Back to Journals » Drug, Healthcare and Patient Safety » Volume 7
Multicenter, noninterventional, post-marketing surveillance study to evaluate dosing of recombinant human follicle-stimulating hormone using the redesigned follitropin alfa pen in women undergoing ovulation induction
Authors Nawroth F, Tandler-Schneider A, Bilger W
Received 30 October 2014
Accepted for publication 3 March 2015
Published 15 April 2015 Volume 2015:7 Pages 63—68
DOI https://doi.org/10.2147/DHPS.S76693
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Shu-Feng Zhou
Frank Nawroth,1 Andreas Tandler-Schneider,2 Wilma Bilger3
1Centre for Reproductive and Prenatal Medicine, Endocrinology and Osteology, Hamburg, Germany; 2Center for Reproductive Medicine, Fertility Center Berlin, Berlin, Germany; 3Medical Affairs, Fertility, Endocrinology and General Medicine, Merck Serono GmbH, Darmstadt, Germany (an affiliate of Merck KGaA, Darmstadt, Germany)
Abstract: This prospective, noninterventional, post-marketing surveillance study evaluated doses of recombinant human follicle-stimulating hormone (r-hFSH) using the redesigned follitropin alfa pen in women who were anovulatory or oligomenorrheic and undergoing ovulation induction (OI) alone or OI with intrauterine insemination. The primary endpoint was the proportion of patients who achieved monofollicular or bifollicular development (defined as one or two follicles 15 mm). Secondary endpoints included characteristics of ovulation stimulation treatment, such as mean total and mean daily r-hFSH doses. Data were analyzed for 3,193 patients from 30 German fertility centers. The proportion of patients with monofollicular or bifollicular development was 71.1% (n=2,270 of a total of 3,193 patients; intent-to-treat population). The mean±standard deviation total and daily doses of r-hFSH were 696.9±542.5 IU and 61.7±29.4 IU, respectively. The three doses prescribed most frequently were: 37.5 IU (n=703 from N=3,189; 22.0%), 50.0 IU (n=1,056 from N=3,189; 33.1%), and 75.0 IU (n=738 from N=3,189; 23.1%) on the first day of stimulation; and 37.5 IU (n=465 from N=3,189; 14.6%), 50.0 IU (n=922 from N=3,189; 28.9%), and 75.0 IU (n=895 from N=3,189; 28.1%) on the last day of stimulation. This noninterventional, post-marketing surveillance study found that monofollicular or bifollicular development was achieved in 71% of patients studied and the small dose increment (12.5 IU) of the redesigned follitropin alfa pen allowed individualized treatment of women undergoing OI.
Keywords: ovulation induction, pen device, recombinant human follicle-stimulating hormone, follitropin alfa
Introduction
Gonadotropins, such as follicle-stimulating hormone (FSH), are used for ovulation induction (OI) in women with World Health Organization group II anovulatory infertility who have failed to conceive using clomifene citrate.1,2 FSH is administered during OI using low-dose protocols to stimulate the recruitment and growth of ovarian follicles. In fertility treatment cycles, the production of a single dominant follicle during ovulation is a key goal to avoid complications such as multiple pregnancies and ovarian hyperstimulation syndrome (OHSS).3,4 The benefits of FSH as a second-line therapy are evident, and the results of a study by Homburg et al also demonstrated superior reproductive outcomes after OI with low-dose FSH compared with clomifene citrate.5 They therefore concluded that FSH may be an appropriate first-line treatment for some anovulatory women, including those with polycystic ovary syndrome.5 Recombinant human FSH (r-hFSH; GONAL-f®, Merck Serono, Darmstadt, Germany, a subsidiary of Merck KGaA, Darmstadt, Germany) is indicated for OI in anovulatory women (including those with polycystic ovary syndrome) who have been unresponsive to clomifene citrate.6 r-hFSH pen devices have been developed to increase the convenience for patients when self-administering treatment.
The redesigned r-hFSH (follitropin alfa) pen (GONAL-f® prefilled pen; Merck Serono, Darmstadt, Germany) is a prefilled, ready-to-use pen designed for self-administration and has a minimum dose increment of 12.5 IU,7,8 which is smaller than for other pen devices and allows smaller dose increments to be administered. The primary objective of this study was to evaluate the doses of r-hFSH using the redesigned follitropin alfa pen, prescribed by physicians in German fertility centers for the treatment of women undergoing OI alone or with intrauterine insemination (IUI).
