Back to Journals » International Journal of Nanomedicine » Volume 17
Multicenter Randomized Open-Label Phase II Clinical Study Comparing Outcomes of NK105 and Paclitaxel in Advanced or Recurrent Breast Cancer
Authors Kosaka Y, Saeki T, Takano T, Aruga T, Yamashita T , Masuda N , Koibuchi Y, Osaki A, Watanabe J, Suzuki R
Received 10 May 2022
Accepted for publication 7 August 2022
Published 27 September 2022 Volume 2022:17 Pages 4567—4578
DOI https://doi.org/10.2147/IJN.S372477
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Phong A Tran
Yoshimasa Kosaka,1– 3 Toshiaki Saeki,2 Toshimi Takano,4,5 Tomoyuki Aruga,6 Toshinari Yamashita,7 Norikazu Masuda,8,9 Yukio Koibuchi,10 Akihiko Osaki,2 Junichiro Watanabe,11,12 Ryu Suzuki13
1Department of Breast and Endocrine Surgery, Kitasato University School of Medicine, Sagamihara-shi, Kanagawa, Japan; 2Department of Breast Oncology, Saitama Medical University International Medical Center, Hidaka-shi, Saitama, Japan; 3Department of Breast Oncology, Japanese Red Cross Sagamihara Hospital, Sagamihara-shi, Kanagawa, Japan; 4Department of Medical Oncology, Toranomon Hospital, Minato-ku, Tokyo, Japan; 5Breast Medical Oncology Department, Breast Oncology Center, Cancer Institute Hospital of Japanese Foundation for Cancer Research, Koto-ku, Tokyo, Japan; 6Department of Breast Surgery, Tokyo Metropolitan Center and Infectious Diseases Center Komagome Hospital, Bunkyo-ku, Tokyo, Japan; 7Department of Breast and Endocrine Surgery, Kanagawa Cancer Center, Yokohama-shi, Kanagawa, Japan; 8Department of Surgery, Breast Oncology, National Hospital Organization Osaka National Hospital, Osaka-shi, Osaka, Japan; 9Department of Breast and Endocrine Surgery, Nagoya University Graduate School of Medicine, Nagoya-shi, Aichi, Japan; 10Department of Breast and Endocrine Surgery, National Hospital Organization Takasaki General Medical Center, Takasaki-shi, Gunma, Japan; 11Division of Breast Oncology, Shizuoka Cancer Center, Sunto-gun, Shizuoka, Japan; 12Department of Breast Oncology, Juntendo University Graduate School of Medicine, Bunkyo-ku, Tokyo, Japan; 13Pharmaceuticals Group, Nippon Kayaku Co., Ltd, Kita-ku, Tokyo, Japan
Correspondence: Yoshimasa Kosaka, Department of Breast Oncology, Japanese Red Cross Sagamihara Hospital, 256 Nakano, Midori-ku, Sagamihara-shi, Kanagawa, 252-0157, Japan, Tel +81-42-784-1101, Email [email protected]
Background: NK105 is a paclitaxel (PTX)-incorporating “core-shell-type” polymeric micellar nanoparticle formulation composed of block copolymers (polyethylene glycol and a polyamino acid). The efficacy and safety of NK105 and paclitaxel in advanced or recurrent breast cancer have never been compared at equivalent dose levels.
Patients and Methods: Patients were randomly assigned to either NK105 or PTX in a 1:1 ratio. The study drug was administered on Day 1, 8, and 15 of a 28-day cycle with 80 mg/m2. The primary endpoint was overall response rate (ORR), secondary endpoints were progression-free survival (PFS), overall survival (OS), and adverse events.
Results: A total of 123 patients (NK105, n=62; PTX, n=61) received one of the two drugs. There was no significant difference in ORR, the median PFS, or OS (NK105 group: 41.9%, 9.1, and 27.5 months, respectively; PTX group: 45.9%, 7.8, and 32.4 months, respectively). Neutropenia occurred more frequently in the NK105 group, but most patients did not require granulocyte-colony stimulating factor or dose-reduction. The median time to onset of peripheral sensory neuropathy (PSN) in the NK105 group was significantly longer than that in the PTX group (p=0.001), and PSN (≥ grade 3) was not observed in the NK105 group.
Conclusion: Weekly NK105 administration was well-tolerated. Efficacy was similar in both groups. The PSN profile was better in the NK105 group.
Keywords: polymeric micellar nanoparticles, chemotherapy-induced peripheral neuropathy, taxane, anti-polyethylene glycol antibodies, overall response rate
Introduction
Paclitaxel (PTX), an antimicrotubule agent, is widely used for the treatment of various types of cancers. PTX is one of the key drugs used in the treatment of breast cancer (BC). In vitro studies have demonstrated that the mechanism of PTX cytotoxicity mainly depends on the intracellular concentration of the drug in tumor cells.1 However, the effectiveness of PTX is limited by various side effects associated with its use.2,3 The major side effects of PTX are hypersensitivity and peripheral sensory neuropathy (PSN).
