-1 microperimeter; Nidek Technologies, Padova, Italy) were performed for all studied patients. For groups receiving monthly treatment, these assessments were performed at baseline, and at months 3, 5, 8 and 12. Groups receiving PRN treatment were assessed at baseline, months 3 and 5, then monthly thereafter. On mfERG, N1-P1 response density was measured as the difference from the first positive peak (P1) to the first negative peak (N1). All mfERG measurements were made within the first ring including P1 latency and P1, N1, and N1-P1 response densities. Response density was calculated as the amplitude/unit area within the first ring (nv/deg2). Due to required maintenance on the mfERG many patients failed to obtain complete data, missing values were imputed based on a last observation carried forward technique. Mean sensitivity on MP was calculated by averaging the stimulus intensity at all 40 measurement points.
Statistical Analysis
Statistical analysis was performed in those patients that completed the 12-month trial. Paired Student’s t-tests were used to compare baseline to final visit response for all cohorts. Given significant heterogeneity in mfERG baseline data, comparisons between cohorts (monthly versus PRN, treatment naïve versus previously treated, and 0.5 mg versus 2.0 mg ranibizumab) was performed utilizing a two-way repeated-measures analysis of variance (ANOVA), all other cohort comparisons were performed with Student’s t-test. For all such statistical analysis, data were considered significant for p-values less than 0.05.
Results
Patients and Treatment
Ninety-one patients were enrolled with 77 completing the study, Table 1 presents baseline characteristics for these patients. Of the patients who failed to complete the study, the majority withdrew or were lost to follow-up after their screening visit with 3 patients passing away prior to completion. Within the PRN treatment group, patients received an average of 9.41 ± 0.34 injections out of a possible 12 injections.
 |
Table 1 Baseline Demographics |
Visual Acuity and Central Foveal Thickness
For all patients that completed the 12-month study, BCVA significantly increased by a mean of 6.57 ± 1.41 ETDRS letters (p < 0.001) and mean CFT decreased by −78.32 ± 12.23 µm (p < 0.005). For the group receiving PRN ranibizumab, BCVA increased by a mean of 6.00 ± 1.25 ETDRS letters (p < 0.005) and CFT decreased by a mean 64.53 ± 13.33 µm (p < 0.005). In patients receiving monthly ranibizumab, BCVA increased by a mean 7.30 ± 2.82 ETDRS letters (p < 0.05) and CFT decreased by a mean of 96.27 ± 22.04 µm (p < 0.005). The mean change in BCVA and CFT at 12 months improved more in the monthly cohort than in the PRN cohort, but neither difference was statistically significant (p = 0.68 and 0.22, respectively). Comparison of treatment naïve and previously treated subgroups demonstrated a greater mean improvement in the treatment-naïve subgroup for both BCVA (p = 0.32) and CFT (p < 0.005). Both 0.5 mg and 2.0 mg ranibizumab demonstrated similar improvements in BCVA (p > 0.99) and CFT (p = 0.71). Figures 1 and 2 respectively demonstrate BCVA and CFT change over time.
Multifocal Electroretinography and Microperimetry
Mean N1-P1 response density and P1 response density on mfERG decreased for all studied patients from 4.22 ± 0.32 to 3.70 ± 0.29 and 2.88 ± 0.23 to 2.41 ± 0.18 nv/deg2, respectively (p < 0.05). Comparing the monthly and PRN, treatment naive and previously treated, 0.5 and 2.0 mg subgroups demonstrated a non-significant difference in the change in mean N1-P1 and P1 response densities (Table 1). Evaluation of mean N1 response density revealed a non-significant improvement in all studied eyes (p = 0.91). Comparison of mean change in N1 response density for the treatment naïve and previously treated cohorts revealed a significantly greater improvement in the treatment-naïve cohort (p < 0.05; Table 2). For all studied patients, P1 latency on mfERG did not significantly change from baseline to final visit (p = 0.69). Phakic and pseudophakic eyes were noted to have similar changes in P1, N1 and N1-P1 response densities over the study (Table 3). Microperimetry demonstrated a significant improvement at 12 months in mean sensitivity for all studied patients, 5.28 ± 0.54 to 7.20 ± 0.57 dB (p < 0.05). Mean sensitivity improved significantly more for the treatment-naive cohort, 3.00 ± 0.75 dB, than the previously treated cohort, 0.06 ± 0.50 dB (p < 0.005). There was no significant difference in the comparison of the monthly and PRN or 0.5 and 2.0 mg cohorts (p = 0.25 and 0.67, respectively). Figures 3 and 4 respectively demonstrate the screening and final visit average measurements for mfERG and MP data.
