Back to Journals » Clinical Ophthalmology » Volume 17
Morphological Macular Changes Under Brolucizumab Treatment for Neovascular Age-Related Macular Degeneration Refractory to Previous Anti-VEGF Treatment Compared with Treatment-Naive Eyes
Authors William A, Verma-Fuehring R, Kuehnel S, Schwabe D, Kampik D, Goebel W, Hillenkamp J
Received 5 November 2022
Accepted for publication 19 January 2023
Published 8 March 2023 Volume 2023:17 Pages 769—777
DOI https://doi.org/10.2147/OPTH.S396304
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Antony William, Raoul Verma-Fuehring, Sophia Kuehnel, Dorothee Schwabe, Daniel Kampik, Winfried Goebel, Jost Hillenkamp
Department of Ophthalmology, University Hospital Würzburg, Würzburg, Germany
Correspondence: Antony William, Department of Ophthalmology, University Hospital Würzburg, Joseph–Schneider Straße 11, Würzburg, 97080, Germany, Tel +4993120120646, Email [email protected]
Purpose: To evaluate the morphological macular changes and fluid dynamics under brolucizumab treatment in eyes refractory to previous anti–vascular endothelial growth factor (anti-VEGF) treatment for neovascular age-related macular degeneration (nAMD) compared with treatment-naive eyes.
Methods: Retrospective study of all eyes treated with brolucizumab for nAMD between 2020 and 2021 with a fixed injection regimen and one year follow-up. Treatment-naive eyes (TN) were compared with eyes refractory to previous treatment with bevacizumab, ranibizumab, or aflibercept (RT). The primary outcome measure was change of best-corrected visual acuity (BCVA). Secondary outcome measures included foveal central thickness (FCT), presence of intra- or subretinal fluid (IRF, SRF) and presence of pigment epithelial detachment (PED) at any time point during treatment in both groups.
Results: Seventeen TN eyes and 17 RT eyes were included. Mean BCVA and mean FCT in TN eyes had significantly improved after 3 months and continued to improve during treatment (p< 0.05 and p=0.001, respectively). In RT eyes, mean BCVA did not change significantly while mean FCT had improved after 3 months of treatment and remained stable thereafter. SRF or PED were more frequent in RT eyes compared with TN eyes (p=0.003 and p=0.005, respectively).
Conclusion: After 3 months of treatment, the BCVA increased significantly only in TN eyes, while the FCT was significantly reduced in both groups. IRF appears to be similarly seen in both groups after the loading phase; however, SRF and PED appear to be more frequent in the RT eyes compared with TN eyes.
Keywords: treatment naïve, TN, refractory treatment, RT
Introduction
Neovascular age-related macular degeneration (nAMD) is a chronic progressive disease which is one of the leading causes of vision loss in industrialized nations.1 In the era of anti–vascular endothelial growth factor (anti-VEGF) therapy, the visual acuity of patients suffering from nAMD has improved significantly; hence, legal blindness prevalence has diminished.2 In the last two decades, the safety and efficacy of different anti-VEGF medications, i.e. bevacizumab, ranibizumab and aflibercept, used to manage nAMD, have been extensively investigated in numerous studies.3–6 Both bevacizumab and ranibizumab inhibit all VEGF-A isoforms, however aflibercept is a recombinant fusion decoy protein consisting of VEGF binding domains of human VEGFR-1 and VEGFR-2 fused to the Fc domain of human immunoglobulin G1 and binds to all forms of VEGF-A but also PlGF-1 and PlGF-2 with a very high affinity, greater than bevacizumab or ranibizumab.7 Moreover, different treatment protocols had been established and investigated to relieve patient’s burden from frequent repetitive injections to manage disease activity such as the pro re nata (PRN) protocol and Treat & Extend regimen.8,9 However, undertreatment remains a key limiting factor seen during treatment schedules in patients with nAMD due to non-adherence.10,11 The matter of nonadherence (defined as lack of adherence to a clinical trial regimen) or nonpersistence (defined as lack of persistence with following recommended clinical trials regimens over time) to intravitreal injection therapy for nAMD has been recently discussed by Okada et al in discussing the discrepancy between the results of real-world data in comparison with the clinical trials.12 Kim et al showed in a systemic meta-analysis of real-world outcomes in nAMD, that the visual acuity gain after 12 months of