PSN impairs patient quality of life, and there is no preventive or curative treatment for this side effect.4 This is a key issue, as PSN induced by taxanes may last several years after the completion of chemotherapy.5,6
NK105 is a PTX-incorporating “core-shell-type” polymeric micellar nanoparticle formulation composed of block copolymers (polyethylene glycol [PEG] and a polyamino acid). It is hoped that NK105 will exhibit a notable “enhanced permeability and retention (EPR)” effect,7 an effect that has been observed in a nonclinical study.8 NK105 can also be used intravenously without the use of a premedication as prophylaxis against hypersensitive reactions.
A Phase III study verified the non-inferiority of NK105 to PTX based on progression-free survival (PFS) in metastatic or recurrent BC.9 NK105 monotherapy showed similar efficacy to PTX monotherapy, but did not meet the primary endpoint, with a non-inferiority margin of 1.215. In the study, the initial dose of NK105 was 65 mg/m2, whereas the initial dose of PTX was 80 mg/m2. The dose of NK105 was selected based on the results of a Phase I study.10 In this phase I study, 80 mg/m2 had been selected as the recommended dose of NK105 in the dose-escalation phase; however, in the subsequent exploratory dose-expansion phase, the high frequency of ≥ grade 3 neutropenia necessitated dose reductions or dose delays. The overall response rate (ORR) in 10 patients with advanced BC in the exploratory dose-expansion phase was 60%, and tumor shrinkage continued in most patients even after the dose had been reduced from 80 mg/m2 to 65 mg/m2. Taking these safety and efficacy data into consideration, an initial dose of 65 mg/m2 was selected for the NK105 group in the subsequent phase III study. The safety profile of NK105 shows that it was well-tolerated, with an incidence of PSN that was favorable when compared to that of PTX.9
To further investigate the value of NK105 against PTX for patients with advanced or recurrent BC, it was necessary to evaluate the efficacy of NK105 80 mg/m2. Because data on the efficacy of 80 mg/m2 NK105 was available from only 10 patients in the exploratory dose-expansion phase of the phase I study,10 we conducted a phase II study to assess the efficacy, safety, and tolerability of weekly NK105 (80 mg/m2) for 3 weeks of every 28-day cycle. The primary objective was to determine the ORR, and the secondary objectives were to determine the PFS and OS and to compare the safety profiles of weekly NK105 (80 mg/m2) and PTX (80 mg/m2).
Materials and Methods
Patients
Women aged 20–74 years with histologically confirmed advanced or recurrent invasive BC, at least one measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, an Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate function of major organs were eligible for enrollment. The major exclusion criteria were: 1) recurrence of BC within 1 year after the last dose of a taxane-based chemotherapy regimen as neo-adjuvant and/or adjuvant therapy; 2) prior treatment with a taxane-based chemotherapy regimen for advanced or recurrent BC; 3) prior chemotherapy with two or more regimens for advanced or recurrent BC; 4) eligibility for anti-human epidermal growth factor receptor 2 therapy or hormone therapy involving the use of cyclin-dependent kinase 4/6 inhibitors; 5) ≥ grade 2 PSN; and 6) preexisting symptoms of diabetic neuropathy.
All patients provided informed consent prior to entry into the study. The study was registered in Japic Clinical Trials Information (no. JapicCTI-183868).
Study Design and Treatment
This randomized, open-label, parallel, phase II study enrolled patients from 26 sites in Japan. Patients were randomly assigned in a 1:1 ratio to receive either NK105 or PTX using an interactive web response system. Randomization was performed using the minimization method, with history of chemotherapy for advanced or recurrent BC and estrogen receptor status as allocation factors.
Patients received either NK105 (80 mg/m2) or PTX (80 mg/m2) on Day 1, 8, and 15 of a 28-day cycle for up to 18 cycles. NK105 (80 mg/m2), which is supplied as a dry powder, was dissolved in 5% glucose solution or saline solution and infused over 30 min. PTX (80 mg/m2), which is supplied as an injection solution, was diluted in 5% glucose solution or saline solution and infused over 1 h with prophylactic premedication (antihistamine, corticosteroid and/or H2 receptor antagonist drugs) to prevent hypersensitivity. Treatment was continued until progressive disease (assessed based on RECIST version 1.1) or an unacceptable adverse event (AE) occurred, or another discontinuation criterion was met. Before the intra-cycle treatments (Day 8, Day 15), each patient was required to satisfy the following criteria: neutrophil count ≥ 500/mm3, platelet count ≥ 75,000/mm3, non-hematologic toxicity associated with study treatment ≤ grade 2. On Day 1 of Cycle 2 and subsequent cycles, patients were also required to satisfy the following criteria: neutrophil count ≥ 1000/mm3, platelet count ≥ 75,000/mm3, non-hematologic toxicity associated with study treatment ≤ grade 2. The dose of NK105 and PTX was reduced by one level (eg, dose reduced from 80 to 65 mg/m2, or from 65 to 50 mg/m2) if any of following criteria were met: neutrophil count < 500/mm3 lasting ≥ 5 days, platelet count < 25,000/mm3, grade 4 febrile neutropenia, non-hematologic toxicity associated with study treatment ≥ grade 3 (anorexia, nausea, vomiting, and diarrhea controllable with supportive care were excluded).