 |
Table 2 Multifocal Electroretinography Cohort Comparisons |
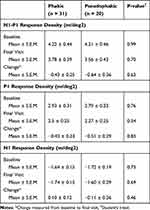 |
Table 3 Lens Status and Multifocal Electroretinography Data |
Complications
Four patients developed adverse reactions, but none required withdrawal due to these complications. Mild adverse reactions included a patient with mild ocular discomfort and another who developed a corneal abrasion following injections. More serious adverse reactions included a vitreous hemorrhage and a patient who developed uveitic glaucoma.
Discussion
In the present study we found that both monthly and PRN dosing of ranibizumab achieved significant improvements in visual acuity and reduction in central macular thickness with a greater, but not significant, improvement in the monthly cohorts. Treatment on a PRN protocol required, on average, three less treatments. The utilization of mfERG and MP can also provide additional information regarding retinal function in response to ranibizumab treatment.
Ranibizumab 0.5mg vs Ranibizumab 2.0mg
Both ranibizumab treatment groups demonstrated similar improvements in BCVA and CFT (Figures 1A and 2A). The 2.0mg dose did not show any clinically meaningful difference in efficacy or safety compared with the 0.5mg ranibizumab dose. These findings are consistent with previous studies.7,16
Prior Treatment vs Naïve Treatment Groups
Consistently, prior treatment groups showed less potential to improve compared to the naïve treatment groups (Figures 1B and 2B). Interestingly, the findings on OCT and MP were the only modalities to demonstrate a significant difference in these cohorts (Figures 2B and 4). The previously treated cohort achieved less robust results due in large part to the chronic nAMD.17 Many of these patients may have developed tachyphylaxis to ranibizumab, which limited their improvement and resolution of recalcitrant fluid.18 Switching those patients that may have developed tachyphylaxis to ranibizumab to another anti-VEGF agent may have been more effective in reducing macular thickness and improving BCVA.19 The notable difference in MP results is due in large part to the significant improvement appreciated in the treatment-naïve cohort (the only studied cohort with such a notable improvement). This is likely representative of the response that recently converted nAMD has to anti-VEGF agents and, as such, may be a useful parameter for future studies on nAMD patients especially in those who are treatment naïve.20
PRN vs Monthly
Treatment protocols for anti-VEGF injections have evolved over the years with options including monthly, PRN, and treat-and-extend (TAE). Monthly injections represent the original treatment strategy whereas the other options such as PRN and TAE dosing decrease the injection burden on patients. In the present study, we evaluated monthly against PRN dosing and found no significant difference in BCVA or CMT at 12 months of treatment (Figures 1C and 2C). Although not statistically significant, there was a trend towards better vision and reduction in CMT with monthly compared to PRN dosing. Overall, patients in the PRN dosing group required an average of 9 out of a possible 12 injections, which included the initial 4 scheduled monthly injections.
Previous studies have similarly evaluated monthly and PRN dosing regimens head to head. The consensus from these previous studies appears consistent with the findings in our study: PRN dosing offers similar treatment efficacy at a lower injection burden than monthly treatment.7,8,16,21,22 A systematic review and network meta-analysis assessing the clinical effectiveness of ranibizumab on a TAE regimen in nAMD found that TAE regimen yields similar effectiveness compared to other treatment regimens such as monthly and PRN dosing for both ranibizumab and aflibercept.11
An additional possible benefit to the decreased treatment burden with PRN dosing not fully evaluated by our study, but highlighted in the IVAN study is the impact on geographic atrophy.8,21 At two years, significantly fewer patients were noted to have new areas of geographic atrophy in the PRN cohort (26%) versus the monthly cohort (34%).21 This has been a point of concern with anti-VEGF injections, but the impact of PRN dosing on this requires more dedicated research.
Our study design supports evidence that alternative treatment options offer similar benefits to monthly anti-VEGF treatments. One limitation of this study is that we do not include a TAE cohort to further compare current treatment protocols.