ranibizumab treatment was lower than that seen in the ANCHOR trial in which patients had received fewer injections and visits.11 Therefore, there is a need for more effective treatment with a lesser injection frequency to improve patient comfort, visual acuity and for better adherence. Recently brolucizumab, a single-chain antibody fragment that inhibits vascular endothelial growth factor-A, has been approved for the treatment of nAMD. Its minute structural antibody fragment unit permits better tissue penetration and higher molar dose.13 The results of the HAWK and HARRIER trials in treatment-naive (TN) eyes showed that brolucizumab is non-inferior and similar in its safety to aflibercept; similarly, different recent studies had shown that brolucizumab is also effective as a treatment option in refractory treatment (RT) eyes to previous anti-VEGF medications.11,16–18,20 However, real-world long-term results about the morphological macular changes and fluid dynamic changes under brolucizumab treatment in TN eyes and RT eyes has not yet been investigated. The aim of this study is to evaluate the morphological macular changes and fluid dynamic changes in eyes treated with brolucizumab for either treatment-naive nAMD or nAMD refractory to previous treatment with bevacizumab, ranibizumab or aflibercept.
Patients and Methods
This is a retrospective, consecutive single-centre study of patients with nAMD treated with brolucizumab. All nAMD eyes that were treated with brolucizumab between March 2020 and April 2022 while treatment was initiated were included. All patients were followed for one year. Thirty-four eyes of 33 patients met the inclusion criteria of nAMD (type I and type II macular neovascularisation, MNV), minimum best-corrected visual acuity (BCVA) of logMAR 1.3, and minimum follow-up of one year. Persistence or increase of the intraretinal fluids (IRF) or subretinal fluids (SRF) or serous retinal pigment epithelial detachment (PED) seen in fovea despite consecutive intravitreal injections of anti-VEGF medications (bevacizumab, ranibizumab or aflibercept) in a 4 weeks interval, was defined as RT. All the patients in the refractory treatment group had received previously at least two different anti-VEGF medications of which each was given at least 5 times every four weeks without a seen anatomical success in terms of persistence or increase of the intraretinal fluids (IRF) or subretinal fluids (SRF) or serous retinal pigment epithelial detachment (PED) seen in fovea.
Exclusion criteria were previous vitreoretinal surgery, previous laser treatment, history of uveitis or rheumatic diseases, choroidal or retinal neovascularization secondary to other retinal diseases or nAMD with type 3 neovascularization and loss of follow-up during the study period. The choice of anti-VEGF agent was left to the discretion of the treating physician. This work adhered to the tenets of the Declaration of Helsinki and approval of the local ethics committee of Wuerzburg-Bayern, Germany. According to German legislation and the requirements of the local institutional review board, complete data anonymization was performed in this study. All patients included in this study had received a loading dose of three intravitreal injections of 6 mg brolucizumab followed by five intravitreal injections and were followed up for one year. After the loading phase, patients were treated according to the HAWK and HARRIER protocol, i.e. injection every 8 weeks (q8w), if disease activity signs were seen in OCT, whereas in the absence of activity, patients were treated every 12 weeks (q12w).13
Visits Schedule and Data Acquisition
Different visits schedules were conducted: at baseline, after the loading phase and after the 4th, 6th and 8th intravitreal injection of brolucizumab. We documented at baseline complete ophthalmological history, including previous intravitreal treatment, vitreoretinal procedures, history of uveitis and rheumatological diseases. A comprehensive ophthalmological examination was performed at baseline, including the BCVA, Spectral Domain OCT (SD-OCT) and fundus photography (FP). Confirmation of the nAMD diagnosis was based on the fluorescein angiography (FA) in the treatment-naive group and OCT findings. After confirmation of the nAMD, patients were assigned into two groups according to the presence or absence of a previous history of treatment with anti-VEGF; treatment-naive (TN) and refractory treatment (RT).