Assessment of Efficacy and Safety
The primary endpoint was the ORR. The ORR was the proportion of patients having complete response or partial response as their best overall response. Tumor assessments were performed upon the completion of every 2 cycles of study drug administration until occurrence of progressive disease according to RECIST version 1.1. Assessments of antitumor efficacy were performed by the investigator and by blinded independent central reviewers, with the former being used for the primary analysis. The secondary efficacy endpoints were PFS and OS. PFS was defined as the period from the day of randomization until the first observation of objective disease progression or death from any cause, whichever occurred first. OS was defined as the period from the day of randomization until the day of death.
Safety data were graded using the Common Terminology Criteria for AEs version 4.03 and were classified using the Medical Dictionary for Regulatory Activities version 20.1. Safety data were summarized descriptively using the safety analysis set, which comprised all randomized patients who received study drug at least once.
Assessment of Pharmacokinetics and Anti-Polyethylene Glycol Antibodies
The plasma concentrations of released PTX and total PTX (both micelle-incorporated and released) in the NK105 group were measured using liquid chromatography-tandem mass spectrometry (LC/MS/MS). Samples were collected within 5 min after completion of infusion to measure the maximum concentration (Cmax) on Day 1 of Cycle 1, Day 1 of Cycle 3, and Day 1 of every third cycle thereafter (eg, Cycles 6, 9, 12, 15, and 18). It was specified that the samples for Cmax measurement should be collected within 5 min after the completion of infusion, based on the results of the aforementioned phase I study.10
The potential immunogenicity was evaluated by measuring the anti-PEG antibodies using electrochemiluminescence.11 Anti-PEG antibodies that are present in the serum bind with the immobilized PEG, the bound ADA is detected using a ruthenium-labeled anti-human IgG and IgM antibody, and this complex is detected by a chemiluminescent signal. Samples were collected at baseline, on Day 8 of Cycle 1, and before dosing in Cycle 3 and every 3 cycles thereafter.
The pharmacokinetic (PK) data were summarized descriptively using the PK analysis set, which comprised all randomized patients who received at least 1 full dose of study drug and who had evaluable PK data and no major protocol violations. The PK data were also summarized by anti-PEG antibody test result.
Statistical Analysis
The ORR for PTX was assumed to be 40% based on the results of the aforementioned phase III study.9 The ORR for NK105 was assumed to be 60% based on the results of the aforementioned phase I study.10 The characteristics of patients in both the phase III and phase I studies were similar to those of the target population in this phase II study. Assuming a 20% between-group difference in ORR, a two-sided significance level of 20%, and a statistical power of 80%, the estimated total sample size required was 112 (56 in each treatment group). The target sample size was therefore set at 120, to take into account withdrawals/dropouts. Statistical analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC).
Efficacy was analyzed using the full analysis set, which consisted of all randomized patients who received the study drug at least once. The 80% confidence interval (CI) of the difference in the ORR was calculated using the Newcombe-Wilson score method. The PFS, OS, and the cumulative incidence of PSN were estimated using the Kaplan–Meier method.
As a post-hoc analysis, Log rank test was used to determine any significant between-group difference in the cumulative incidence of PSN. The ORRs in the primary and recurrent BC subgroups were evaluated as a post-hoc analysis to assess whether different proportions of patients with primary and recurrent BC were affected in the NK105 and PTX groups.
Results
Patients and Treatment
From March 2018 to Aug 2019, a total of 125 female patients at 26 sites in Japan were randomly assigned centrally in a 1:1 ratio to receive either NK105 or PTX. The follow-up period was completed on April 20, 2021. Of all randomized patients, a total of 123 patients received the study drug at least once and were included in the full analysis set and the safety analysis set (Supplementary Figure 1). The main baseline characteristics are summarized in Table 1. The median age of the patients was 61 years in the NK105 group and 55 years in the PTX group. A total of 66 patients (53.7%) had recurrent disease (39 patients [62.9%] in the NK105 group and 27 patients [44.3%] in the PTX group). All patients had metastatic lesions and were stage IV. The main baseline characteristics of the two groups were comparable (Table 1).
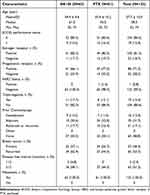 |
Table 1 Baseline Patient Characteristics in the Full Analysis Set |
The median duration of treatment was 6.8 months (range, 0–21.7) in the NK105 group and 7.2 months (range, 0–19.1) in the PTX group. The median dose intensity was 53.15 mg/m2/week in the NK105 group and 54.09 mg/m2/week in the PTX group. The median relative dose intensity was 100.0% for both NK105 and PTX. Supplementary Tables 1 and 2 show the dose intensity and relative dose intensity by age. There was no association between age and relative dose intensity.
