Back to Journals » Clinical Interventions in Aging » Volume 16
Morphological Irregularity of Unruptured Intracranial Aneurysms is More Related with Aneurysm Size Rather Than Cerebrovascular Atherosclerosis: A Case-Control Study
Authors Qi P , Feng X, Lu J, Wang J, Hu S, Wang D
Received 20 January 2021
Accepted for publication 24 March 2021
Published 20 April 2021 Volume 2021:16 Pages 665—674
DOI https://doi.org/10.2147/CIA.S301326
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Zhi-Ying Wu
Peng Qi,1 Xin Feng,2 Jun Lu,1 Junjie Wang,1 Shen Hu,1 Daming Wang2
1Department of Neurosurgery, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 2Department of Neurosurgery, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, China; Graduate School of Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Daming Wang
Graduate School of Peking Union Medical College, No. 9 Dongdansantiao, Dongcheng District, Beijing, 100730, People’s Republic of China
Tel +86 10-85136281
Fax +86 10-85132621
Email [email protected]
Objective: It remains unclear whether irregular morphological features of intracranial aneurysms (IAs) are associated with atherosclerosis. We investigated the effect of cerebrovascular atherosclerosis stenosis (CAS) on irregular morphology of IAs.
Patients and Methods: This single-center case-control study included consecutive patients with IAs at our institution from September 2011 to September 2018. Cases were patients with irregular IAs, and age- and location-matched controls were patients with regular IAs. Conditional logistic regression models were used to assess the relationship between angiographic variables of CAS and aneurysmal irregularity.
Results: A total of 140 cases of irregular IAs and 140 controls were included in the analysis. Sixteen patients with irregular IAs (11.4%) and eleven patients with regular IAs (7.9%) had > 50% parent artery stenosis; however, the differences were not statistically significant between these two groups. In addition, no significant between-group differences were observed in distributions of the cerebrovascular stenosis, number of arterial stenoses, and location of the stenosis. In the final adjusted conditional logistic regression model, only aneurysm size (≥ 7 mm) was significantly associated with irregular IA morphology (P = 0.022). Moreover, 89 cases of irregular IAs and 89 controls were included in the analysis of unruptured IAs (UIAs). In the final adjusted conditional logistic regression model, only aneurysm size (≥ 7 mm) was significantly associated with irregular UIA morphology (P = 0.020).
Conclusion: Our findings indicate that the morphological irregularity of unruptured intracranial aneurysms is more related with aneurysm size rather than cerebrovascular atherosclerosis. Further studies are needed to use prospective data to identify causative factors responsible for aneurysmal irregularity.
Keywords: atherosclerotic stenosis, intracranial aneurysms, irregular morphology, risk factors, aneurysm size
Introduction
Intracranial aneurysm (IA) is an abnormal focal dilation of an artery in the brain and is the result of arterial wall degeneration. IA rupture is often a lethal and highly disabling event. Irregular morphology is a significant independent predictor of IA rupture,1 and 17–76% of IAs have been demonstrated to have irregular morphology.2,3 A wide range of morphological changes in the aneurysm wall has been demonstrated, including irregularity of aneurysm wall during the pre-stage of lobulation and the presence of multilobulated aneurysms. Notably, studies have indicated that atherosclerosis is associated with morphological changes causing fibrous tissue deposition.4,5 A systematic review in autopsy and angiography studies investigating the prevalence of incidental aneurysms reveals that atherosclerotic diseases seem to increase the risk of incidental aneurysms.6 In addition, the North American Symptomatic Carotid Endarterectomy Trial found that 3.1% of patients with carotid stenosis had cerebral aneurysms,7 possibly because atherosclerotic diseases and intracranial aneurysms share common risk factors. However, few studies have examined whether atherosclerosis leads to morphological changes in aneurysmal walls. It remains unclear whether irregular morphological features, such as lobulation and/or daughter sac, are associated with atherosclerosis.