In every visit after the baseline visit a complete ophthalmological examination including BCVA, anterior and posterior segment findings, SD-OCT findings and signs of intraocular inflammation or retinal vasculitis were documented. SD-OCT measurements were performed using Spectralis HRA-OCT® (Heidelberg Engineering, Heidelberg, Germany) after pupillary dilation with tropicamide (Mydriaticum Stulln®, Stulln, Germany). For OCT, each patient underwent 20°x20° degree 2 raster scans with 49 B-scans with a 117 µm distance between every scan using the built-in volumetric software protocol. Foveal central thickness was defined as the thickness of the innermost 1-mm ring of the built-in ETDRS macular map. Qualitative assessment of SD-OCT parameters foveal central thickness (FCT), IRF, SRF, and serous PED, were assessed through two investigators (FV&KS) for each patient during the duration of the study according to the latest consensus guidelines.14
Outcomes Measures and Statistical Analysis
For statistical analysis Student’s two-tailed t-test, matched pairs test, one-way-ANOVA analysis and Fischer exact test analysis were calculated at a significance level of p <0.05. Normal distribution of data was confirmed by a Shapiro–Wilk test at baseline for age, BCVA and FCT (p>0.05). For all parameters ranges are given as standard deviations (SD). All statistical tests were performed using JMP® software (version 16.0, SAS Institute Inc., Cary, NC, USA).
Results
Thirty-four eyes of 33 patients were enrolled in this study, from which 17 eyes were treatment-naive (TN) and 17 eyes were refractory treatment to previous anti-VEGF (RT). The mean age of the patients was 79.15±7.29 years. In the RT group, all the patients received a mean of 2.1±0.2 different previous intravitreal medications.
At the baseline, in the TN group the mean BCVA was logMAR 0.6±0.1, mean FCT was 350.5±43.44 µm. Also, IRF was present in 9 eyes (53%), SRF in 14 eyes (82.4%) and serous PED in 7 eyes (41.2%). In contrast, in the RT group, the mean BCVA in logMAR was 0.46±0.1, the mean FCT 314±42.15 µm. Nevertheless, IRF was seen in 7 eyes (41.2%), SRF in 14 eyes (82.4%) and serous PED in 7 eyes (41.2%).
At the baseline, there was no statistically significant difference between both groups concerning BCVA, FCT, IRF, SRF, Serous PED, p>0.05. The demographic characteristics of the patients’ results are summarized in Table 1.
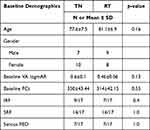 |
Table 1 General Demographics and Results of All the Eyes at Baseline |
After the loading phase, the mean BCVA in the TN group was 0.39±0.1 logMAR, FCT was 195.6±27.8 µm, IRF was seen in 3 eyes (17.6%), SRF in 7 eyes (41.2%) and serous PED in 5 eyes (29.4%). In the RT group, the BCVA after the loading phase was 0.43±0.06 logMAR, FCT was 216.5±28.1 µm, IRF was seen in 3 eyes (17.6%), SRF in 7 eyes (41.2%) and serous PED in 16 eyes (94.1%). The visual acuity increased significantly from baseline after the loading phase only in the TN group (BCVA from 0.6±0.1 to 0. 39±0.1 logMAR, p=0.01).
In contrast, in the RT group no significant change in the visual acuity was seen from baseline after the loading phase (0.46±0.1 to 0.43±0.06logMAR, p>0.05), Figure 1.
Comparably, the presence of serous PED was significantly lower in the TN group than in the RT group, p<0.0001 (PED was seen in 5 eyes in TN versus 16 eyes in RT). Nevertheless, a statistically significant reduction in the FCT was seen in both groups, with p<0.0001 after the loading phase, Figure 2. Similarly, after the loading phase the treatment interval was higher in the TN group, 47.5% had a q12w compared with 29.4% in the RT group, however, this was not statistically significant, p=0.22.