Materials and methods
Study design
This was a prospective, multicenter, noninterventional, post-marketing surveillance study conducted in patients undergoing OI alone or OI with IUI. Patients received treatment according to local routine practice (standard care), including measurement of developing follicles by transvaginal ultrasound. Ethical approval was obtained before commencement of the study from the Ethical Committee of the Ärztekammer Hamburg (identification number PV3951). The study was initiated on March 5, 2012 and ended on March 31, 2014.
Patients
The women included were anovulatory or oligomenorrheic and had used the redesigned follitropin alfa pen (300 IU, 450 IU, 900 IU) for OI followed by either sexual intercourse at the optimum time or IUI. Only one cycle was reported per patient. Exclusion criteria included concomitant use of other gonadotropins or clomifene citrate, and all contraindications listed in the approved prescribing information (dated May 2011) for the redesigned follitropin alfa pen (these match those listed in the current prescribing information5).
Data collection
Routine pseudoanonymized clinical data were collected prospectively using the electronic RecDate ADVANCE database,9 which has been certified by the German in vitro fertilization registry.
Outcomes
Patient characteristics and demographics at baseline were recorded, including age, body mass index, FSH levels, antral follicle count (AFC; sum of follicles in both ovaries <11 mm in diameter in the early follicular phase), anti-Müllerian hormone, and fertility history.
Primary endpoint
The primary endpoint was the proportion of patients who achieved monofollicular or bifollicular development, ie, one or two follicles of ≥15 mm diameter at final transvaginal ultrasound assessment prior to triggering of ovulation (ideally on the day of administration of human chorionic gonadotropin but could have occurred earlier).
Secondary endpoints
The secondary endpoints evaluated included: the total number of follicles and number of follicles ≥15 mm in diameter (assessed at final transvaginal ultrasound assessment prior to triggering of ovulation); treatment characteristics (including mean daily and mean total r-hFSH dose, r-hFSH dose on first and last days of stimulation, duration of stimulation); proportions of patients according to incremental doses received on the first and last days of stimulation; and clinical pregnancy (35 days after human chorionic gonadotropin) rates per cycle. A post hoc analysis evaluated the proportion of patients according to the first dose adjustment received.
Safety
The incidences of OHSS, adverse events, and serious adverse events were recorded during the study.
Data analysis
Sample size
The sample size calculation (final analysis) was based on the response rate for monofollicular or bifollicular development as follows: using results of a multiple regression analysis, a nomogram was developed for individual doses using biomarkers for known values (eg, AFC and r-hFSH dose), with response rate for monofollicular or bifollicular development as the result. Using an alpha of 0.05, ten biomarkers/predictors, an anticipated effect size of 0.01, and statistical power of 0.9, the minimum sample size was estimated as 2,064. A larger sample size of 2,500 patients was chosen to allow for missing values (no formal calculation of the percentage of missing values was made).
Statistical methods
No statistical hypotheses were formulated. Absolute and relative frequencies were determined for nominal and ordinal characteristics. For quantitative variables, the median (range) and mean ± standard deviation were calculated. If appropriate, parameters were classified and also treated as ordinal characteristics. In addition, a multiple regression analysis was conducted to relate the rate of monofollicular or bifollicular development to potential biomarkers of ovarian response; data were analyzed for five baseline variables (age, body mass index, FSH levels, AFC, and anti-Müllerian hormone).
Results
Patients
Data were analyzed for 3,193 patients from 30 German in vitro fertilization centers; this was the total number of patients with complete data at study end. All patients received at least one dose of r-hFSH. Planned treatment (OI alone in 2,003 patients; OI plus IUI in 1,190 patients) was completed by 2,801 patients (1,713 of 2,003 patients receiving OI alone; 1,088 of 1,190 patients receiving OI plus IUI). Table 1 shows the patient characteristics and cause(s) of infertility. The median (range) AFC was 3.0 (0.0–34.0). Ovulation induction performed in this study was the first treatment cycle for 48.9% (1,561 of 3,193) of patients.
Follicular development data were unavailable for 372 patients. The final transvaginal ultrasound measurement of follicle size was performed on days 8–14 for most patients (n=2,005 from N=2,821; 71.1%).
Efficacy outcomes
Primary endpoint
In the intent-to-treat population, the number (%) of patients with monofollicular or bifollicular development (one or two follicles ≥15 mm) was 2,270 of 3,193 (71.1%). For the subpopulation of patients for whom data on follicular development were available (n=2,821), the proportion of patients with monofollicular or bifollicular development was 2,270 of 2,821 (80.5%).
Secondary endpoints
The mean ± standard deviation total number of follicles per patient was 3.2±4.7 and the mean ± standard deviation number of follicles ≥15 mm in diameter was 1.2±0.9. The mean ± standard deviation duration of stimulation was 10.9±5.4 days. The mean ± standard deviation total and daily doses of r-hFSH were 696.9±542.5 IU and 61.7±29.4 IU, respectively (n=3,192).
