Back to Journals » Research and Reports in Urology » Volume 13
Multidisciplinary Therapy in Men with Newly Diagnosed Oligometastatic Prostate Cancer
Authors Watanabe K, Kawaguchi G, Ikeda Y, Hara N, Nishiyama T
Received 16 May 2021
Accepted for publication 7 July 2021
Published 10 August 2021 Volume 2021:13 Pages 565—571
DOI https://doi.org/10.2147/RRU.S320433
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jan Colli
Kazuhiro Watanabe,1,* Gen Kawaguchi,2,* Yohei Ikeda,3 Noboru Hara,1 Tsutomu Nishiyama1
1Department of Urology, Uonuma Institute of Community Medicine, Niigata University Medical and Dental Hospital, Minamiuonuma, Niigata, Japan; 2Department of Radiotherapy, Uonuma Institute of Community Medicine, Niigata University Medical and Dental Hospital, Minamiuonuma, Niigata, Japan; 3Department of Diagnostic Radiology, Uonuma Institute of Community Medicine, Niigata University Medical and Dental Hospital, Minamiuonuma, Niigata, Japan
*These authors contributed equally to this work
Correspondence: Tsutomu Nishiyama
Department of Urology, Uonuma Institute of Community Medicine, Niigata University Medical and Dental Hospital, Urasa 4132, Minamiuonuma, Niigata, 949-7302, Japan
Tel +81-25-777-3200
Fax +81-25-777-5067
Email [email protected]
Purpose: To assess the use of aggressive local therapy with systemic therapy for patients with oligometastatic prostate cancer.
Patients and Methods: Patients with oligometastatic prostate cancer received systemic therapy centered on androgen deprivation therapy (ADT). After six months or more of ADT, the patients received radiation therapy or surgery for the prostate, and radiation therapy for all metastatic sites. ADT was continued for 2– 3 years after local therapy.
Results: Twelve patients who were judged to be able to undergo radiotherapy or surgical treatment of the prostate and radiation therapy for all metastatic sites and one case of pubic bone recurrence after radical prostatectomy were included. Bone metastases (n = 11) and para-aortic lymph node metastases (n = 2) were found. The number of bone metastases was one (n = 7), two (n = 3), and three (n = 1). Radiation therapy (70– 74 Gy) was performed on the prostate in 11 of 12 patients. A prostatectomy was performed on one patient who was judged to be unable to receive a sufficient dose to the metastatic site when radical radiation was applied to the prostate. Radiation therapy (45– 60 Gy) was performed on all metastatic sites. Prostate-specific antigen (PSA) levels increased again during treatment in three patients. There was a significant difference in PSA levels before local therapies between the three patients who developed castration-resistant prostate cancer (CRPC) during the course of treatment and the eight patients who did not progress to CRPC (p = 0.012). There was also a significant difference in PSA levels after local therapies between the three patients who developed CRPC during the course of treatment and the eight patients who did not progress to CRPC (p = 0.012). Four patients completed treatment. In one patient in whom the testosterone level recovered to the normal level the PSA level remained the level below the measurement sensitivity.
Conclusion: Aggressive local therapy in combination with systemic therapy centered on ADT is a promising treatment option for oligometastatic prostate cancer.
Keywords: prostate cancer, oligometastases, multidisciplinary therapy
Introduction
Prostate cancer is one of the most common cancers in men. Metastatic prostate cancer remains incurable despite advances in the systemic management of hormone-sensitive prostate cancer and castration-resistant prostate cancer (CRPC) patients.
Oligometastasis was first defined by Hellmann and Weichselbaum in 1995 as a distinct biological and clinically relevant state between localized and widespread systemic disease.1 Oligometastatic prostate cancer is one of the clinical states observed along the spectrum of the natural history of prostate cancer. Local therapy to the prostate for oligometastatic prostate cancer may slow the progression of the disease and improve overall survival.2 A multimodal treatment strategy with systemic therapy and local therapy can eliminate detectable disease in selected patients with metastatic spread at diagnosis.3,4
In the present study, we administered aggressive local therapy for oligometastatic prostate cancer in addition to systemic therapy centered on ADT.