After the 8th intravitreal brolucizumab injection (1 year), the BCVA in the TN group was 0.24±0.1logMAR, FCT 162.5±23.4 µm, IRF was seen in 2 eyes (11.8%), SRF in 2 eyes (11.8%) and serous PED in 6 eyes (35.3%). On the other hand, in the RT group, the BCVA was 0.42±0.1logMAR, FCT 217.5±25.6 µm, IRF was seen in 4 eyes (23.5%), SRF in 10 eyes (59%) and serous PED in 14 eyes (82.3%). After the 8th intravitreal brolucizumab injection, there was a significant increase in the visual acuity in TN group from the loading phase (0.42±0.06 to 0.24±0.1 logMAR) (p=0.003). In contrast, in the RT group, there was no significant change seen in the visual acuity (0.43±0.06 to 0.42±0.1logMAR, p>0.05), Figure 1.
In addition, after one year there was a further substantial reduction in the FCT in the TN from the loading phase (−42.8 ± 15.1 µm), in comparison to the RT group (−5.81± 25.6 µm), with p=0.001, Figure 2. The incidence of the IRF was similar in both groups; however, a statistically significant difference in the presence of SRF and PED was seen in the RT group compared with the TN group; p=0.003 and p=0.005, respectively.
Lastly, after one year the treatment interval was significantly shorter in the RT group in comparison to the TN group, 8.66 ± 0.5 weeks in RT versus 12.3 ± 0.6 weeks in TN, p=0.0001, with only 6.6% in RT group reaching a treatment interval of q12w in comparison to 82.2% in the TN. The patients’ results throughout the study duration are summarized in Table 2.
 |
Table 2 Results of All Eyes in the Treatment-Naive and Refractory Treatment Groups at the Different Visits Schedules |
Drug-Related Events
In our study, the incidence of drug-related events was seen only in a single patient in the TN group while none was seen in the RT. Intraocular inflammation in the anterior and posterior segment occurred with occlusive retinal vasculitis and drop of the BCVA logMAR (from 1 to 1.5). The clinical findings of the anterior chamber showed anterior chamber cells (3+), diffuse corneal keratic precipitate, while in the posterior segment, there were vitreous cells (2+) according to the SUN classification and peripapillary retinal vasculitis. The patient was treated with intensive local and systemic steroid (intravenous prednisolone 250 mg for 3 days then switched to oral prednisolone 1 mg/kg oral) and then tapered over 6 weeks.
Under the steroid treatment, the intraocular inflammation resolved; however, the BCVA had dropped to 1.5 logMAR.
Discussion
Recently, published results of the phase III trials of HAWK and HARRIER showed that brolucizumab is non-inferior and similar in its safety to aflibercept and more than 50% of the brolucizumab patients maintained an interval of q12w through 48 weeks.13 However, several studies showed certain risk of intraocular inflammation, retinal vasculitis and retinal vascular occlusion (4.0%) after brolucizumab. These side effects occurred more frequently than after ranibizumab (1.5%) and (1.1%) with aflibercept.15–17 Despite those adverse events, several recent studies have reported favourable anatomical responses following a shift to brolucizumab or switch in refractory nAMD.18,19 Currently, the long-term morphological macular changes and fluid dynamic changes of brolucizumab in patients with TN nAMD and RT are undetermined.
To the best of our knowledge, this current study is among the earliest to investigate the long-term morphological macular changes and fluid dynamic changes under brolucizumab in treatment-naive and eyes refractory to other anti-VEGF agents.