Multifocal Electroretinography
The use of mfERG has been noted to be valuable in the evaluation of many retinal pathologies notably as a screening tool for hydroxychloroquine toxicity.23,24 Among the important measures on mfERG are the P1 response density which has been shown to correlate with bipolar cell function,25 N1 response density with correlations to both photoreceptor and bipolar function,25 and N1-P1 response density representing these ON functions as a whole. Multifocal ERG testing can be a more objective measure of retinal function.25
In our study we found a significant decrease in both N1-P1 response density at the end of the 1-year study for patients of all subgroups; however, P1 latency on mfERG did not significantly change from baseline to final visit (Figure 3A and B). This was despite the improvement seen in both BCVA and CMT arguing for some level of permanent damage to bipolar cells, possibly correlating with fibrotic or atrophic changes, despite the resolution of fluid.25 Interestingly, these results were consistent even in the treatment-naïve patients who would most be expected to demonstrate an improvement in mfERG data.
There are several factors that could account for these changes. Response densities are known to decrease with age26 and studies have also shown microstructural and functional progression despite stable morphological features in both non-neovascular and neovascular AMD.27,28 In our present study, many patients—especially the previously treated patients—had developed fibrosis or a macular scar, inhibiting the function of the inner layer. This contributed to a decrease in the intensity of baseline mfERG responses and it would be expected that the use of ranibizumab would have limited efficacy in treating these entities.
The possible deleterious impact of anti-VEGF injections on retinal health has been well documented, most notably in the form of geographic atrophy progression and advancement of fibrosis.29–31 The depreciations in mfERG measurements across cohorts may be representative of this negative impact. It is important to note that the only mfERG measurement with a significant difference when compared across cohorts was the N1 response density between the treatment naïve and previously treated groups. Being a representation of both photoreceptor and bipolar health, this value may be less affected by the possible acute fibrotic or atrophic retinal changes to anti-VEGF therapy. This data is limited by its small sample size; however, follow-up evaluation of treatment-naïve patients based on time since neovascular conversion and BCVA may be beneficial as these additional characteristics should correlate with expected response in retinal function.
Cataract progression can also affect mfERG with response density losses through reduction in retinal illuminance and increase in intraocular scatter.26 Although we observed no significant progression of cataract throughout the duration of the study, undetected increases in lens opacities could also have played a role in the reduced response density. However, evaluation of mfERG data based on baseline lens status noted similar decreases in both phakic and pseudophakic eyes, which supports the notion that the changes were due to diminishing macular function.
Previous studies have evaluated the utility in monitoring treatment response in nAMD with mixed results. One study showed that mfERG significantly improved in patients with nAMD during the initiation phase of intravitreal ranibizumab with P1 response density of the central ring significantly increasing after the first of three injections.32 However, Reinsberg et al demonstrated that monthly injections with ranibizumab over one year had no statistically significant effects on P1 response density.28 Over the 12-month enrollment in the study, mfERG results showed retinal health did not improve with either the PRN or monthly ranibizumab injection protocol despite improvements in other endpoints. The mfERG results may prove to be the most sensitive measure of retinal stimulus and reveal loss of structural integrity with chronic nAMD. It is the only direct measure of retinal function that is unbiased by subjectiveness. Investigation of an agent that can improve retinal health and subsequently vision is of further interest. This more objective measure of retinal function may prove particularly useful as we try to identify agents that truly preserve retinal function with the treatment of nAMD.
Microperimetry
MP is a subset of visual field testing which evaluates retinal sensitivity while the fundus is directly examined, allowing correlation between macular pathology and corresponding functional abnormalities. Early macular function loss can be detected by MP before significant visual impairment is established and changes on MP have been documented as a foretelling of progression of intermediate AMD to advanced and exudative stages.33 MP has become a common way to measure macular function in assessment of natural history and treatment outcomes in macular disease.
Mean sensitivity improved significantly for all studied patients over this 12-month study (Figure 4). This MP data is consistent with the findings in other studies with improvement in retinal sensitivity measured using this technique.14,34 Overall there was a consistent trend of improvement in all cohorts with the treatment-naïve cohort being the only one with a statistically significant improvement at 12 months. These findings suggest that MP may be a useful adjunctive measure of retinal function and an additional clinical trial endpoint for future nAMD research. On the other hand, visual field testing, of which MP is a subset, is subjective. There is a learning curve and with repeat testing the results can improve in the absence of improved retinal or optic nerve function.35,36 This makes MP a more subjective endpoint – similar to visual acuity.
An important consideration for both mfERG and MP results is that in patients with macular pathology such as nAMD their results may be impacted by fixation difficulties.37
Limitations
Our study is limited by the lack of inclusion of a TAE group for full evaluation of current treatment protocols. In addition, there was a significant difference in baseline characteristics between the monthly and PRN cohorts in regards to mfERG. However, in our final evaluation of change in all of these measures, there was not a statistically significant difference when evaluated across the cohorts. A final limitation, which was discussed above, is the inherent difficulty with obtaining MP and mfERG in patients with nAMD due to possible fixation impairment. It is unclear the impact this had in our population but should be kept in mind when evaluating results.
