Back to Journals » Clinical Epidemiology » Volume 11
Mortality in 43,598 men with infertility – a Swedish nationwide population-based cohort study
Authors Lundberg FE , Johansson ALV , Ludvigsson JF
Received 27 March 2019
Accepted for publication 10 June 2019
Published 26 July 2019 Volume 2019:11 Pages 645—657
DOI https://doi.org/10.2147/CLEP.S210180
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Henrik Sørensen
Frida E Lundberg,1 Anna LV Johansson,1 Jonas F Ludvigsson1,2
1Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Solna, Sweden; 2Department of Pediatrics, Orebro University Hospital, Orebro, Sweden
Background: Previous studies indicate a higher risk of comorbidity in men with infertility; however, research on mortality is scarce and the few studies that do exist have rarely differentiated between infertility and infertility-related diagnoses.
Objective: To examine mortality in men with an infertility or infertility-related diagnosis.
Design, setting, and participants: Population-based cohort study of men born in 1944–1992 in Sweden. We used Cox regression estimated hazard ratios (HRs) for infertility while adjusting for number of children, education, year of birth, country of birth, diabetes, hypertension, liver disease and end-stage renal disease. In all, 43,598 men with a diagnosis of infertility and 57,733 men with an infertility-related diagnosis were compared with 2,762,254 men (reference group) without such diagnoses.
Outcome measures: All-cause and cause-specific mortality at age 20 to 69 years.
Results and limitations: The 2,863,585 men in the study were followed for a median time of 22.0 years. During follow-up, 439 men with a diagnosis of infertility died, corresponding to a crude incidence rate of 1.56 deaths per 1,000 person-years. These figures can be compared with 1,400 deaths in men with an infertility-related diagnosis (1.96 deaths/1,000 person-years) and 99,463 deaths in reference individuals (2.17 deaths/1,000 person-years). Overall, men with a diagnosis of infertility did not have a higher risk of death (adjusted [a]HR=0.98; 95% confidence interval [95% CI]=0.89–1.08), but had a higher risk of death before age 30 (20–29 years) (aHR=3.26; 95% CI=2.42–4.41). This early excess mortality was largely explained by cancer diagnosed before infertility. Having an infertility-related diagnosis was associated with death (aHR=1.23; 95% CI=1.17–1.30). Limitations include the lack of general screening for infertility in Sweden and the lack of information on semen parameters.
Conclusion: Men with a diagnosis of infertility are not at a higher risk of death than the general population, although having a diagnosis related to infertility may be linked to a higher risk of death.
Patient summary: Men with a diagnosis of infertility do not seem to have a higher risk of death though an infertility-related diagnosis in men is associated with the risk of death.
Keywords: cancer, death, infertility
Introduction
It is estimated that 12–28% of couples trying to conceive are diagnosed with infertility.1,2 The World Health Organization has defined infertility as “a disease of the reproductive system defined by the failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse”.3
In infertile couples male infertility is the sole or contributing explanation in about half of the cases.2 Causes of male infertility include not only genetic defects (including certain syndromes), cancer, trauma, infections, hormonal problems, and systemic diseases but also mechanical obstruction and anatomical aberrations, sexual dysfunction, varicocele, and importantly, oligospermia.2,4
Male infertility has previously been linked to an excess risk of cancer, including testicular and prostate cancer.5,6 Prostate cancer represents 33% of all male cancers and hence may influence overall survival in men with infertility.7 Infertility may also have social consequences (for instance, it might trigger depression and psychiatric comorbidity). In a study by Lotti et al men in infertile couples were found to have a higher degree of self-reported anxiety and depression.8 Male infertility and mortality may have non-genetic shared risk factors, including comorbidity and lifestyle factors. Given the importance of genetic factors in both infertility and DNA repair, it is also possible that infertility is linked to cancer.9,10 Men with poor semen quality11 and their first-degree relatives12 appear to have a higher risk of cancer; and a low sperm count has also been linked to a higher risk of hospitalization.13 Glazer et al found a positive association between male infertility and the risk of chronic disease.14
Despite these investigations, large-scale studies of mortality in men with infertility are rare. In a Danish study in 2009, Jensen et al reported higher mortality in men with poor semen quality.15 The authors suggested that this association could be due to confounding by lifestyle and social factors (such as health and education level). Men evaluated for infertility in a US study11 were at lower risk of death (standardized mortality ratio [SMR]=0.39; 95% CI=0.30–0.49). However, neither of these studies adjusted for socioeconomic status.