Patients and Methods
Patients
We recruited patients who had untreated metastatic prostate cancer or who relapsed after radical prostate treatment for localized prostate cancer and had not received prior systemic treatment, and had less than five bone metastases, and/or metastatic para-aortic lymph nodes. In the selection of target patients, patients who were judged to be able to undergo prostate treatment by radiotherapy or prostatectomy and radiotherapy for all the metastatic sites were enrolled in the multidisciplinary treatment. This study was approved by the Ethics Committee (Niigata University School of Medicine Ethics Committee Approval No. 2479). All patients provided informed consent to participate in this research and for the details to be published. This study was conducted in accordance with the Declaration of Helsinki.
Diagnostic Procedures
Patients with untreated prostate cancer pathologically diagnosed by prostate biopsy were examined for metastases by routine thoraco-abdomino-pelvic computed tomography (CT) and bone scintigraphy. Systemic therapy centered on ADT was performed for 6 months or longer, and evaluation was performed again using thoraco-abdomino-pelvic CT, bone scintigraphy, and whole-body magnetic resonance imaging (MRI). The presence of metastasis in patients who had relapsed after radical prostate treatment for localized prostate cancer and did not receive prior systemic treatment was examined by thoraco-abdomino-pelvic CT, bone scintigraphy, and whole-body MRI.
Systemic Therapy
No patient was CRPC at the time of patient recruitment. All patients received systemic therapy centered on ADT. Degarelix was used as the gonadotropin releasing hormone (GnRH) antagonist, and leuprorelin or goserelin was used as the GnRH agonist. All patients received bicalutamide 80mg/day, an antiandrogen agent, in combination with ADT. After the start of ADT, docetaxel 70 mg/m2 was administered to patients who gave their consent after sufficient explanation six times in total. Local therapy was performed more than 6 months following the start of ADT. ADT was planned to be continued for 2 to 3 years after the end of local therapy. In the patients who progressed to CRPC, the additional treatments including lifelong ADT were continued thereafter.
Local Therapy
TrueBeam® ver. 2.7 was used as the radiotherapy device, and Eclipse ver. 13.6 was used as the radiation treatment planning system (Varian Medical Systems, CA, USA). The policy was to prescribe a curative dose to the primary lesion of the prostate and all lesions of up to five bone metastases and lymph node metastases.
The prostate was prescribed 70 Gy in 35 fractions to 74 Gy in 37 fractions at the isocenter. Other metastatic sites were prescribed 60 Gy in 30 fractions if possible. Metastases that were close to the small intestine were administered a reduced dose of 54 Gy in 27 fractions, which was a tolerable dose. If there were lymph node metastases before the start of hormone therapy and they shrank below 5 mm, a prophylactic dose of 45 Gy in 25 fractions was prescribed to the lymph node area. If it was judged that a sufficient dose could not be delivered to each of the metastatic lesions based on the size, extent of extension, or positional relationship with the prostate, a curative dose was prescribed only to the bone metastases after radical prostatectomy and lymph node dissection. The prostate was treated with dynamic conformal arc radiation therapy. The whole pelvic lymph node area and other metastases were treated with fixed multiple-field.
Statistics
The PSA levels of patients who progressed to CRPC during the course of treatment and the PSA levels of patients who did not progress to CRPC were examined using the Mann–Whitney U-test. IBM SPSS Statistics Ver.27. was used as the statistics software.
Results. (Table 1)
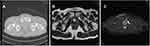
We included 12 patients who were judged to be able to perform radiation therapy or surgical treatment for the prostate and radiation therapy to all metastatic sites and one patient with skeletal recurrence after radical prostatectomy. Their ages ranged from 72 to 85 years, with an average of 77 years. Pathological diagnosis by prostate biopsy was Grade Group 4 (n = 7) and Grade Group 5 (n = 6). Pretreatment PSA levels in patients with untreated metastatic prostate cancer ranged from 16 to 260 ng/mL. The pretreatment PSA level of untreated patients who relapsed after radical prostatectomy for localized prostate cancer was 1.64 ng/mL. In this patient, thoraco-abdomino-pelvic CT and bone scintigraphy did not identify the metastatic site; however, it was identified by whole-body MRI (Figure 1). All thirteen patients received combination therapy with ADT and bicalutamide. Docetaxel therapy was used in combination in six patients.