Conclusions
Both PRN and monthly treatment of nAMD with ranibizumab is associated with significant improvement in visual function as assessed by BCVA and MP and improvement in macular thickening on HR-OCT. Monthly treatment showed a nonsignificant trend towards better BCVA and reduction in CMT compared to PRN treatment whereas treatment on a PRN protocol required an average of three less injections compared to monthly. Prior treatment groups showed less potential to improve compared to the naive treatment groups and the 2.0mg dose of ranibizumab showed no clinically meaningful difference in efficacy compared with the 0.5mg dose. Despite the improvements noted on other modalities, the mfERG data demonstrated a decline in almost all measures. These measures of visual function may be useful in assessing retinal health and response to treatment in future clinical trials.
Data Sharing Statement
The authors intend to share the study protocol, statistical analysis plan, and de-identified aggregate data presented in the manuscript immediately following publication and ending 36 months following article publication with researchers who provide a methodologically sound proposal. This data is available from the corresponding author on reasonable request.
Acknowledgments
This study was funded as an investigator-sponsored trial by Genentech, Inc., South San Francisco, CA. Support for third-party editorial assistance for this manuscript, provided by Envision Scientific Solutions, was provided by Genentech, Inc. Trial registration: clinicaltrials.gov identifier: NCT00764738.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded as an investigator-sponsored trial by Genentech, Inc., South San Francisco, CA. Support for third-party editorial assistance for this manuscript, provided by Envision Scientific Solutions, was provided by Genentech, Inc.
Disclosure
The following authors have no financial disclosures: MGA, JW, SMO, RPG, SK. The authors report no conflicts of interest for this work.
References
1. Pennington KL, DeAngelis MM. Epidemiology of age-related macular degeneration (AMD): associations with cardiovascular disease phenotypes and lipid factors. Eye Vis (Lond). 2016;3(34):1–20.
2. Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485.
3. Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26(8):859–870. doi:10.1097/01.iae.0000242842.14624.e7
4. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi:10.1056/NEJMoa054481
5. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi:10.1056/NEJMoa062655
6. Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. doi:10.1016/j.ophtha.2012.03.053
7. Busbee BG, Ho AC, Brown DM, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120(5):1046–1056. doi:10.1016/j.ophtha.2012.10.014
8. Chakravarthy U, Harding SP, Rogers CA, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119(7):1399–1411. doi:10.1016/j.ophtha.2012.04.015
9. Krebs I, Schmetterer L, Boltz A, et al. A randomised double-masked trial comparing the visual outcome after treatment with ranibizumab or bevacizumab in patients with neovascular age-related macular degeneration. Br J Ophthalmol. 2013;97(3):266–271. doi:10.1136/bjophthalmol-2012-302391
10. Gupta OP, Shienbaum G, Patel AH, et al. A treat and extend regimen using ranibizumab for neovascular age-related macular degeneration clinical and economic impact. Ophthalmology. 2010;117(11):2134–2140.