Previously published studies were directed towards brolucizumab use as a switch or shift treatment in refractory nAMD but these studies were limited by short follow-up periods and individual treatment protocols.18–20
In our study, we applied the HAWK and HARRIER protocols for better comparability with large prospective multi-centre trials. For instance, in the SHIFT study from Bulirsch et al after administrating a single brolucizumab injection, patients were then treated directly subsequently with a treat and extend protocol.18 The different treatment protocols that are already known from the previous anti-VEGF drugs raised the question: which treatment protocol shall be implemented in the patients receiving brolucizumab? According to the HAWK and HARRIER study protocols, all patients received a starting loading phase of three injections then followed by q12w unless disease activity was identified, resulting in permanent adjustment to q8w.13 Treating refractory nAMD patients with only a starting single dose of brolucizumab injection and then controlling the patients in 8 weeks to access disease activity is still controversial. The current prospective multicentric FALCON study is evaluating the pros and cons of such an approach.21
We recognized through our current study that after the loading phase of brolucizumab, there was a statistically significant reduction in the FCT in both groups, p=0.0001. However, the BCVA only increased significantly in the TN group, p=0.01. The morphological macular changes in the various anatomical compartments were similar in both groups, apart from the serous PED, which was significantly more prevalent in the RT group. A higher percentage of patients in the TN group (47.5%) reached a q12w interval compared with 29.4% in the RT group after the loading phase, yet this difference was not statistically significant.
Furthermore, an adequate morphological response to the retinal fluids in all the retinal compartments was achieved aside from the serous PED in the RT; thus, our results are consistent with the previously published studies of HAWK and HARRIER.13 A non-increase in the visual acuity and the significant presence of serous PED in RT is similar to the results published by Dugel et al, where a subgroup of eyes with poor response to initial treatment during the loading phase was correlated to the presence of dynamic fluctuations in the IRF and SRF activity in OCT.22 Since patients in the RT group were previously treated with different anti-VEGF medications and were refractory in terms of persistent fluids under frequent treatment, visual recovery might be limited even if morphological und structural improvement are later seen.23
After the 8th intravitreal brolucizumab injection, there was a furthermore significant improvement in the visual acuity and significant reduction in the FCT in the TN group from the loading phase, p= <0.05, which was not seen in the RT group. However, we noticed more fluid dynamics fluctuations in terms of presence of serous PED & SRF, which were statistically significant in the RT group compared with the TN group, p=0.04 and 0.006, respectively. Based on the presence of the above macular changes that were significantly seen in the RT group, it was not unexpected for us to observe that the majority of the patients in the RT groups had an injections interval that was statistically significantly shorter (q8w) compared with that seen in TN groups (q12w). It is crucial to mention that at the baseline, there was no statistically significant difference in the intraretinal fluids in the different retinal compartments in both groups, however, after the loading phase there was a significant presence of serous PED in RT group in comparison to the TN group and further, throughout the study, a higher incidence of serous PED and SRF was seen in RT group patients.
Simider et al has previously discussed the importance of fluid dynamics changes on the visual acuity in patients with nAMD and determined IRF resolves earlier with anti-angiogenic therapy, followed by SRF. In contrast, PED decreased at a slower rate and intensity. Recurrence of IRF during follow-up showed no additional negative effect on function; however, recurrence of SRF during follow-up showed a tendency for an additional negative effect on function.24 Our results are fairly similar to the results of Simider et al, in which we have noticed that the presence of IRF after the loading phase are similar in both groups, however, a higher tendency of serous PED and SRF was evident after the loading phase and increased through one year of treatment in RT after extension of the injections intervals.
Our results in the RT group after one year were also similar to the results Bilgic et al, in which they reported that the majority of the patients in the switch group (68.8%) had received brolucizumab in q8w interval. However, this study was shorter in duration than ours.20
Although the injection interval in the RT group was shorter than that seen in the TN group, patients in the RT group benefited from a switch to brolucizumab. Since patients in the RT group had previously suffered from the persistence of intraretinal fluids under a 4 weeks treatment regimen, however, a significant reduction of the FCT and stability of the visual acuity was seen and a longer treatment interval of 8 weeks has been reached under brolucizumab treatment. Through our results, we were able to show that a switch to brolucizumab therapy in the patients with RT nAMD achieves anatomical success, increases the treatment interval and hence favours the relief of patient’s burden and a better adherence. It is important to mention that the clinical guidelines in the management of nAMD endorse that fluid on OCT indicates activity of the disease and recommend retreatment.25
In our case series, we documented only in a single case an intraocular inflammation with retinal vasculitis; it was in the TN group, and responded well to local and systemic steroid treatment according to the recent management guidelines published by Holz et al.26 The onset of brolucizumab-related adverse effect was similar to that reported previously by Mones et al within the first 6 months after the first injection.27
The main limitations of our current study are the retrospective nature and the small number of patients recruited; however, it provides a comprehensive and long-term follow-up.