After systemic therapy for six months or longer, the patients received radiation therapy for the prostate or prostatectomy, and radiation therapy for all metastatic sites. Image evaluation was performed again before local therapy. In addition to thoraco-abdomino-pelvic CT and bone scintigraphy, whole-body MRI was performed. No new additional metastatic site was detected. Bone metastases (n = 11) and para-aortic lymph node metastases (n = 2) were found. The number of bone metastases was one (n = 7), two (n = 3), and three (n = 1). In 11 patients with untreated prostate cancer who were followed for 12 months or more after local treatment, the PSA levels before the start of local therapy were 0.01 or less to 5 ng/mL. Of these, the PSA levels of the three patients who developed CRPC during the course of treatment were 0.25 to 5 ng/mL. The PSA levels of the eight patients who did not progress to CRPC were 0.01 or less to 0.25 ng/mL. There was a significant difference between the two groups (p = 0.012) (Table 2).
 |
Table 1 Patients Characteristics |
 |
Table 2 PSA Levels of Patients with and without Progress to CRPC |
As local therapy, radiation therapy (70–74 Gy) was performed in 11 of 12 cases for the prostate (± pelvic lymph node metastasis site). A radical prostatectomy was performed on one patient who was judged to be unable to receive a sufficient dose to the metastatic site when radical radiation was applied to the prostate. Irradiation (54–60 Gy) was performed on all bone metastatic sites and 45 Gy was applied on the whole pelvic lymph node and para-aortic lymph node area in two patients. In 11 patients with untreated prostate cancer who had passed 12 months or more after local therapy, PSA levels after the end of local therapy were 0.01 or less to 1.55 ng/mL. Of these, the PSA levels of the three patients who developed CRPC during the course of treatment were 0.25 to 1.55 ng/mL. The PSA levels of the eight patients who did not progress to CRPC were 0.01 or less to 0.16 ng/mL. There was a significant difference between the two groups (p = 0.012) (Table 2).
The observation periods after the end of local therapy were 3 to 53 months, with an average of 29.2 months. PSA levels increased again during treatment in three patients; therefore, bicalutamide was changed to enzalutamide. One patient changed from bicalutamide to enzalutamide because a slight re-elevation of PSA level was observed immediately before local therapy. In two patients, their PSA level increased again three months and 12 months after local therapy. No new metastatic site was identified by image re-evaluation at the time of change from bicalutamide to enzalutamide. After change to enzalutamide, PSA levels remained low in all three patients. Four patients completed the therapy. The PSA level remained below the measurement sensitivity in one patient in whom the testosterone level recovered to the normal level.
Discussion
Retrospective analyses and prospective case-control studies of large databases such as the Surveillance, Epidemiology, and End Results database and the US National Cancer Database reported an improved overall survival of radiation therapy for local prostate of oligometastatic prostate cancer.2,5–7 Based on these results, various guidelines recommend local primary radiation therapy for the prostate in combination with ADT.
Secondary analysis of the Southwest Oncology Group 8894 study involving 1286 men with metastatic prostate cancer indicated a significant survival advantage in men who had previously undergone radical prostatectomy.8 In addition, the use of total prostatectomy for metastatic prostate cancer was supported by a retrospective analysis and prospective studies.9
Reducing the amount of metastases in metastatic site treatment for metastatic prostate cancer may also improve patient outcomes. Survival in patients with bone metastatic CRPC was improved in the Alpharadin in Symptomatic Prostate Cancer Patients trial that investigated the addition of 233Ra to standard care.10 In addition, improved survival of metastatic site treatment by stereotactic ablative radiotherapy (SABR) or surgery was shown in patients with oligo-recurrent metastatic prostate cancer.11,12 In the present study, normal multi-field irradiation was performed instead of SABR. The advantages of the normal irradiation instead of the SABR are that the time for one treatment can be shortened and that it is relatively easy to irradiate over a wide area. On the other hand, the disadvantages are that the number of treatments increase and the treatment intensity is weakened. Recently, intensity-modulated radiation therapy and SABR are beginning to be introduced as a curative treatment modality for localized prostate cancer; however, we treated with dynamic conformal arc radiation therapy for the prostate with 70 Gy - 74 Gy at the isocenter.13 70–74 Gy as the prescribed doses is maximum dose as the definitive therapy for the prostate with dynamic conformal arc radiation therapy. Schick et al showed that oligometastatic patients may be successfully treated with high-dose irradiation (>64 Gy) to the metastatic lesions.14 Therefore, 60 Gy as metastases-directed therapy for bone metastases is appropriate dose with normal multi-field irradiation.