For these reasons, we investigated the risk of death in men with a diagnosis of infertility and in men with a diagnosis related to infertility. We hypothesized that having a diagnosis of, or related to, infertility would be a risk factor for death, even after adjusting for relevant covariates (level of education, income, and country of birth).
Methods
Study design
We assessed a population-based cohort involving all men born in Sweden between 1944 and 1992 who were registered in the Total Population Register of Sweden.16 We calculated mortality rates incorporating data from the nationwide Cause of Death Register.17 Large-scale register linkages were made possible through the unique personal identity number (PIN) assigned to all Swedish permanent residents.18 This approach allowed us to examine individuals over time with almost no loss of follow-up.
Setting
Sweden has a publicly funded healthcare system with universal access to both hospital-based and primary care for adults and children.19,20 Couples seeking treatment for infertility are normally referred to a fertility clinic at the hospital, where both partners go through individualized diagnostic testing for infertility. Male infertility is evaluated by semen analysis, sometimes combined with an examination of the genitals.21 Hence, infertility is usually investigated in a hospital setting.
Exposures: infertility and infertility-related diagnoses
Data on exposures were obtained from the Swedish Patient Register.22 This register began in 1964 and has been nationwide since 1987. It also includes hospital-based outpatient care since 2001. We used International Classification of Disease (ICD) codes to identify individuals with infertility (diagnosis of male infertility) and infertility-related diagnoses (ie hypopituitarism, testicular hypofunction, cryptorchidism, hypospadias, Klinefelter syndrome, varicocele, and testicular torsion) (Table S1). While we are unaware of any validation of male infertility diagnoses, most diagnoses in the Swedish Patient Register have a positive predictive value ranging from 85–95%.22
Number of children
Number of children and date of birth for each child were obtained from the Swedish Multigeneration Register;23 through this register, we could also identify men without children.
Study participants
The study population comprised all men born in 1944–1992 who were registered in the Swedish Total Population Register,16 alive and residing in Sweden at the start of study follow-up (age of 20 years or on Jan 1, 1991, whichever occurred last). We excluded individuals with a re-used PIN to minimize the risk of incorrect linkages.
Cause of death
We used ICD codes to identify cause of death, specifically examining the risk of death from cancer, cardiovascular disease, and external causes (Table S2). Suicide was separated from other external causes of death (eg accidental poisonings, car accidents, falling or drowning) using an indicator variable for deliberate self harm in the Cause of Death Register.
Covariates and adjustment
We obtained data on covariates from the Swedish Patient Register, the Swedish Cancer Register, and from other relevant government registers (primarily the Total Population Register and the Education Register). Attained age was used as the underlying timescale. Multivariable-adjusted analyses included highest educational attainment 1990–2010 and country of birth as fixed covariates and diabetes, hypertension, liver failure, and end-stage renal disease as time-varying covariates. Disposable income at age 32–36 years was included as a fixed covariate in a separate model. Each covariate was included based on a priori knowledge of associations with the exposure and outcome, and categorized in accordance with Table 1. Date of diagnosis for chronic diseases was obtained from the Swedish Patient Register while information on date and site of any cancer diagnosis was obtained from the Swedish Cancer Register (Table S1).
Statistical methods
Frequencies, percentages, medians and interquartile ranges were calculated for the background variables by the exposure groups. To compare covariates between the exposure groups, the Chi-square test or analysis of variance (ANOVA) were used. Follow-up started at Jan 1, 1991 or the age of 20 years, whichever occurred last, and ended at date of death, first emigration, or end of study (Dec 31, 2012), whichever occurred first. The maximum age of follow-up was 69 years. The main outcome was all-cause death. As secondary outcomes, we assessed death due to cancer, cardiovascular disease, suicide and other external causes, for which person-time was censored for other causes of death. Mortality rates were estimated as events per 1,000 person-years. Using Cox regression, we estimated hazard ratios (HRs), comparing the mortality rates in men with or without infertility while adjusting for potential confounders and attained age as the underlying timescale. Infertility and infertility-related diagnoses were treated as time-varying exposures in which a person contributed person-time to the unexposed group until diagnosis of infertility (or related diagnosis).
To investigate whether any associations found were driven by not having children we performed analyses stratified by birth of the first child. We assessed non-proportional hazards by age and calendar period. We stratified the analysis on death before or after age 30, since the plotted mortality rates across age indicated a different pattern in young adult males. We also stratified the analysis by the year 2001 to investigate potential differences before and after the inclusion of outpatient care in the Swedish Patient Register. Finally, we assessed the potential confounding by disposable income in a sensitivity analysis, where the models were adjusted for income. Likelihood ratio tests were used to assess the interactions.