Efficacy
Table 2 summarizes the tumor response. The investigator-assessed ORR was 41.9% in the NK105 group and 45.9% in the PTX group. In the blinded independent central review assessments, only 118 of the 123 patients were evaluated for ORR because in the other 5 patients the independent central reviewers did not recognize any measurable lesions in the screening radiographic images according to RECIST ver. 1.1. Overall, 26 patients in the NK105 group and 28 patients in the PTX group achieved a partial response. The blinded independent central reviewer-assessed ORR was 44.1% in the NK105 group and 42.4% in the PTX group (Table 2). Four patients in the NK105 group and 3 patients in the PTX group achieved complete response, and 22 patients in the NK105 group and 22 patients in the PTX group achieved partial response. There was no significant between-group difference with respect to ORR. In the post-hoc analysis, the difference in tumor response between primary and recurrent BC that was seen with NK105 was not consistent with that seen with PTX (Supplementary Tables 3, 4).
 |
Table 2 Summary of Tumor Response in the Full Analysis Set |
The median PFS was 9.1 months (95% CI, 6.0–12.2) in the NK105 group and 7.8 months (95% CI, 5.9–15.2) in the PTX group (Figure 1). The median OS was 27.5 months (95% CI, 22.8–31.1) in the NK105 group and 32.4 months (95% CI, 22.1–NE) in the PTX group (Figure 2).
 |
Figure 1 Kaplan–Meier curves for progression-free survival in the full analysis set. Abbreviations: CI, confidence interval; PTX, paclitaxel. |
 |
Figure 2 Kaplan–Meier curves for overall survival in the full analysis set. Abbreviations: CI, confidence interval; NE, not estimable; PTX, paclitaxel. |
Safety
Table 3 shows the AEs that occurred in at least 20% of patients. In the safety analysis set, all patients in both treatment groups experienced AEs. Grade 3 or higher AEs occurred in 49 (79.0%) patients in the NK105 group and 34 (55.7%) patients in the PTX group. Four patients (6.5%) in the NK105 group and 7 patients (11.5%) in the PTX group discontinued treatment due to AEs (Supplementary Table 5). Serious AEs were reported in 10 patients (16.1%) in the NK105 group and 8 patients (13.1%) in the PTX group. No treatment-related deaths occurred in either group.
 |
Table 3 Adverse Events Occurring in 20% or More of the Patients in Either Group |
The incidence of hematologic AEs in the NK105 group was higher than that in the PTX group. The most common hematologic AE was neutropenia (79.0% vs 55.7%). None of the patients discontinued treatment due to any hematologic AEs. Three patients (4.8%) in the NK105 group and 1 patient (1.6%) in the PTX group required dose reduction due to neutropenia. Six patients (9.7%) in the NK105 group and 1 patient (1.6%) in the PTX group were administered granulocyte-colony stimulating factor at least once for the treatment of neutropenia, not for prophylactic purposes. Two patients (3.2%) in the NK105 group experienced grade 3 febrile neutropenia. The incidence of serious infection was similar in both groups: 2 patients (3.2%) in the NK105 group and 2 patients (3.3%) in the PTX group.
The most common non-hematologic AEs were alopecia and PSN. The incidence of PSN in the NK105 group was lower than that in the PTX group (64.5% vs 82.0%), and the incidence of grade 3 PSN as well was lower in the NK105 group than in the PTX group (0% vs 9.8%). In the post-hoc analyses, the median time to onset of PSN in the NK105 group was significantly longer than that in the PTX group (2.6 months vs 1.2 months, p=0.001, Figure 3). Grade 2 or higher PSN events tended to occur later in the NK105 group than in the PTX group (Figure 4).
 |
Figure 3 Cumulative incidence of peripheral sensory neuropathy. Abbreviations: CI, confidence interval; PSN, peripheral sensory neuropathy; PTX, paclitaxel. |
 |
Figure 4 Cumulative incidence of grade 2 or higher peripheral sensory neuropathy. Abbreviations: CI, confidence interval; NE, not estimable; PSN, peripheral sensory neuropathy; PTX, paclitaxel. |
Hypersensitivity reactions were reported in 11 patients (17.7%) in the NK105 group and 12 patients (19.7%) in the PTX group. Of note, 15 patients (24.2%) in the NK105 group and all 61 (100%) patients in the PTX group received some kind of premedication to prevent hypersensitivity.
Pharmacokinetics
PK analyses were conducted in 61 of the 64 randomized patients in the NK105 group, as no PK data had been obtained from the other 3 patients, who had not received at least 1 full dose of the study drug.