With regard to systemic therapy, all patients received systemic therapy centered on ADT. All patients added bicalutamide 80mg/day. Local therapy was performed more than 6 months following the start of ADT. ADT is continued for 2 to 3 years after the end of local therapy. In our country, prior to wide use of new androgen receptor signaling pathway inhibition therapy, combination androgen blockade with bicalutamide 80mg/day in combination with ADT was the standard hormonal therapy.15 The introduction of new hormone therapies such as new androgen receptor-targeted agents has been shown to significantly improve progression-free survival.16–18 In our study, bicalutamide was used in addition to ADT; however, PSA levels were re-elevated in three patients who developed CRPC, and were changed from bicalutamide to enzalutamide. After changing to enzalutamide, the patients who developed CRPC had low and stable PSA levels. Thus, it is possible to use a new androgen receptor-targeted agent in combination with ADT from the initial stage of treatment may lead to an improvement in treatment results. In addition, changes in PSA levels during the course of treatment may be useful markers for inferring progression to CRPC (Table 2).19 The number of patients in our study is low and the observation period is short, the included patients are all alive. Since it is not possible to evaluate the therapeutic effect of bone metastases using images, the evaluation of therapeutic effect used changes in PSA as a biochemical marker with good results.
Evidence found in STAMPEDE trial suggests that prostate radiotherapy improves overall survival for men with metastatic prostate cancer who have a low metastatic burden with lifelong androgen deprivation therapy and with up-front docetaxel if possible.2 In our study, after the start of ADT, docetaxel 70 mg/m2 was administered to patients who gave their consent after sufficient explanation six times in total based on the result of STAMPEDE trial. In our country, patients with advanced prostate cancer are often given docetaxel 70 mg/m2.20
A multidisciplinary trial for untreated oligometastatic prostate cancer reported good results.3,4 Our initial results suggest that multidisciplinary treatment with aggressive prostate and metastasis-oriented therapeutic approaches in combination with systemic therapy centered on ADT for patients with oligometastatic prostate cancer improves patient outcomes.
Oligometastatic disease is a restricted disease state in which, dependent on the definition used, between one and five metastases are detected on imaging. However, the sensitivity of the number of metastases also differs depending on the diagnostic imaging method used (Figure 1). In our country, we cannot use positron emission tomography (PET)-CT for evaluation of bone metastasis. We used whole-body MRI in addition to thoraco-abdomino-pelvic CT and bone scintigraphy instead of PET-CT in this study. Patients with CRPC without metastasis by conventional bone scintigraphy and CT were evaluated by PET diagnostic method targeting prostate-specific membrane antigen (PSMA).21 Distant metastases were detected in 55% of CRPC patients without metastasis by conventional methods. In addition, in a study in which SABR was performed on metastatic sites in patients with oligometastatic prostate cancer diagnosed by conventional bone scintillation and CT diagnostic methods, the patients without newly detected metastatic sites using PSMA-PET had improved progression-free survival compared with the patients with newly detected untreated metastatic sites using PSMA-PET.11 Image reassessment using whole-body MRI of our three CRPC-advanced patients did not reveal a new metastatic site. A more sensitive diagnostic method, such as PSMA-PET, may have diagnosed a new metastatic site. Improvement of diagnostic methods such as PSMA-PET will contribute to the improvement of treatment results for oligometastatic prostate cancer in the future.
Accumulating high-level evidence will support progression-free survival and overall survival benefits of local therapy for prostate and metastatic sites in combination with systemic therapy in patients with oligometastatic prostate cancer.22
Conclusion
Oligometastatic prostate cancer is a clinical condition observed along the natural history of prostate cancer progression. Multidisciplinary therapy for oligometastatic prostate cancer is considered to be a promising treatment. Some patients with distant metastases can complete treatment and be observed without treatment. Improvement of diagnostic methods will contribute to the improvement of treatment results for oligometastatic prostate cancer in the future.