In the analysis of cause-specific death due to cancer, we specifically examined the association between infertility and death due to cancer stratified by death before or after age 30 as well as cancer diagnosis before diagnosis of infertility or diagnosis related to infertility in that infertility or related problems in young men may be caused by cancer treatment.
The regression analyses included only men with complete information on all covariates. Missing data was present in variables country of birth (0.02%), education (6.09%) and income (7.52%). Men with missing information on education were mainly short-term foreign-born residents appearing as “immortals” in the included registers, ie with incomplete follow-up for death and emigration, and were thus excluded from the analyses.
Data were prepared using SAS software (version 9.4, SAS Institute Inc., Cary, NC, USA) and Stata software version 15 IC (StataCorp. 2017, College Station, TX, USA) was used for the statistical analyses. All tests were two-sided and the significance level was set to 0.05.
This study was approved by the Regional Ethical Review Board in Stockholm, Sweden (ethical approval 2013/1849–31/2, amendment 2014/118–32).
Results
Background data
The study base comprised 3,049,364 men born in 1944–1992 who were alive and residing in Sweden at the start of follow-up (Figure 1). We excluded men with missing on education (n=185,761) and country of birth (n=18), leaving 2,863,585 men for the analyses. Of these, 43,598 had a diagnosis of infertility (1.5%) and 57,733 an infertility-related diagnosis (2.0%) and 2,762,254 had no diagnosis of infertility or infertility-related diagnosis (reference individuals) during follow-up (Table 1). The mean age at start of follow-up was 20.0 years for men with a diagnosis of or related to infertility, and 23.9 years for the reference men. The men were followed for a median time of 22.0 years: median age at the end of follow-up was 40.7 years for men with a diagnosis of infertility, 38.1 years for men with an infertility-related diagnosis, and 44.8 years for the reference men. A larger proportion of men with an infertility diagnosis had higher education (≥3 years, 28.1%) compared with men with an infertility-related diagnosis (16.6%) and reference men (16.2%). The proportion of men born in the Nordic countries was similar in all three groups. Men with a diagnosis of infertility more often had children at the end of follow-up (69.3%) compared with those with an infertility-related diagnosis (47.6%) and reference individuals (60.2%). The most common infertility-related diagnoses were cryptorchidism, varicocele, and testicular torsion. The proportion of men who were censored because of emigration was lower in men with a diagnosis of infertility (1.3%) than in men with an infertility-related diagnosis (3.9%) and reference individuals (5.3%). Cancer was the cause of death in 44.4% (diagnosis of infertility), 23.5% (infertility-related diagnosis) and 26.3% (reference group), with cardiovascular death constituting 18.2% (men diagnosed with infertility), 19.4% (men with an infertility-related diagnosis), and 24.9% (in reference individuals) of deaths in the study.
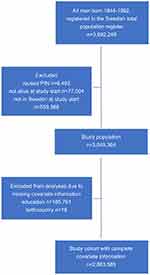 |
Figure 1 Flow chart of study population. |
 |
Table 1 Characteristics of men born in Sweden between 1944 and 1992 by infertility status |
All-cause mortality
During a follow-up of 281,656 years, 439 men with a diagnosis of infertility died (Table 2), corresponding to a crude incidence rate of 1.56 deaths per 1,000 person-years. This figure can be compared with 1,400 deaths during 712,620 person-years in men with an infertility-related diagnosis (1.96 deaths per 1,000 person-years) and 99,449 deaths during 45,822,503 person-years in reference individuals (2.17 deaths per 1,000 person-years). After adjusting for confounders, men with a diagnosis of infertility did not have a higher mortality rate (HR=0.98; 95% confidence interval [95%CI]=0.89–1.08), whereas having an infertility-related diagnosis was associated with a higher mortality rate (HR=1.23; 95%CI=1.17–1.30).
 |
Table 2 All-cause mortality in men with a diagnosis of infertility or an infertility-related diagnosis |
When splitting time by birth of the first child, the rate of death in men with an infertility-related diagnosis was higher in those with no children (HR=1.20; 95% CI=1.12–1.29) and in those who had at least one child (HR=1.11, 95% CI=1.03–1.20) compared with the reference group. Men with a diagnosis of infertility did not have a higher mortality rate either before or after having a child.