The arithmetic means of the Cmax values for total PTX and released PTX in the NK105 group ranged from 22.10 to 23.76 µg/mL and 3.095 to 3.430 µg/mL, respectively, during the course of study treatment.
Anti-Polyethylene Glycol Antibodies
Anti-PEG antibodies were detected at baseline in 45 of 62 patients (72.6%) in the NK105 group and 43 of 61 patients (70.5%) in the PTX group. The Cmax of the total PTX (the total of both micelle-incorporated and released PTX) is shown in Supplementary Figure 2 for patients in the PK analysis population consisting of patients who had anti-PEG antibodies measured after receiving NK105. Anti-PEG antibodies did not affect the Cmax of the total PTX after a single dose, and similar results were obtained after multiple doses of NK105. In addition, anti-PEG antibodies had no impact on the incidence of hypersensitivity reactions.
Discussion
To the best of our knowledge, this is the first report of a phase II study comparing the efficacy, safety, and tolerability of weekly NK105 80 mg/m2 (Day 1, 8, and 15) every 4 weeks with those of PTX administered using the same dosage and administration in patients with advanced or recurrent BC. The relationship between efficacy and safety was also evaluated using the Cmax and anti-PEG antibody profiles.
In this study, the efficacy of NK105 was comparable to that of PTX in terms of the ORR, PFS, and OS. In this study, NK105 80 mg/m2 afforded an improvement in efficacy compared to that obtained with NK105 65 mg/m2, as reported in a previous phase III study as it yielded a higher ORR (41.9% versus 31.6%), a shorter median time to best response (1.92 months versus 2.84 months), and a longer median PFS (9.1 months versus 8.4 months).9 The patient characteristics and the median duration of NK105 treatment were similar between this study and the previous phase III study. This suggested that increasing the dose of NK105 from 65 mg/m2 to 80 mg/m2 increases its efficacy. In this study, the median age of the patients in the NK105 group was higher than that in the PTX group. There were many studies indicating a significant association between age and OS, PFS, and breast cancer-specific survival. Therefore, age could be a potential prognostic factor of advanced breast cancer.12 Although there have been no findings to date that age affects the efficacy of NK105, the higher proportion of older patients in the NK105 group might have affected the efficacy assessments. The ORRs that have been reported with PTX 80 mg/m2 weekly monotherapy in BC range between 20% and 43.3%.9,13–15 The reported ORR that has been achieved with monotherapy with the comparable drug nab-PTX at a weekly dose of 100 mg/m2 in BC is approximately 45%,16,17 which is comparable to that of NK105 80 mg/m2. The use of a taxane in combination with a drug having a different mechanism of action can yield improved efficacy. For example, the use of nab-PTX in combination with an anti-PD-L1 antibody afforded favorable efficacy in triple-negative BC.17 Therefore, future studies should also investigate the efficacy of regimens consisting of NK105 administered in combination with drugs that have different mechanisms of action from that of PTX.
The AEs that were reported in the NK105 80 mg/m2 group in this study were all known events. Although the increase in the NK105 dose from 65 mg/m2 to 80 mg/m2 led to an increased incidence of hematologic AEs, none of the patients with hematologic AEs required discontinuation of NK105 treatment due to these AEs, nor was there any increase in the incidence of serious infection. Therefore, we believe that the hematologic toxicity of NK105 80 mg/m2 is manageable. On the other hand, the PSN profile of NK105 was more favorable than that of PTX. Although the incidence of grade 2 events was increased by the increase in the NK105 dose from 65 mg/m2 to 80 mg/m2, none of the patients developed ≥ grade 3 PSN events. The median time to onset of PSN was significantly longer in the NK105 group.
Studies have reported an extremely high incidence of PSN with taxanes (range, 11%–87%),6 and the reported median time to onset of PSN is approximately 5 weeks.18 Grade 3 and higher PSN events have also been reported with the use of the comparable drug nab-paclitaxel.16,17 Generally, appearance of signs of PSN should prompt consideration of dose reduction or treatment discontinuation. However, this may potentially reduce the antitumor efficacy and adversely affect the OS.4 In some patients, the symptoms of PSN may persist for a long time, adversely affecting the quality of life.19 Management of PSN is an increasingly important issue owing to the increasing number of cancer survivors due to advances in treatment, including chemotherapies.6,19,20 It is noteworthy that no grade 3 or higher PSN events occurred with weekly dosing with NK105 80 mg/m2, and PSN events tended to occur later with NK105 than with taxanes.21 In addition to the duration of infusion and the cumulative dose, diabetes has also been reported to be a risk factor for PSN.22 Patients with preexisting symptoms of diabetic neuropathy are believed to be at higher risk of developing PSN while receiving chemotherapy.23,24 Because patients with ≥ grade 2 PSN and preexisting symptoms of diabetic neuropathy were excluded from this study, it is important to investigate whether or not PSN is alleviated in these patients, as well.