In this case-control study, we investigated the effects of cerebrovascular atherosclerosis stenosis (CAS) on the formation of irregular IA morphology using quantitative indicators of CAS, such as the severity of stenosis, parent artery stenosis, number of stenosis cerebral arteries, and location of cerebrovascular stenosis. The findings would lead to a more detailed understanding of the relationship between CAS and irregular morphology of IAs and help to identify factors affecting morphological changes in aneurysmal walls.
Patients and Methods
Patient Selection
This study was approved by the Medical Ethics Committee of Beijing Hospital, and its protocol was in accordance with the principles of the Declaration of Helsinki. This single-center case-control study included consecutive patients with IAs evaluated or treated at our institution from September 2011 to September 2018. Subjects were divided into the following two groups: patients with irregular IAs (cases) and those with regular IAs (controls). One control was randomly selected from the patients with regular IAs and was matched to each patient with an irregular IA based on age (<50, 50–70, or ≥70 years old) and the location of IAs. Locations of IAs were divided into internal carotid-posterior communicating artery, anterior communicating artery, middle cerebral artery, internal carotid artery, basilar tip and basilar-superior cerebellar artery, vertebral artery-posterior inferior cerebellar artery and vertebrobasilar junction, and anterior cerebral artery.
The exclusion criteria included: (1) dissecting, fusiform, traumatic, mycotic, or partially thrombosed aneurysms; (2) aneurysms without readable and clear three-dimensional rotational angiography allowing an evaluation of lesion geometry and morphology; (3) aneurysms associated with cerebral arteriovenous malformation, arteriovenous fistula, or moyamoya disease.
Data Collection and Definitions
All aneurysms were diagnosed by digital subtraction angiography (DSA). Morphological features and stenosis were assessed using images from 2D/3D DSA by three experienced readers who worked in our center. Three interventional neuroradiologists (DM W, LJ W, and P Q) in this study had more than 10 years of experience.
Shape was classified as irregular when small bleb(s) or secondary aneurysm(s) were protruding from the saccular intracranial aneurysm fundus in any angiography image projection, or when the aneurysm fundus was clearly bi- or multilobular.8 Each reader made his morphological assessment independently, and the final assessment was determined by results agreed by two or three readers.
Angiographic variables for CAS included the severity of atherosclerosis stenosis (less than 50%, 50–70%, or 70% or more), number of arteries with stenosis (single or multiple), parent arterial stenosis (less than 50%, 50–70%, or 70% or more), and location of stenosis (anterior or posterior circulation). Extracranial artery stenosis was defined as ≥50% stenosis according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria.9 Anatomic severity of intracranial artery stenotic lesions was determined as ≥50% stenosis according to the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) method.10
We measured the aneurysm size, width, neck size (mm), aspect ratio (AR), and location. The AR was defined as the height of an aneurysm compared with its average neck size. Bifurcation aneurysms were defined as aneurysms that were located at parent artery bifurcations and therefore originated from more than one parent vessel (internal carotid artery terminus, internal carotid-posterior communicating artery, middle cerebral artery bifurcation, anterior communicating artery, and apex of the basilar artery).
We investigated other potential risk factors modulating aneurysm morphology. Social-demographic characteristics included age and sex. Clinical characteristics included cardiac comorbidities (coronary artery disease or myocardial infarction); history of cerebral ischemic events (transient ischemic attack, amaurosis fugax, or stroke); hypertension, hypercholesterolemia, or diabetes mellitus (patient or medical records indicated hypertension, hypercholesterolemia, or hyperglycemia, for which drug treatment, lifestyle, or other approaches had been provided); alcohol use (current or previous intake >5 drinks per day); and family history of IAs. Current smokers were defined as patients who smoked at the time of treatment or smoked ≥100 cigarettes during the past year. Former smokers were defined as patients who had smoked ≥100 cigarettes but had not smoked during the past year.11
Statistical Analyses
Statistical analyses were performed using SPSS Statistics for Windows (Version 23.0; IBM Corp, Armonk, New York, US). Continuous variables were analyzed using the Student’s t-test or Mann–Whitney U-test. Categorical variables were analyzed using Fisher’s exact test or the Pearson chi-square test. Associations between CAS and irregular shapes were assessed using Fisher’s exact test or the linear-by-linear association test. Because our study was performed on matched samples, the Cochran-Mantel-Haenszel test was also performed. Variables with P values of <0.20 in the univariate logistic regression analysis were evaluated in the multivariate analysis. Because the samples were matched, we used conditional logistic regression to calculate univariate and multivariate odds ratios (ORs) with 95% confidence intervals (CI). A P-value < 0.05 was considered statistically significant.