Our current study demonstrated that brolucizumab is an effective treatment option in management of both treatment-naive patients and refractory treatment patients. Treatment-naive patients benefit from a significant improvement in the visual acuity after the loading phase and after 1 year and benefit from significant reduction in the FCT after the loading phase and after 1 year. Treatment-naive patients have favourable morphological effect in terms of sustained improvement in morphology with reduction and subsequent stability of the retinal fluids. On the other hand, refractory treatment patients under previous anti-VEGF therapy also benefit from shift to brolucizumab, improvement in the morphological macular changes and achieve a longer treatment interval. Brolucizumab-related adverse effects cannot be ignored; however, its use has a considerable benefit that cannot be overlooked. The recent published guidelines from Holz et al are helpful in classifying the form of uveitis that can occur and the treatment necessary according to each form. The effectiveness of brolucizumab in controlling the signs of activity in patients through a more prolonged interval of q16w, for example, in inactive nAMD, remains ambiguous.
Ethical Approval and Informed Consent
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. For this type of study, formal consent is not required (retrospective study).
Funding
No funding was received for this research.
Disclosure
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grant; participation in speaker’s bureaus; membership, employment or other equity interest) or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in subject matter or materials discussed in the manuscript.
References
1. Lim LS, Mitchell P, Seddon JM, et al. Age-related macular degeneration. Lancet. 2012;379(9827):1728–1738. doi:10.1016/S0140-6736(12)60282-7
2. Bloch SB, Larsen M, Munch IC. Incidence of legal blindness from age-related macular degeneration in Denmark: year 2000 to 2010. Am J Ophthalmol. 2012;153(2):209–213 e2. doi:10.1016/j.ajo.2011.10.016
3. Martin DF, Maguire MG, Fine SL; Comparison of Age-related Macular Degeneration Treatments Trials Research. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. doi:10.1016/j.ophtha.2012.03.053
4. Eleftheriadou M, Vazquez-Alfageme C, Citu CM, et al. Long-term outcomes of aflibercept treatment for neovascular age-related macular degeneration in a clinical setting. Am J Ophthalmol. 2017;174:160–168. doi:10.1016/j.ajo.2016.09.038
5. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi:10.1056/NEJMoa054481
6. Silva R, Berta A, Larsen M, et al. Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: results with ranibizumab from the TREND study. Ophthalmology. 2018;125(1):57–65. doi:10.1016/j.ophtha.2017.07.014
7. Pozarowska D, Pozarowski P. The era of anti-vascular endothelial growth factor (VEGF) drugs in ophthalmology, VEGF and anti-VEGF therapy. Cent Eur J Immunol. 2016;3(3):311–316. doi:10.5114/ceji.2016.63132
8. Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009;148(1):43–58 e1. doi:10.1016/j.ajo.2009.01.024
9. Abedi F, Wickremasinghe S, Islam AFM, et al. Anti-VEGF treatment in neovascular age-related macular degeneration: a treat-and-extend protocol over 2 years. Retina. 2014;34(8):1531–1538. doi:10.1097/IAE.0000000000000134
10. Spooner KL, Mhlanga C, Hong T, et al. The burden of neovascular age-related macular degeneration: a patient’s perspective. Clin Ophthalmol. 2018;12:2483–2491. doi:10.2147/OPTH.S185052
11. Kim LN, Mehta H, Barthelmes D, et al. Metaanalysis of real-world outcomes of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration. Retina. 2016;36(8):1418–1431. doi:10.1097/IAE.0000000000001142
12. Okada M, Mitchell P, Finger RP, et al. Nonadherence or nonpersistence to intravitreal injection therapy for neovascular age-related macular degeneration: a mixed-methods systematic review. Ophthalmology. 2021;128(2):234–247. doi:10.1016/j.ophtha.2020.07.060
13. Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: phase 3, multicenter, randomized double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72–84.