Figure 2 shows the unadjusted mortality rates by age. We found a higher mortality rate before the age of 30 years in men with a diagnosis of infertility. No such pattern was seen in men with an infertility-related diagnosis. A stratified analysis (Table 2) revealed that having a diagnosis of infertility was associated with an higher mortality rate up to age 30 years (HR=3.26; 95% CI=2.42–4.41), but not after age 30 (HR=0.91; 95% CI=0.83–1.01). Men with an infertility-related diagnosis had a higher mortality rate both before (HR=1.29; 95% CI=1.14–1.46) and after age 30 years (HR=1.22, 95% CI=1.15–1.29). Results from the analysis stratified by calendar period (Table 2) did not show higher mortality in men with a diagnosis of infertility before 2001 (HR=0.76, 95% CI=0.53–1.09) or from 2001 and later (HR=1.00, 95% CI=0.91–1.11). Men with an infertility-related diagnosis had higher mortality rates both in the earlier (HR=1.19; 95% CI=1.06–1.33) and later calendar period (HR=1.24, 95% CI=1.17–1.32).
Cause-specific mortality
Cancer mortality was higher in both men with a diagnosis of infertility and in those with an infertility-related diagnosis (Table 3). However, cancer mortality was only higher in individuals with a diagnosis of cancer preceding the infertility or related diagnosis, especially among men who died from cancer before age 30. The most common cancer types among infertile men were brain tumors, hematological cancers, and tumors of bone, cartilage, mesothelium, and soft tissue.
 |
Table 3 Cause-specific mortality in men with a diagnosis of infertility or an infertility-related diagnosis |
Cardiovascular mortality rate was not higher among men with infertility or related diagnoses. The rate of suicide was higher in men with an infertility-related diagnosis (HR=1.18, 95% CI=1.01–1.37), while the mortality rate due to other external causes was not (HR=1.04, 95% CI=0.91–1.18). Having a diagnosis of infertility was inversely associated with death due to suicide (HR=0.69, 95% CI=0.50–0.94) and other external causes (HR=0.55, 95% CI=0.40–0.74).
Sensitivity analyses
Adjusting for disposable income did not influence the associations between infertility and mortality (Table S3).
Discussion
Main findings
In this population-based study of nearly 3 million men aged 20–69 years we found no association between a diagnosis of infertility and mortality. In contrast, infertility-related diagnoses were associated with excess mortality. Overall, risk estimates for death decreased after adjustment for potential confounders and therefore we cannot rule out that the observed positive association for infertility-related diagnoses is due to the possibility of unaccounted confounding.
Comparison with previous studies
We confirm earlier reports that diagnosed infertility is not a risk factor for early death. However, in contrast to some studies, we did not observe a protective effect against death either. For instance, Eisenberg et al reported 61% lower mortality in men evaluated for infertility.11 That paper was based on two US cohorts (California and Texas) and out of 11,935 men, 69 (0.58%) died during follow-up. Very few (19.9%) of the participants in that study were followed before age 30 (20–29) years, which is when we observed an excess mortality in men with a diagnosis of infertility. Although the Eisenberg et al study adjusted for comorbidity, it failed to take socioeconomic status into account. The authors investigated cause-specific death but only observed a statistically significant decrease in infectious disease risk. The Danish study of Jensen et al15 did not include any data on infertility in general, nor did it discuss any explanation for the lower risk of death from infectious diseases. Latif and colleagues have demonstrated an inverse association between sperm count and hospitalization in two studies, using both registry-based13 and questionnaire-based24 data in men seeking help for infertility. One of these studies also found that mortality was higher in men with a low sperm concentration.13 However, infertility was not the focus of the studies by Latif et al In fact, these researchers repeatedly point out that they adjusted their analyses for fertility status and that that did not change the association between sperm count and morbidity. Our study did not contain any data on sperm count. Our findings of an increased mortality in infertility-related disorders are consistent with earlier research. When Jasim et al25 examined 23,515 patients with hypopituarism as part of a meta-analysis, they found a 55% higher mortality in this group. There are few studies on males with disorders of sex development.26,27 Mortality was not increased among 69 men with 46,XX disorders of sex development in a Danish study,26 although it should be noted that statistical power was low and that the 95%CI was wide (HR=0.6, 95% CI=0.2–2.5). Another study reported a 50% higher risk of death among men with Klinefelter syndrome in Denmark and Britain,27 similar to the 38% higher mortality rate seen in our study.