Results
Patient Selection
A total of 468 patients with 638 IAs were evaluated or treated at our institution during the study period. After applying our exclusion criteria, 336 patients with 507 unruptured or ruptured IAs (147 irregular and 360 regular IAs) were included in this study. However, seven patients with irregular IAs did not have matched controls. Consequently, our study population was composed of 140 patients with regular IAs and 140 patients with irregular IAs. Table 1 shows the distribution of demographic and clinical characteristics in both groups.
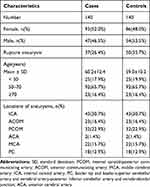 |
Table 1 Distribution of Age, Sex and Locations of Cases and Controls |
Among the 507 IAs, 383 were unruptured IAs (UIAs), including 95 irregular UIAs. However, six cases of irregular UIAs did not have matched controls. Consequently, 89 cases of regular UIAs and 89 cases of irregular UIAs were included in this study. Table 2 shows the distribution of demographic and clinical characteristics in these two groups.
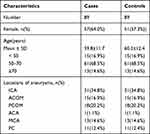 |
Table 2 Distribution of Age, Sex and Locations of Cases and Controls in Unruptured IAs |
Association Between CAS and Irregular Shapes of Unruptured and Ruptured IAs
Table 3 shows the frequency and odds of risk factors in patients with irregular and regular IAs. The following covariates met the predetermined level of significance and entered the stepwise forward selection for the conditional logistic model: alcohol use (P = 0.117), multiple aneurysms (P = 0.055), aneurysm size (P < 0.001), location of bifurcation (P = 0.086), and parent arteries with 70% or more stenosis (P = 0.086). No significant association was found between the severity of atherosclerosis stenosis (less than 50%/50–70%), number of arteries with stenosis (single/multiple), or location of stenosis (anterior/posterior circulation) and the risk of formation of irregular shapes of unruptured and ruptured IAs.
 |
Table 3 Frequency and Odds of Vascular Risk Factors in Cases Compared to Controls |
The distribution of the severity of atherosclerosis stenosis, number of arteries with stenosis, and location of the stenosis in the irregular and regular groups are shown in Figures 1–3. Patients with irregular IAs had more severe parent artery stenosis (Figure 1). Nine patients with irregular IAs (6.4%) had 50–70% parent artery stenosis, and seven patients with irregular IAs (5%) had 70% or more parent artery stenosis. Six patients with regular IAs (4.3%) had 50–70% parent artery stenosis, and five patients with regular IAs (3.6%) had 70% or more parent artery stenosis. No statistically significant difference was observed between the regular and irregular groups (50–70%, P = 0.750; ≥70%, P = 0.086). Similar results were observed in the distribution of the severity of atherosclerosis stenosis, number of arteries with stenosis (Figure 2), and location of the stenosis (Figure 3) in the regular and irregular groups, and the differences were not statistically significant between these two groups.
 |
Figure 1 The distribution of the severity of atherosclerosis stenosis in the irregular and regular groups. |
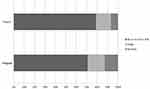 |
Figure 2 The distribution of number of arteries with stenosis, and location of the stenosis in the irregular and regular groups. |
 |
Figure 3 The distribution of location of the stenosis in the irregular and regular groups. |
The conditional logistic regression analysis revealed that only aneurysm size (≥7 mm) was significantly associated with irregular IA morphology (OR, 1.5; 95% CI, 1.1–2.1; P = 0.022) after adjusting for alcohol use, multiple aneurysms, location of bifurcation, and parent arteries with ≥70% stenosis.