Although different physician-assessed PSN and patient-reported PSN results have been obtained in previous studies,25,26 in the present study, only physician-assessed PSN was evaluated based on the Common Terminology Criteria for AEs. Since evaluating PSN was not the primary objective of this study, the sample size was not sufficient to detect a between-group difference with respect to the onset of PSN. Therefore, due caution is warranted when interpreting the PSN data.
In recent years, some reports have suggested that anti-PEG antibodies may affect the PK profiles, therapeutic efficacy, and adverse reactions of PEG-modified proteins,11,27 and the FDA has recommended that anti-PEG antibody assessments also be performed with PEGylated protein drug products. Therefore, we measured the anti-PEG antibodies on an exploratory basis in this study. Although more than 70% of the patients in both groups in this study were positive for anti-PEG antibodies prior to study drug administration, we found no evidence the presence or absence of anti-PEG antibodies had any effect on the pharmacokinetics or adverse events, including hypersensitivity. Although NK105 is a PEGylated micellar formulation, it is noteworthy that the Cmax remains almost constant in repeated dosing. Therefore, the presence or absence of anti-PEG antibodies does not appear to be a key consideration in NK105 therapy.
The EPR effect has been well accepted as one of the universal pathophysiological characteristics of solid tumors.28,29 The EPR effect significantly increases the accumulation of drugs in tumors and reduces the off-target effects.30 The PSN profile of weekly NK105 was better than that of PTX. It appears that the difference in particle size between NK105 and PTX may have resulted in their differential distribution to the dorsal root ganglion,31,32 resulting in a lower incidence of PSN in the NK105 group compared to the PTX group.
On the other hand, the efficacy of weekly NK105 monotherapy was comparable to that of PTX monotherapy. Inter-heterogeneity or intra-heterogeneity has been reported in breast cancer.33,34 There has been much discussion of the concept and application of the EPR effect in clinical use. In particular, heterogeneity of the solid tumor bulk, tumor blood vessel abnormalities vary both within and between tumors, and tumor stage is the most important factor determining whether or not nanomedicine will be effective, due to the EPR effect.35,36
Conclusions
Weekly NK105 (80 mg/m2) administration is well-tolerated. Efficacy, as evidenced by both the ORR and other measures, was almost the same in the NK105 and PTX groups. The safety profile, and especially the PSN profile, of NK105 was better than that of PTX.
Data Sharing Statement
No further data will be shared.
Ethics Approval and Informed Consent
Written informed consent was obtained from each patient prior to entry into the study. The study was conducted in compliance with the principles of the declaration of Helsinki, and with Good Clinical Practice. The protocol and informed consent form were approved by the institutional review boards of all participating sites (see Supplementary Table 6) and by the regulatory authorities.
Consent for Publication
This article contains no personal information.
Acknowledgments
We thank all the patients who agreed to take part in the trial, and their families, the investigators (see Supplementary Table 6) and staff members who assisted at each site. We thank Mr. Lee Dancy and Enago for English-language editing services. This work was supported by Nippon Kayaku Co. Ltd.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Nippon Kayaku Co. Ltd., which provided the study drugs (NK105 and PTX). Nippon Kayaku Co. Ltd. contributed to the conception and design of the study, and was responsible for managing the research, including the analysis of the data.
Disclosure
Y.K. reports having received honoraria from Chugai, AstraZeneca, Eisai, Pfizer, Kyowa Kirin, Novartis, Taiho, and Nippon Kayaku; and financial support from Nippon Kayaku for attending meetings. T.S. reports having received grants from Eisai, Kyowa Kirin, Taiho, Chugai, Nippon Kayaku, AstraZeneca, NRG Oncology Japan, Sawai, JBCRG, Daiichi Sankyo, Novartis, and West Japan Oncology Group; honoraria from Aska, AstraZeneca, Eisai, Ono, Taiho, Takeda, Chugai, Eli Lilly, Nippon Kayaku, Novartis, Pfizer, MiRTeL, and Meiji; and financial support from Nippon Kayaku for attending meetings. T.T. reports having received grants from Chugai, Daiichi Sankyo, Ono, MSD, and Eisai, and honoraria from Chugai, Daiichi Sankyo, Eisai, Eli Lilly, and Celltrion Healthcare. T.A. reports having received honoraria and support for attending meetings from Pfizer, Chugai, AstraZeneca, Eli Lilly, Eisai, Daiichi Sankyo, MSD, Taiho, Celltrion, Nihon Medi-Physics, Kirin, Konica Minolta REALM, and Nippon Kayaku. T.Y. reports having received grants from Chugai, Taiho, Kyowa Kirin, and Nippon Kayaku; honoraria from Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Novartis Pharma, Taiho, AstraZeneca, Pfizer Japan, and Nippon Kayaku; and support for attending meetings from Nippon Kayaku. N.M. reports having received grants from AstraZeneca, Daiichi Sankyo, Eli Lilly, MSD, Sanofi, Chugai, Eisai, Kyowa Kirin, Novartis, Pfizer, and Nippon Kayaku; honoraria from AstraZeneca, Chugai, Eisai, Eli Lilly, Pfizer, and Nippon Kayaku; and board memberships from the Japanese Breast Cancer Society and the Japan Breast Cancer Research Group Association. Y.K. reports having received honoraria and support for attending meetings from Nippon Kayaku. A. O. reports having received grants from AstraZeneca, Eisai, Kyowa Kirin, Taiho, Chugai, Nippon Kayaku, Covance Japan, Maruho, Bayer, Sanofi, Eli Lilly, MSD, Takeda, Sawai, Daiichi Sankyo, Pfizer, and West Japan Oncology Group; honoraria from AstraZeneca, Eisai, Daiichi Sankyo, Chugai, Nippon Kayaku, Pfizer, Shionogi, and Eli Lilly; and financial support from Nippon Kayaku for attending meetings. J.W. reports having received grants and honoraria from AstraZeneca, Eisai, MSD, Gilead, Daiichi Sankyo, and Eli Lilly; honoraria from Chugai, Pfizer, Taiho, Novartis, Takeda, and Nippon Kayaku; and support for attending meetings from Nippon Kayaku. R.S. is an employee of Nippon Kayaku Co. Ltd. The authors report no other conflicts of interest in this work.