14. Spaide RF, Jaffe GJ, Sarraf D, et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology. 2020;127(5):616–636. doi:10.1016/j.ophtha.2019.11.004
15. Leclaire MD, Lauermann J, Alten F, et al. Intraokuläre Entzündung mit okklusiver retinaler Vaskulitis nach intravitrealer Brolucizumab-Injektion [Intraocular inflammation with occlusive retinal vasculitis following intravitreal injection of brolucizumab]. Ophthalmologe. 2022;119(3):296–299. German. doi:10.1007/s00347-021-01341-4
16. Witkin AJ, Hahn P, Murray TG, et al. Occlusive Retinal Vasculitis Following Intravitreal Brolucizumab. J Vitreoretin Dis. 2020;4(4):269–279. doi:10.1177/2474126420930863
17. Haug SJ, Hien DL, Uludag G, et al. Retinal arterial occlusive vasculitis following intravitreal brolucizumab administration. Am J Ophthalmol Case Rep. 2020;18:100680. doi:10.1016/j.ajoc.2020.100680
18. Bulirsch LM, Saßmannshausen M, Nadal J, et al. Short-term real-world outcomes following intravitreal brolucizumab for neovascular AMD: SHIFT study. Br J Ophthalmol. 2021;106(9):1288–1294.
19. Book M, Ziegler M, Rothaus K, et al. Erste Erfahrungen mit Brolucizumab bei neovaskulärer altersabhängiger Makuladegeneration und Therapierefraktärität unter der bisherigen Anti-VEGF-Therapie [Real-life experiences with Brolucizumab in recalcitrant neovascular age-related macular degeneration]. Ophthalmologe. 2022;119(3):258–264. German. doi:10.1007/s00347-021-01474-6
20. Bilgic A, Kodjikian L, March de Ribot F, et al. Real-world experience with brolucizumab in wet age-related macular degeneration: the REBA study. J Clin Med. 2021;10(13):2758. doi:10.3390/jcm10132758
21. Holz FG, Schmitz-Valckenberg S, Wolf A, et al. A randomized, open-label, multicenter study of switching to brolucizumab with or without a loading dose for patients with suboptimal anatomically controlled neovascular age-related macular degeneration-the FALCON study. Graefes Arch Clin Exp Ophthalmol. 2022;260(8):2695–2702. doi:10.1007/s00417-022-05591-z
22. Dugel PU, Jaffe GJ, Sallstig P, et al. Brolucizumab versus aflibercept in participants with neovascular age-related macular degeneration: a randomized trial. Ophthalmology. 2017;124(9):1296–1304. doi:10.1016/j.ophtha.2017.03.057
23. Gale RP, Pearce I, Eter N, et al. Anatomical and functional outcomes following switching from aflibercept to ranibizumab in neovascular age-related macular degeneration in Europe: SAFARI study. Br J Ophthalmol. 2020;104(4):493–499. doi:10.1136/bjophthalmol-2019-314251
24. Simader C, Ritter M, Bolz M, et al. Morphologic parameters relevant for visual outcome during anti-angiogenic therapy of neovascular age-related macular degeneration. Ophthalmology. 2014;121(6):1237–1245. doi:10.1016/j.ophtha.2013.12.029
25. Schmidt-Erfurth U, Chong V, Loewenstein A, et al. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br J Ophthalmol. 2014;98(9):1144–1167. doi:10.1136/bjophthalmol-2014-305702
26. Holz FG, Heinz C, Wolf A, et al. Intraokulare Entzündungen bei Brolucizumab-Anwendung [Intraocular inflammation with brolucizumab use: patient management-diagnosis-therapy]. Ophthalmologe. 2021;118(3):248–256. German. doi:10.1007/s00347-021-01321-8
27. Mones J, Srivastava SK, Jaffe GJ, et al. Risk of inflammation, retinal vasculitis, and retinal occlusion-related events with brolucizumab: post hoc review of HAWK and HARRIER. Ophthalmology. 2021;128(7):1050–1059. doi:10.1016/j.ophtha.2020.11.011
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