Association Between CAS and Irregular Morphology of UIAs
Table 4 shows the frequency and odds of risk factors in irregular and regular UIA patients. The following covariates met the predetermined level of significance and entered the stepwise forward selection for the conditional logistic model: alcohol use (P = 0.136), multiple aneurysms (P = 0.132), aneurysm size (P < 0.001), cerebral ischemic comorbidities (P = 0.117), and parent arteries with 70% or more stenosis (P = 0.086). We did not detect significant associations between parent artery stenosis, severity of atherosclerosis stenosis (less than 50%/50–70%), number of arteries with stenosis (single/multiple), or location of stenosis (anterior/posterior circulation) with the risk of the formation of irregularly shaped UIAs.
 |
Table 4 Frequency and Odds of Vascular Risk Factors in Cases Compared to Controls in Unruptured IAs |
Patients with irregular UIAs had more severe parent artery stenosis. Eleven patients with irregular UIAs (12.3%) had more than 50% parent artery stenosis, and four patients with regular UIAs (4.7%) had more than 50% parent artery stenosis; however, no statistically significant differences were observed between these two groups (50–70%, P = 0.434; ≥70%, P = 0.229). Similar results were noted in the distribution of the severity of atherosclerosis stenosis, number of arteries with stenosis, and location of the stenosis in the regular and irregular UIA groups, and the differences were not statistically significant between these two groups either.
The conditional logistic regression analysis revealed that only aneurysm size (≥7 mm) was significantly associated with irregular morphology of UIAs (OR, 1.5; 95% CI, 1.1–2.1; P = 0.020) after adjusting for alcohol use and multiple aneurysms.
Discussion
Aneurysms presenting with irregular shapes have a higher risk of rupture than those with regular spherical shapes.12 The irregular intracranial aneurysmal morphology usually includes daughter sac, lobulation, or other irregular shapes. Morita et al found that aneurysms with daughter sac were associated with a higher rate of rupture than those with smooth wall.13 Notably, multilobulated aneurysms are the most frequently reported aneurysms among ruptured aneurysms, followed by aneurysms with single sac with irregular margin, daughter sac, and single sac with smooth margin (44.9%, 25.9%, 18%, and 11.2%, respectively).3 However, the precise factors contributing to the formation of IAs are unclear. One common feature of saccular IAs is the atherosclerotic change within the aneurysm wall.4 Killer-Oberpfalzer et al14 and Kosierkiewicz et al15 demonstrated that atherosclerotic lesions were present in all saccular IAs, and advanced atherosclerosis with diffuse intimal thickening (proliferating smooth muscle cells) and macrophages/lymphocytes were noted in both the smallest and largest aneurysms. In addition, Mizoi et al observed thick-walled large aneurysms (>10 mm) with irregular surfaces, on which whitish/yellowish atherosclerotic plaques obstructed the visualization of blood.16 Some studies found that an irregular shape was significantly related to UIA wall enhancement, which may indicate more atherosclerotic plaque formation.17–19 Daizo et al performed a prospective study and found that higher luminal concentrations of lipoprotein(a) in the aneurysm sac were significantly associated with increased wall enhancement of UIAs, which confirmed that atherosclerotic remodeling of the aneurysm wall may be associated with aneurysm sac.20,21 However, in this present study, we found no association between CAS features (cerebrovascular stenosis, number of arteries with stenosis, parent arteries stenosis, and location of stenosis) and the formation of irregular morphology of IAs.