The mean ± standard deviation daily doses of r-hFSH (n=3,189) were: 57.6±28.1 IU on the first day of stimulation and 66.5±34.5 IU on the last day of stimulation. The dose of r-hFSH received during stimulation was unchanged for most patients (n=2,003 from N=3,189; 62.8%). The mean ± standard deviation number of dose changes was 1.1±2.4; the median (range) was 0.0 (0.0–22.0).
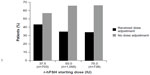
On the first day of stimulation, 12.5 IU (lowest possible dose) was prescribed for 74 of 3,189 (2.3%) patients, and the three doses prescribed most frequently were 37.5 IU (n=703 from N=3,189; 22.0%), 50.0 IU (n=1,056 from N=3,189; 33.1%), and 75.0 IU (n=738 from N=3,189; 23.1%, Figure 1A). On the last day of stimulation, 12.5 IU was prescribed for 52 of 3,189 (1.6%) patients, and the three doses prescribed most frequently were 37.5 IU (n=465 from N=3,189; 14.6%), 50.0 IU (n=922 from N=3,189; 28.9%), and 75.0 IU (n=895 from N=3,189; 28.1%, Figure 1B). Figure 2 shows the proportion of patients who received a 37.5, 50.0, or 75.0 IU dose on the first day of stimulation according to whether they received/did not receive a dose adjustment.
Post hoc evaluation of patients who had a dose adjustment revealed: in patients who received 37.5 IU on the first day of stimulation, the first dose adjustment was most commonly to 50.0 IU (n=190 from N=302; 62.9%) and 75.0 IU (n=102 from N=302; 33.8%); in patients who received 50.0 IU on the first day of stimulation, the first dose adjustment was most commonly to 62.5 IU (n=73 from N=363; 20.1%) and 75.0 IU (n=241 from N=363; 66.4%); in patients who received 75.0 IU on the first day of stimulation, the first dose adjustment was most commonly to 50.0 IU (n=54 from N=250; 21.6%) or 100.0 IU (n=64 from N=250; 25.6%).
Outcome data were unavailable for 862 cycles. The clinical pregnancy rate per started cycle (35 days after human chorionic gonadotropin) was 13.8% (n=441 from N=3,193). In patients for whom outcome data were available, the clinical pregnancy rate was 18.9% (n=441 from N=2,331).
Regression analysis
The multiple regression analysis for age, body mass index, FSH levels, AFC, and anti-Müllerian hormone was conducted in the subpopulation of patients for whom data on follicular development were available (n=2,821; Table 2).
Safety
There were six (n=6 from N=3,193; 0.2%) instances of OHSS; one case was grade III OHSS and required hospitalization (reported as a serious adverse event); four cases were grade I OHSS and in one case ovum pick-up was performed. Eight adverse events were reported during the study; these were early ovulation (n=1), no response (n=1), OHSS leading to hospitalization (n=1), OHSS where ovum pick-up was performed (n=1), and grade I OHSS (n=4).
Discussion
This noninterventional, post-marketing surveillance study found that monofollicular or bifollicular development was achieved in 71% of the women who received r-hFSH using the redesigned follitropin alfa pen for OI (OI alone or with IUI). The doses received by patients were generally low, with average doses on the first and last days of stimulation being about 60–70 IU. On the first day of stimulation, a low dose of r-hFSH (75 IU or lower) was received by most patients (91%); in fact, a large proportion (68%) of patients received a dose that was less than the approved starting dose of 75 IU. The incidence of OHSS during the study was low and there were only two other adverse events reported during this study. The use of low r-hFSH doses in OI helps to reduce the risk of OHSS.4,10 OHSS may be prevented by using alternative methods of OI, such as laparoscopic ovarian drilling or in vitro maturation. However, the possible risks of alternative methods of OI should be considered and balanced against any potential benefits.
Self-administered injection devices aim to provide a convenient option for treatment. The redesigned follitropin alfa pen is a ready-to-use, prefilled pen with several new features.7 A study of patient and nurse usability factors associated with the redesigned follitropin alfa pen using simulated injections found that there were no unexpected operational risks during its use and no major concerns regarding risk of dosing errors or misuse.11
OI using a low-dose, step-up r-hFSH protocol remains a valuable treatment option for anovulatory or oligomenorrheic women.12 The ability to use smaller dose increments of r-hFSH during such protocols would provide a greater opportunity for individualized treatment. A feature of the redesigned follitropin alfa pen is the small 12.5 IU minimum dose increment.7 The option of using doses lower than 75 IU was utilized by physicians for many patients in the current study, despite the recommended starting dose for this indication being 75–150 IU. The lowest possible single dose when using the redesigned follitropin alfa pen, ie, 12.5 IU, was administered in a small proportion of patients (approximately 2%) on the first and last days of stimulation at the physicians’ discretion. In addition, the 12.5 IU dose increment was used by physicians during prescription of higher doses, for example, 87.5 IU, and at the first dose adjustment, for example, 37.5 to 50 IU, thus allowing individualized treatment.