Acknowledgment
This research project received no specific grant from funding agencies in the public or commercial sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13(1):8–10. doi:10.1200/JCO.1995.13.1.8
2. Parker CC, James ND, Brawley CD, et al.; Systemic Therapy for Advanced or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE) investigators. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled Phase 3 trial. Lancet. 2018;392(10162):2353–2366. doi:10.1016/S0140-6736(18)32486-3
3. O’Shaughnessy MJ, McBride SM, Vargas HA, et al. A pilot study of a multimodal treatment paradigm to accelerate drug evaluations in early-stage metastatic prostate cancer. Urology. 2017;102:164–172. doi:10.1016/j.urology.2016.10.044
4. Reyes DK, Rowe SP, Schaeffer EM, et al. Multidisciplinary total eradication therapy (TET) in men with newly diagnosed oligometastatic prostate cancer. Med Oncol. 2020;37:60. doi:10.1007/s12032-020-01385-7
5. Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur Urol. 2014;65:1058–1066. doi:10.1016/j.eururo.2013.11.012
6. Rusthoven CG, Jones BL, Flaig TW, et al. Improved survival with prostate radiation in addition to androgen deprivation therapy for men with newly diagnosed metastatic prostate cancer. J Clin Oncol. 2016;34(24):2835–2842. doi:10.1200/JCO.2016.67.4788
7. Cho Y, Chang JS, Rha KH, et al. Does radiotherapy for the primary tumor benefit prostate cancer patients with distant metastasis at initial diagnosis? PLoS One. 2016;11(1):e0147191. doi:10.1371/journal.pone.0147191
8. Thompson IM, Tangen C, Basler J, Crawford ED. Impact of previous local treatment for prostate cancer on subsequent metastatic disease. J Urol. 2002;168(3):1008–1012. doi:10.1016/S0022-5347(05)64562-4
9. Heidenreich A, Pfister D, Porres D. Cytoreductive radical prostatectomy in patients with prostate cancer and low volume skeletal metastases: results of a feasibility and case-control study. J Urol. 2015;193(3):832–838. doi:10.1016/j.juro.2014.09.089
10. Parker C, Nilsson S, Heinrich D, et al.; ALSYMPCA Investigators. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi:10.1056/NEJMoa1213755
11. Phillips R, Shi WY, Deek M, et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: the ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6:650–659. doi:10.1001/jamaoncol.2020.0147
12. Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter Phase II Trial. J Clin Oncol. 2018;36(5):446–453. doi:10.1200/JCO.2017.75.4853
13. Foerster R, Zwahlen DR, Buchali A, et al. Stereotactic body radiotherapy for high-risk prostate cancer: a systematic review. Cancers (Basel). 2021;13(4):759. doi:10.3390/cancers13040759
14. Schick U, Jorcano S, Nouet P, et al. Androgen deprivation and high-dose radiotherapy for oligometastatic prostate cancer patients with less than five regional and/or distant metastases. Acta Oncol. 2013;52(8):1622–1628. doi:10.3109/0284186X.2013.764010
15. Akaza H, Hinotsu S, Usami M, et al.; Study Group for the Combined Androgen Blockade Therapy of Prostate Cancer. Combined androgen blockade with bicalutamide for advanced prostate cancer: long-term follow-up of a phase 3, double-blind, randomized study for survival. Cancer. 2009;115(15):3437–3445. doi:10.1002/cncr.24395
16. Fizazi K, Tran N, Fein L, et al.; LATITUDE Investigators. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352–360. doi:10.1056/NEJMoa1704174
17. Chi KN, Agarwal N, Bjartell A, et al.; TITAN Investigators. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 381;2019:13–24. doi:10.1056/NEJMoa1903307
18. Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: a randomized, Phase III Study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974–2986. doi:10.1200/JCO.19.00799
19. Hamano I, Hatakeyama S, Narita S, et al. Impact of nadir PSA level and time to nadir during initial androgen deprivation therapy on prognosis in patients with metastatic castration-resistant prostate cancer. World J Urol. 2019;37(11):2365–2373. doi:10.1007/s00345-019-02664-3
20. Yamashita T, Shiota M, Machidori A, et al. Efficacy and safety of 4-weekly docetaxel for castration-resistant prostate cancer. Cancer Invest. 2021;39(3):251–256. doi:10.1080/07357907.2020.1871486
21. Fendler WP, Weber M, Iravani A, et al. Prostate-specific membrane antigen ligand positron emission tomography in men with nonmetastatic castration-resistant prostate cancer. Clin Cancer Res. 2019;25(24):7448–7454. doi:10.1158/1078-0432.CCR-19-1050
22. NCT03678025. Standard systemic therapy with or without definitive treatment in treating participants with metastatic prostate cancer. Available from: https://ClinicalTrials.gov/show/NCT03678025.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.