Alexei et al found that computational fluid dynamic and fluid–structure interaction simulations identified a synergetic effect of high stenosis of parent artery in inducing greater aneurysm inflow velocity and deeper jet penetration, greater dome pressure, and greater tensile stress in the aneurysm wall.20 Chakravarty et al also found that the wall shear stress and several time-variant patterns of streamlines and vorticity contours of the flow phenomena are highly influenced by the severity of the stenosis and the angle of bifurcation.22 In this study, we observed that patients with irregular IAs had more severe parent artery stenosis (Tables 2 and 4). Analysis of ruptured and unruptured IAs revealed that 16 patients with irregular IAs (16.4%) and 11 patients with regular IAs (7.9%) had more than 50% parent artery stenosis. Analysis of UIAs indicated that 11 patients with irregular UIAs (12.3%) and 4 patients with regular UIAs (4.7%) had more than 50% parent artery stenosis. However, the differences were not statistically significant between the regular and irregular groups. Further, we observed that patients with irregular IAs tended to have more severe CAS. Analysis of ruptured and unruptured IAs revealed that 38 patients with irregular IAs (27.2%) and 33 patients with regular IAs (23.5%) had more than 50% CAS, and the same trend was also observed in the analysis of UIAs; however, these differences were not statistically significant between the regular and irregular groups. The slight increase in atherosclerosis stenosis may be related to hemodynamic changes, such as faster blood flow in the stenotic parent artery. Another possible reason is that the severity of parent artery stenosis only partially reflects the severity of atherosclerosis in the aneurysm wall.
Gu et al found that the volume change rate and AR were independent correlative factors for the formation of IAs with irregular shapes.23 In this study, the conditional logistic regression analysis revealed that only aneurysm size (≥7 mm) was significantly associated with irregular IA morphology (OR, 1.5; 95% CI, 1.1–2.1; P = 0.022) after adjusting for alcohol use, multiple aneurysms, location of bifurcation, and parent arteries with ≥70% stenosis. This finding is in line with that in our previous study.24 This is a meaningful discovery, which may mean that morphological irregularity of unruptured intracranial aneurysms is more related with aneurysm size rather than cerebrovascular atherosclerosis (Figure 4). The growth rate of irregular aneurysms is significantly higher than that of regular aneurysms.2,25–27 In fact, it has been demonstrated that aneurysms have a stable period of growth; after the stable period, aneurysms show a rapid increase in size, mostly due to the formation of daughter sac.25
Some studies stated that aneurysms configurations can be detected by autonomous ganglia. Various autonomic nerve fibers, body fluids and chemical factors can maintain the innervation of cerebrovascular nerves. The parasympathetic cranial nerves cause vasodilation, and the sympathetic signals of the stellate ganglia can constrict cerebral arteries. Additionally, trigeminal nerve endings provide dense, vasodilatory innervation to cerebral vessels. Innervation of the cerebrovascular serves a regulatory function by altering lumen diameter, permeability, sensory and secretory functions; thus, it is predicted that these nerves play important roles in determining aneurysms configurations characteristics.28–30 However, these hypotheses are difficult to confirm in our case series, and further research is needed.
The strengths of our study include the age- and location-matched case-control design and extensive adjustment for potential confounders. In addition, our study is the first to investigate the association between CAS and irregularities of aneurysms. To avoid potential bias caused by possible morphological changes after aneurysm rupture, we also analyzed a series of UIAs, and the results for both UIAs and ruptured IAs were similar. However, this study has some limitations. First, the limited statistical power of the study undermines the ability to detect potential associations. Many studies have suggested that hemodynamic factors and pathological features in the IA wall are important factors associated with the irregular shape formation; however, we did not adjust for hemodynamic features in our study. These factors may have led to an underestimation of the association in this study. Second, this study was a retrospective study, and all patients with aneurysms came from a single center; accordingly, the generalizability of the present findings is limited. Therefore, further studies are needed to confirm the association between irregular IA morphology and atherosclerosis. Third, we only investigated the risk factors based on previous studies. However, some potential risk factors that affect the formation of irregular shapes of IAs (ie, genetic factors, hemodynamic change, aneurysm wall enhancement) have not been included in this study. Fourth, we used four features of CAS, ie, the severity of atherosclerotic stenosis, parent-artery stenosis, number of stenotic arteries, and anterior/posterior circulation stenosis, as indicators for the assessment of CAS; however, this approach may still lack sufficient persuasiveness and typicality to clarify the relationship between aneurysmal irregularity and atherosclerotic stenosis. Thus, more prospective data or related scoring models may be needed in future studies.