11. Danyliv A, Glanville J, McCool R, et al. The clinical effectiveness of ranibizumab treat and extend regimen in nAMD: systematic review and network meta-analysis. Adv Ther. 2017;34(3):611–619. doi:10.1007/s12325-017-0484-0
12. Thavikulwat AT, Jacobs-El N, Kim JS, et al. Evolution of geographic atrophy in participants treated with ranibizumab for neovascular age-related macular degeneration. Ophthalmol Retina. 2017;1(1):
13. Ferris FL
14. Alexander P, Mushtaq F, Osmond C, Amoaku W. Microperimetric changes in neovascular age-related macular degeneration treated with ranibizumab. Eye (Lond). 2012;26(5):678–683. doi:10.1038/eye.2012.7
15. Gerth C. The role of the ERG in the diagnosis and treatment of age-related macular degeneration. Doc Ophthalmol. 2009;118(1):63–68. doi:10.1007/s10633-008-9133-x
16. Ho AC, Busbee BG, Regillo CD, et al. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2014;121(11):2181–2192. doi:10.1016/j.ophtha.2014.05.009
17. Dirani A, Mantel I. Ranibizumab treatment history as predictor of the switch-response to aflibercept: evidence for drug tolerance. Clin Ophthalmo. 2018;12:593–600. doi:10.2147/OPTH.S160367
18. Eghoj MS, Sorensen TL. Tachyphylaxis during treatment of exudative age-related macular degeneration with ranibizumab. Br J Ophthalmol. 2011;96(1):21–23. doi:10.1136/bjo.2011.203893
19. Gasperini JL, Fawzi AA, Khondkaryan A, et al. Bevacizumab and ranibizumab tachyphylaxis in the treatment of choroidal neovascularisation. Br J Ophthalmol. 2012;96(1):
20. Holz FG, Figueroa MS, Bandello F, et al. Ranibizumab treatment in treatment-naive neovascular age-related macular degeneration: results from luminous, a global real-world study. Retina. 2019:
21. Chakravarthy U, Harding SP, Rogers CA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382(9900):1258–1267. doi:10.1016/S0140-6736(13)61501-9
22. The CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–1908. doi:10.1056/NEJMoa1102673
23. Young B, Eggenberger E, Kaufman D. Current electrophysiology in ophthalmology: a review. Curr Opin Ophthalmol. 2012;23(6):497–505. doi:10.1097/ICU.0b013e328359045e
24. Vincent A, Robson AG, Holder GE. Pathognomonic (diagnostic) ERGs. A review and update. Retina. 2013;33(1):5–12. doi:10.1097/IAE.0b013e31827e2306
25. Hood DC, Frishman LJ, Saszik S, Viswanathan S. Retinal origins of the primate multifocal ERG: implications for the human response. Invest Ophthalmol Vis Sci. 2002;43(5):1673–1685.
26. Gerth C, Garcia SM, Ma L, Keltner JL, Werner JS. Multifocal electroretinogram: age-related changes for different luminance levels. Graefes Arch Clin Exp Ophthalmol. 2002;240(3):202–208. doi:10.1007/s00417-002-0442-6
27. Gerth C, Delahunt PB, Alam S, Morse LS, Werner JS. Cone-mediated multifocal electroretinogram in age-related macular degeneration: progression over a long-term follow-up. Arch Ophthalmol. 2006;124(3):345–352. doi:10.1001/archopht.124.3.345
28. Reinsberg M, Hilgers R, Ludeke I, et al. Testing the clinical value of multifocal electroretinography and microperimetry and the effects of intravitreal therapy with ranibizumab on macular function in the course of wet age-related macular degeneration: a 1-year prospective study. Clin Ophthalmol. 2017;11:621–629. doi:10.2147/OPTH.S123513
29. Gemenetzi M, Lotery AJ, Patel PJ. Risk of geographic atrophy in age-related macular degeneration patients treated with intravitreal anti-VEGF agents. Eye (Lond). 2017;31(1):1–9. doi:10.1038/eye.2016.208
30. Grunwald JE, Daniel E, Huang J, et al. The CATT research group risk factors for the development of GA in CATT. Ophthalmology. 2014;121(1):150–161. doi:10.1016/j.ophtha.2013.08.015
31. Cohen SY, Oubraham H, Uzzan J, et al. Causes of unsuccessful ranibizumab treatment in exudative age-related macular degeneration in clinical settings. Retina. 2012;32(8):1480–1485.
32. Campa C, Hagan R, Sahni JN, et al. Early multifocal electroretinogram findings during intravitreal ranibizumab treatment for neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:3446–3451.
33. Dinc UA, Yeneral M, Gorgun E, Oncel M. Assessment of macular function by microperimetry in intermediate age-related macular degeneration. Eur J Ophthalmol. 2008;18(4):595–600.
34. Parravano M, Oddone F, Tedeschi M, et al. Retinal functional changes measured by microperimetry in neovascular age-related macular degeneration treated with ranibizumab: 24-month results. Retina. 2010;30(7):
35. Wu Z, Ayton LN, Guymer RH, Luu CD. Intrasession test-retest variability of microperimetry in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54(12):7378–7385. doi:10.1167/iovs.13-12617
36. Chen FK, Patel PJ, Xing W, et al. Test–retest variability of microperimetry using the nidek MP1 in patients with macular disease. Invest Ophthalmol Vis Sci. 2009;50(7):3464–3472. doi:10.1167/iovs.08-2926
37. Vujosevic S, Pucci P, Casciano M, et al. Long-term longitudinal modifications in mesopic microperimetry in early and intermediate age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2017;255(2):301–309. doi:10.1007/s00417-016-3466-z
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