References
1. Giannakakou P, Robey R, Fojo T, Blagosklonny MV. Low concentrations of paclitaxel induce cell type-dependent p53, p21 and G1/G2 arrest instead of mitotic arrest: molecular determinants of paclitaxel-induced cytotoxicity. Oncogene. 2001;20(29):3806–3813. doi:10.1038/sj.onc.1204487
2. Zhang D, Yang R, Wang S, Dong Z. Paclitaxel: new uses for an old drug. Drug Des Dev Ther. 2014;1(8):279–284.
3. Marupudi NI, Han JE, Li KW, Renard VM, Tyler BM, Brem H. Paclitaxel: a review of adverse toxicities and novel delivery strategies. Expert Opin Drug Saf. 2007;6(5):609–621. doi:10.1517/14740338.6.5.609
4. Ibrahim EY, Ehrlich BE. Prevention of chemotherapy-induced peripheral neuropathy: a review of recent findings. Crit Rev Oncol Hematol. 2022;2(145):102831.
5. Kerckhove N, Collin A, Condé S, Chaleteix C, Pezet D, Balayssac D. Long-term effects, pathophysiological mechanisms, and risk factors of chemotherapy-induced peripheral neuropathies: a comprehensive literature review. Front Pharmacol. 2017;2(8):86.
6. Zajączkowska R, Kocot-Kępska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of chemotherapy-induced peripheral neuropathy. Int J Mol Sci. 2019;20(6):1451. doi:10.3390/ijms20061451
7. Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392.
8. Hamaguchi T, Matsumura Y, Suzuki M, et al. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br J Cancer. 2005;92(7):1240–1246. doi:10.1038/sj.bjc.6602479
9. Fujiwara Y, Mukai H, Saeki T, et al. A multi-national, randomised, open-label, parallel, phase III non-inferiority study comparing NK105 and paclitaxel in metastatic or recurrent breast cancer patients. Br J Cancer. 2019;120(5):475–480. doi:10.1038/s41416-019-0391-z
10. Mukai H, Kato K, Esaki T, et al. Phase I study of NK105, a nanomicellar paclitaxel formulation, administered on a weekly schedule in patients with solid tumors. Investig New Drugs. 2016;34(6):750–759. doi:10.1007/s10637-016-0381-4
11. Hong L, Wang Z, Wei X, Shi J, Li C. Antibodies against polyethylene glycol in human blood: a literature review. J Pharmacol Toxicol Methods. 2020;102:106678. doi:10.1016/j.vascn.2020.106678
12. Cuyún Carter G, Mohanty M, Stenger K, et al. Prognostic Factors in Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative (HR+/HER2-) Advanced Breast Cancer: a Systematic Literature Review. Cancer Manag Res. 2021;13:6537–6566. doi:10.2147/CMAR.S300869
13. Decker T, Overkamp F, Rösel S, et al. A randomized phase II study of paclitaxel alone versus paclitaxel plus sorafenib in second- and third-line treatment of patients with HER2-negative metastatic breast cancer (PASO). Bio Med Cent Cancer. 2017;17(1):499.