Conclusions
Our findings indicate that the morphological irregularity of unruptured intracranial aneurysms is more related with aneurysm size rather than cerebrovascular atherosclerosis. Further studies should use more prospective data and employ related scoring models to disentangle the causative factors contributing to aneurysmal irregularity.
Ethical Statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Patient Consent for Publication
All patients provided informed consent in accordance with the Declaration of Helsinki.
Funding
This work was supported by [Beijing Hospital Clinical Research 121 Program] grant number [121-2016006].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Akio M, Takaaki K; UCAS Japan Investigators, et al.. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366:2474–2482.
2. Backes D, Rinkel GJE, Greving JP, et al. ELAPSS score for prediction of risk of growth of unruptured intracranial aneurysms. Neurology. 2017;88(17):1600–1606. doi:10.1212/WNL.0000000000003865
3. Abboud T, Rustom J, Bester M, et al. Morphology of ruptured and unruptured intracranial aneurysms. World Neurosurg. 2016;99:610–617. doi:10.1016/j.wneu.2016.12.053
4. Chalouhi N, Ali MS, Jabbour PM, et al. Biology of intracranial aneurysms: role of inflammation. J Cerebral Blood Flow Metabol. 2012;32(9):1659–1676. doi:10.1038/jcbfm.2012.84
5. Downregulation of T Cell Immunoglobulin and Mucin Protein. 3 in the pathogenesis of intracranial aneurysm. Inflammation. 2015;38(1):368–374. doi:10.1007/s10753-014-0040-x
6. Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke. 1998;29:251–256. doi:10.1161/01.STR.29.1.251
7. Kappelle LJ, Eliasziw M, Fox AJ, Barnett HJ. Small, unruptured intracranial aneurysms and management of symptomatic carotid artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Group. Neurology. 2000;55:307–309. doi:10.1212/WNL.55.2.307
8. Antti Lindgren E, Timo K, Joel B, et al. Irregular shape of intracranial aneurysm indicates rupture risk irrespective of size in a population-based cohort. Stroke. 2016;47:1219–1226. doi:10.1161/STROKEAHA.115.012404
9. Rothwell PM, Eliasziw M, Gutnikov SA, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003;361:107–116. doi:10.1016/S0140-6736(03)12228-3
10. Chimowitz MI, Kokkinos J, Strong J, et al. The warfarin-aspirin symptomatic intracranial disease study. Neurology. 1995;45(8):1488–1493. doi:10.1212/WNL.45.8.1488
11. Amato MS, Boyle RG, Levy D. How to define e-cigarette prevalence? Finding clues in the use frequency distribution. Tob Control. 2016;25(e1):e24. doi:10.1136/tobaccocontrol-2015-052236
12. Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10(7):626–636. doi:10.1016/S1474-4422(11)70109-0