The multiple regression analysis conducted here did not find any clear relationship between the rate of monofollicular or bifollicular development and potential biomarkers of ovarian response in the population studied. However, as might be expected, there was a slight trend to suggest a reduced rate of monofollicular or bifollicular development with increasing age.
Study strengths include use of the RecDate database, which provided prospective documentation of data from approximately 18% of all fertility clinics in Germany. Possible limitations include descriptive data evaluation and the fact that the study was observational in nature, with no blinding and no comparator arm. In addition, the findings of the multiple regression analysis should be treated with caution due to the low number of patients in the subcategories analyzed.
In summary, this noninterventional study found that monofollicular or bifollicular development was achieved in a large proportion of women who received r-hFSH using the redesigned follitropin alfa pen for OI. The small dose increment (12.5 IU) of the redesigned follitropin alfa pen was utilized by clinicians and allowed individualized treatment of women undergoing OI.
Acknowledgments
This paper is published on behalf of the GONAL-f® Prefilled Pen NIS group. The authors thank Elmar Beck, Anfomed, Germany, for statistical support, and Jocelyn Woodcock of Caudex Medical, Oxford, UK (supported by Merck KGaA, Darmstadt, Germany) for her assistance with preparation of this paper. The statistical analysis was performed by Anfomed.
Author contributions
All authors contributed to drafting of the manuscript and critical discussions, and approved the manuscript before submission for publication. FN, WB, and the sponsor contributed to the study design. All authors and the sponsor were involved in data interpretation. The sponsor collected the data and all authors had full access to data.
Disclosure
This study was sponsored by Merck KGaA, Darmstadt, Germany. WB is an employee of Merck Serono GmbH, Germany. FN and AT-S report no conflicts of interest in this work.
References
Messinis IE. Ovulation induction: a mini review. Hum Reprod. 2005;20(10):2688–2697. | |
van Santbrink EJ, Fauser BC. Ovulation induction in normogonadotropic anovulation (PCOS). Best Pract Res Clin Endocrinol Metab. 2006;20(2):261–270. | |
Mono-ovulatory cycles: a key goal in profertility programmes. Hum Reprod Update. 2003;9(3):263–274. | |
Mathur R, Kailasam C, Jenkins J. Review of the evidence base of strategies to prevent ovarian hyperstimulation syndrome. Hum Fertil (Camb). 2007;10(2):75–85. | |
Homburg R, Hendriks ML, Konig TE, et al. Clomifene citrate or low-dose FSH for the first-line treatment of infertile women with anovulation associated with polycystic ovary syndrome: a prospective randomized multinational study. Hum Reprod. 2012;27(2):468–473. | |
European Medicines Agency. Gonal-f; follitropin alfa: Product information. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000071/WC500023748.pdf. Accessed October 17, 2013. | |
Abbotts C, Salgado-Braga C, Audibert-Gros C. A redesigned follitropin alfa pen injector for infertility: results of a market research study. Patient Prefer Adherence. 2011;5:315–331. | |
Christen M, Schertz JC, Arriagada P, Keitel J, Muller H. The redesigned follitropin alpha pen injector for infertility treatment. Expert Opin Drug Deliv. 2011;8(6):833–839. | |
Pak SJ, Warlich J, van Rooij TN. [RecDate – an IT-solution for the documentation and quality management of reproductive medicine]. Zentralbl Gynakol. 2001;123(8):482–486. German. | |
Homburg R, Howles CM. Low-dose FSH therapy for anovulatory infertility associated with polycystic ovary syndrome: rationale, results, reflections and refinements. Hum Reprod Update. 1999;5(5):493–499. | |
Schertz JC, Saunders H, Hecker C, Lang B, Arriagada P. The redesigned follitropin alfa pen injector: results of the patient and nurse human factors usability testing. Expert Opin Drug Deliv. 2011;8(9):1111–1120. | |
van Santbrink EJ, Fauser BC. Is there a future for ovulation induction in the current era of assisted reproduction? Hum Reprod. 2003; 18(12):2499–2502. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.