Strengths and limitations
Our study offers several improvements compared with previous research. Through the PIN,18 we were able to perform exact matching (as opposed to probabilistic matching used in one US study11). The Total Population Register covers all residents in Sweden and also tracks emigrations, which allows for a virtually complete follow-up of our study participants. During follow-up of 281,000 person-years in men with a diagnosis of infertility and 814,000 person-years in men with an infertility-related diagnosis, there were 409 and 1,356 deaths, respectively. The large numbers allowed us to calculate precise risk estimates, as well as to examine cause-specific death. We had hypothesized that male infertility would be associated with a higher risk of death. We could not confirm this hypothesis in the overall analysis but in a stratified analysis we noted a 4.58-fold higher risk of death in men with a diagnosis of infertility before the age of 30 years. Early death from cancer may signal impaired DNA repair, which has been hypothesized by others to also negatively affect spermatogenesis and thereby male fertility.11 However, because the association was only present in men diagnosed with infertility following cancer, the infertility was most likely caused by the cancer treatment. Lack of data on the cause of infertility, radiotherapy and chemotherapy precluded us from exploring this line of thought further.
We present results from two statistical models that adjusted for potential confounders. Such an approach is important given that research has shown that individuals of high socioeconomic status are more likely to seek medical attention for infertility. However, even with adjustment for level of education and income, we cannot rule out residual confounding. For instance, our study shows that men with a diagnosis of infertility more often had children at the end of follow-up than men without infertility. While we can assume that the men diagnosed with infertility had tried to have children, we have no information on whether men in the comparison group had tried to have children. Infertility and mortality may share a number of risk factors associated with lifestyle, including smoking, alcohol consumption, high BMI and obesity, lack of physical exercise, chronic diseases, medication use, and dietary factors.28 Unfortunately, we did not have any data on most of these potential confounders, but were able to adjust for comorbidity, such as diabetes mellitus, end-stage renal disease, hypertension, and liver failure.
The purpose of this study was to examine male infertility in the general population and its potential link to mortality. We did not have data on semen parameters, hormonal evaluation, or on how the diagnostic work-up had been performed for each patient; nor did we have granular data on the cause of infertility other than ICD codes from the Swedish Patient Register or data on fertility status in the partner. Because there is no general screening for fertility problems or semen quality, we cannot rule out that only a selected group of men with fertility problems seek medical attention for their problems. Eisenberg et al found that men undergoing infertility investigations had lower mortality and that higher mortality occurred only in men with affected sperm parameters.11 Men seeking medical examinations for potential infertility problems are more likely to have a partner, be health aware, have higher socioeconomic status, and more highly educated, but may differ regarding other factors, including region of residence, for which we could not adjust. Still, infertile men without children (indicating more severe infertility) had a lower (albeit not statistically significant) HR for death in our study (HR=0.92; 95% CI=0.80–1.06).
Because diagnoses from outpatient care were only available since 2001, many infertile men were likely classified as non-infertile in the earlier period. This likelihood could bias the results towards the null, although any such effect is likely small given the large comparison group. In addition, because most diagnoses of infertility were made in outpatient care, our results may not be generalizable to infertile men diagnosed before 2001. However, the small proportion of men who were diagnosed with infertility in inpatient care before 2001 did not have higher mortality (HR=0.85,95% CI=0.58–1.24).
Conclusion
In conclusion, this nationwide study found no excess risk of death in men with a diagnosis of infertility. Men with an infertility-related diagnosis had a slightly higher risk of death compared with men without such a diagnosis. Further research is needed to investigate the underlying mechanisms of this association.
Transparency declaration
The lead author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Ethics approval
The study was approved by the Ethical Review Board in Stockholm, Sweden (ethical approval 2013/1849-31/2, amendment 2014/118-32).
Data sharing statement
No additional data available because of Swedish regulations.
Abbreviation list
CI, confidence interval; HR, Hazard ratio; IQR, Interquartile range.
Acknowledgments
This project was supported by the EU-FP7 Health Program (agreement 259679), the Swedish Research Council (K2011-69X-21871-01-6 and SIMSAM 340-2013-5867) and the Strategic Research Program in Epidemiology Young Scholar Awards, Karolinska Institutet. None of the funding organizations have had any role in the design and conduct of the study; in the collection, management, and analysis of the data; or in the preparation, review, and approval of the manuscript.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
All authors have completed the Unified Competing Interest form and declare: no support from any organization for the submitted work [or describe if any]; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; and no other relationships or activities that could appear to have influenced the submitted work.