13. Morita A, Kirino T, Hashi K, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366:2474–2482.
14. Killer-Oberpfalzer M, Aichholzer M, Weis S, et al. Histological analysis of clipped human intracranial aneurysms and parent arteries with short-term follow-up. Cardiovasc Pathol. 2012;21(4):299–306. doi:10.1016/j.carpath.2011.09.010
15. Kosierkiewicz TA, Factor SM, Dickson DW. Immunocytochemical studies of atherosclerotic lesions of cerebral berry aneurysms. J Neuropathol Exp Neurol. 1994;53(4):399–406. doi:10.1097/00005072-199407000-00012
16. Mizoi K, Yoshimoto T, Nagamine Y. Types of unruptured cerebral aneurysms reviewed from operation video-recordings. Acta Neurochir (Wien). 1996;138(8):965–969. doi:10.1007/BF01411286
17. Kai Q, Jianping S, Zixiao Y, et al. Validation of wall enhancement as a new imaging biomarker of unruptured cerebral aneurysm. Stroke. 2019;50:1570–1573. doi:10.1161/STROKEAHA.118.024195
18. Weiying Z, Du Y, Guo Q, et al. The clinical and morphologic features related to aneurysm wall enhancement and enhancement pattern in patients with anterior circulation aneurysms. World Neurosurg. 2020;134:e649–e656. doi:10.1016/j.wneu.2019.10.156
19. Wang G-X, Li W, Sheng L, et al. Relationships between aneurysmal wall enhancement and conventional risk factors in patients with intracranial aneurysm: a high-resolution MRI study. J Neuroradiol. 2019;46:25–28. doi:10.1016/j.neurad.2018.09.007
20. Alexei A, Kenichi K, Kilian G-K, et al. Proximal stenosis is associated with rupture status in middle cerebral artery aneurysms. World Neurosurg. 2018;109:e835–e844. doi:10.1016/j.wneu.2017.10.108
21. Daizo I, Mario Z, Roa JA, et al. Concentration of Lp(a) (Lipoprotein[a]) in aneurysm sac is associated with wall enhancement of unruptured intracranial aneurysm. Stroke. 2021;52(4):1465–1468. doi:10.1161/STROKEAHA.120.032304
22. Chakravarty S, Sen S. Analysis of pulsatile blood flow in constricted bifurcated arteries with vorticity-stream function approach. J Med Eng Technol. 2008;32:10–22. doi:10.1080/03091900600700822
23. Yan G, Yonggang Z, Meng L, et al. Dynamic volume change rate and aspect ratio are correlated to the formation of an irregular morphology of unruptured intracranial aneurysm. J Comput Assist Tomogr. 2018;undefined:undefined.
24. Feng X, Zhang B, Guo E, et al. Bifurcation location and growth of aneurysm size are significantly associated with an irregular shape of unruptured intracranial aneurysms. World Neurosurg. 2017;107:255–262. doi:10.1016/j.wneu.2017.07.063
25. Żyłkowski J, Kunert P, Jaworski M, et al. Changes of size and shape of small, unruptured intracranial aneurysms in repeated computed tomography angiography studies. Videosurg Other Miniinvasive Tech. 2015;10(2):178–188. doi:10.5114/wiitm.2015.52707
26. Brinjikji W, et al. Risk factors for growth of intracranial aneurysms: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2016;37(4):615–620. doi:10.3174/ajnr.A4575
27. Backes D, Rinkel GJE, Laban KG, et al. Patient- and aneurysm-specific risk factors for intracranial aneurysm growth: a systematic review and meta-analysis. Stroke. 2016;47(4):951–957. doi:10.1161/STROKEAHA.115.012162
28. Ilhan Y, Metehan E. Onen Mehmet R et al. Inverse association between basilar artery volume and neuron density in the stellate ganglion following bilateral common carotid artery ligation: an experimental study World Neurosurg. 2017;100:138–143.
29. Ilhan Y, Metehan E, Resid OM, et al. Inverse association between basilar artery volume and neuron density in the stellate ganglion following bilateral common carotid artery ligation: an experimental study. World Neurosurg. 2017;100:138–143. doi:10.1016/j.wneu.2016.12.034
30. Ozkan U, Aydin MD, Gündoğdu C, et al. Histopathologic changes in oculomotor nerve and ciliary ganglion in aneurysmatic compression injuries of oculomotor nerve. Minim Invasive Neurosurg. 2004;47:107–110. doi:10.1055/s-2004-818435
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.