14. Perez EA, Vogel CL, Irwin DH, Kirshner JJ, Patel R. Weekly paclitaxel in women age 65 and above with metastatic breast cancer. Breast Cancer Res Treat. 2002;73(1):85–88. doi:10.1023/A:1015230212550
15. Sato K, Inoue K, Saito T, et al. Multicenter phase II trial of weekly paclitaxel for advanced or metastatic breast cancer: the Saitama breast cancer clinical study group (SBCCSG-01). Jpn J Clin Oncol. 2003;33(8):371–376. doi:10.1093/jjco/hyg075
16. Gradishar WJ, Krasnojon D, Cheporov S, et al. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol. 2009;27(22):3611–3619. doi:10.1200/JCO.2008.18.5397
17. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–2121. doi:10.1056/NEJMoa1809615
18. de la Morena Barrio P, Conesa MÁ, González-Billalabeitia E, et al. Delayed recovery and increased severity of paclitaxel-induced peripheral neuropathy in patients with diabetes. J Natl Compr Canc Netw. 2015;13(4):417–423. doi:10.6004/jnccn.2015.0057
19. Grisold W, Cavaletti G, Windebank AJ. Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro Oncol. 2012;14(Suppl 4):iv45–54. doi:10.1093/neuonc/nos203
20. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol. 2014;32(16):1739–1747. doi:10.1200/JCO.2013.52.4629
21. Inoue M, Matsumoto K, Tanaka M, et al. Analysis of chemotherapy-induced peripheral neuropathy using the Japanese adverse drug event report database. Sci Rep. 2021;11(1):11324. doi:10.1038/s41598-021-90848-6
22. Lee JJ, Swain SM. Peripheral neuropathy induced by microtubule-stabilizing agents. J Clin Oncol. 2006;24(10):1633–1642. doi:10.1200/JCO.2005.04.0543
23. Kus T, Aktas G, Kalender ME, et al. Taxane-induced peripheral sensorial neuropathy in cancer patients is associated with duration of diabetes mellitus: a single-center retrospective study. Support Care Cancer. 2016;24(3):1175–1179. doi:10.1007/s00520-015-2898-z
24. Hershman DL, Till C, Wright JD, et al. Comorbidities and risk of chemotherapy-induced peripheral neuropathy among participants 65 years or older in Southwest Oncology Group clinical trials. J Clin Oncol. 2016;34(25):3014–3022. doi:10.1200/JCO.2015.66.2346
25. Nyrop KA, Deal AM, Reeder-Hayes KE, et al. Patient-reported and clinician-reported chemotherapy-induced peripheral neuropathy in patients with early breast cancer. Current clinical practice. Cancer. 2019;125(17):2945–2954. doi:10.1002/cncr.32175
26. Shimozuma K, Ohashi Y, Takeuchi A, et al. Feasibility and validity of the Patient Neurotoxicity Questionnaire during taxane chemotherapy in a phase III randomized trial in patients with breast cancer N-SAS BC 02. Support Care Cancer. 2009;17(12):1483–1491. doi:10.1007/s00520-009-0613-7
27. Schellekens H, Hennink WE, Brinks V. The immunogenicity of polyethylene glycol: facts and fiction. Pharm Res. 2013;30(7):1729–1734. doi:10.1007/s11095-013-1067-7
28. Duncan R. Polymer conjugates for tumour targeting and intracytoplasmic delivery. The EPR effect as a common gateway? Pharm Sci Technol Today. 1999;2(11):441. doi:10.1016/S1461-5347(99)00211-4
29. Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2011;63(3):131–135. doi:10.1016/j.addr.2010.03.011
30. Yang M, Li J, Gu P, Fan X. The application of nanoparticles in cancer immunotherapy: targeting tumor microenvironment. Bioact Mater. 2020;6(7):1973–1987. doi:10.1016/j.bioactmat.2020.12.010
31. Nakamura I, Ichimura E, Goda R, et al. An in vivo mechanism for the reduced peripheral neurotoxicity of NK105: a paclitaxel-incorporating polymeric micellar nanoparticle formulation. Int J Nanomedicine. 2017;12:1293–1304. doi:10.2147/IJN.S114356
32. Taheri A, Rad A, Sadeghi E, Varshosaz J. Comparison of efficacy and peripheral neuropathy of solvent-based paclitaxel with paclitaxel poliglumex and NK105. A systematic review and meta-analysis. Curr Pharm Des. 2021;27(17):2041–2055. doi:10.2174/1381612826666200917145551
33. Martelotto LG, Ng CK, Piscuoglio S, Weigelt B, Reis-Filho JS. Breast cancer intra-tumor heterogeneity. Breast Cancer Res. 2014;16(3):210. doi:10.1186/bcr3658
34. Zardavas D, Irrthum A, Swanton C, Piccart M. Clinical management of breast cancer heterogeneity. Nat Rev Clin Oncol. 2015;12(7):381–394. doi:10.1038/nrclinonc.2015.73
35. Wu J. The enhanced permeability and retention (EPR) effect: the significance of the concept and methods to enhance its application. J Pers Med. 2021;11(8):771. doi:10.3390/jpm11080771
36. Swetha KL, Roy A. Tumor heterogeneity and nanoparticle-mediated tumor targeting: the importance of delivery system personalization. Drug Deliv Transl Res. 2018;8(5):1508–1526. doi:10.1007/s13346-018-0578-5
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