References
1. Gurunath S, Pandian Z, Anderson RA, Bhattacharya S. Defining infertility–a systematic review of prevalence studies. Hum Reprod Update. 2011;17(5):575–588.
2. Lotti F, Maggi M. Sexual dysfunction and male infertility. Nat Rev Urol. 2018;15:287–307.
3. Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92(5):1520–1524.
4. Anawalt BD. Approach to male infertility and induction of spermatogenesis. J Clin Endocrinol Metab. 2013;98(9):3532–3542.
5. Kanto S, Takahashi K, Maehara I, Fukuzaki A, Kyono K, Arai Y. Incidental testicular cancers that subsequently developed in oligozoospermic and azoospermic patients: report of three cases. Fertil Steril. 2007;88(5):1374–1376.
6. Mancini M, Carmignani L, Gazzano G, et al. High prevalence of testicular cancer in azoospermic men without spermatogenesis. Hum Reprod. 2007;22(4):1042–1046.
7. Official statistics of Sweden. Cancer Incidence in Sweden 2014. Stockholm, Sweden: The National Board of Health and Welfare; 2015.
8. Lotti F, Corona G, Castellini G, et al. Semen quality impairment is associated with sexual dysfunction according to its severity. Hum Reprod. 2016;31(12):2668–2680.
9. Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14(11):1197–1213.
10. Gunes S, Al-Sadaan M, Agarwal A. Spermatogenesis, DNA damage and DNA repair mechanisms in male infertility. Reprod Biomed Online. 2015;31:309–319.
11. Eisenberg ML, Li S, Behr B, et al. Semen quality, infertility and mortality in the USA. Hum Reprod. 2014;29(7):1567–1574.
12. Anderson RE, Hanson HA, Patel DP, et al. Cancer risk in first- and second-degree relatives of men with poor semen quality. Fertil Steril. 2016;106(3):731–738.
13. Latif T, Jensen TK, Mehlsen J, et al. Original contribution semen quality as a predictor of subsequent morbidity: a danish cohort study of 4,712 men with long-term follow-up. Am J Epidemiol. 2017;186(8):910–917.
14. Glazer CH, Bonde JP, Eisenberg ML, et al. Male infertility and risk of nonmalignant chronic diseases: a systematic review of the epidemiological evidence. Semin Reprod Med. 2017;35:282–290.
15. Jensen TK, Jacobsen R, Christensen K, et al. Good semen quality and life expectancy: a cohort study of 43,277 men. Am J Epidemiol. 2009;170(5):559–565.
16. Ludvigsson JF, Almqvist C, A-K EB, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31:125–136.
17. Westerling R. Comparing Swedish hospital discharge records with death certificates: implications for mortality statistics. Int J Epidemiol. 2000;29:495–502.
18. Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667.
19. Anell A. The public-private pendulum - patient choice and equity in Sweden. N Engl J Med. 2015;372(1):1–4.
20. Wettergren B, Blennow M, Hjern A, Söder O, Ludvigsson JF. Child health systems in Sweden. J Pediatr. 2016;177S:S187–202.
21. Gottlieb C, Fridström M. Ofrivillig Barnlöshet. Stockholm: Svensk förening för obstetrik och gynekologi; 2010.
22. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450.
23. Ekbom A. The Swedish multi-generation register. In: Dillner J, editor. Methods in Biobanking. Totowa, NJ: Humana Press; 2011:215–220.
24. Latif T, Lindahl-Jacobsen R, Mehlsen J, et al. Semen quality associated with subsequent hospitalizations - Can the effect be explained by socio-economic status and lifestyle factors? Andrology. 2018;6(3):428–435.
25. Jasim S, Alahdab F, Ahmed AT, et al. Mortality in adults with hypopituitarism: a systematic review and meta-analysis. Endocrine. 2017;56:33–42.
26. Berglund A, Johannsen TH, Stochholm K, et al. Incidence, prevalence, diagnostic delay, morbidity, mortality and socioeconomic status in males with 46,XX disorders of sex development: a nationwide study. Hum Reprod. 2017;32(8):1751–1760.
27. Bojesen A, Gravholt CH. Morbidity and mortality in Klinefelter syndrome (47,XXY). Acta Paediatr. 2011;100(6):807–813.
28. Sermondade N, Faure C, Fezeu L, et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update. 2013;19(3):221–231.
Supplementary materials
 |
Table S1 Diagnosis codes for male infertility, related disorders and chronic diseases |
 |
Table S2 Diagnosis codes for causes of death |
 |
Table S3 All-cause mortality in men with income information according to infertility status |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

