Back to Journals » International Journal of General Medicine » Volume 9
Morgellons disease: a filamentous borrelial dermatitis
Authors Middelveen MJ , Stricker RB
Received 6 July 2016
Accepted for publication 15 September 2016
Published 14 October 2016 Volume 2016:9 Pages 349—354
DOI https://doi.org/10.2147/IJGM.S116608
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Video abstract presented by Raphael B Stricker
Views: 37692
Marianne J Middelveen, Raphael B Stricker
International Lyme and Associated Diseases Society, Bethesda, MD, USA
Abstract: Morgellons disease (MD) is a dermopathy characterized by multicolored filaments that lie under, are embedded in, or project from skin. Although MD was initially considered to be a delusional disorder, recent studies have demonstrated that the dermopathy is associated with tickborne infection, that the filaments are composed of keratin and collagen, and that they result from proliferation of keratinocytes and fibroblasts in epithelial tissue. Culture, histopathological and molecular evidence of spirochetal infection associated with MD has been presented in several published studies using a variety of techniques. Spirochetes genetically identified as Borrelia burgdorferi sensu stricto predominate as the infective agent in most of the Morgellons skin specimens studied so far. Other species of Borrelia including Borrelia garinii, Borrelia miyamotoi, and Borrelia hermsii have also been detected in skin specimens taken from MD patients. The optimal treatment for MD remains to be determined.
Keywords: Morgellons disease, dermatitis, Lyme disease, Borrelia burgdorferi, spirochetes
Introduction
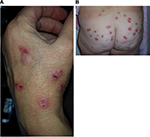
Morgellons disease (MD) is an emerging dermopathy with worldwide distribution. The name “Morgellons” is derived from a disease recognized in the seventeenth century in French children by Sir Thomas Browne. These children were noted to have “coarse hairs” protruding from their backs.1 The distinguishing feature of MD is the appearance of skin lesions with filaments that lie under, are embedded in, or project from skin (Figures 1 and 2). Filaments can be white, black, or brightly colored.2–6 Furthermore, MD patients exhibit a variety of manifestations that resemble symptoms of Lyme disease (LD), such as fatigue, joint pain, and neuropathy.2–6 A study found that 98% of MD subjects had positive LD serology and/or a tickborne disease diagnosis,5 confirming the clinical association between MD and spirochetal infection. Conversely, 6% of LD patients in an Australian study were found to have MD.7
The similarity between MD and an animal disease, bovine digital dermatitis (BDD), an acknowledged spirochetal infection that is associated with ulcerative lesions exhibiting keratin projections, was previously explored.6 Treponemal spirochetes are the primary etiologic agents of BDD.8,9 A causal relationship between spirochetal infection and filament formation was confirmed by duplication of the clinical disease via experimental infection with pure cultured treponemes.8,9 This prompted further investigation into the possibility of a spirochetal etiology for MD to discover if a similar disease process occurred at the cellular level.10–13
Histopathology of MD
Histological studies on MD tissue show that MD filaments are not textile fibers. They are biofilaments of human cellular origin produced by epithelial cells and stem from deeper layers of the epidermis, the upper layers of the dermis, and the root sheath of hair follicles.6,10,11 Histological studies established that these filaments are predominantly composed of collagen and keratin,10,11 and are nucleated at the base of attachment to epithelial cells,11 thus demonstrating human cellular origin. Staining of embedded filaments with Congo red resulted in apple-green birefringence suggestive of an amyloid component, although this remains to be confirmed by more specific studies (unpublished data). Staining of embedded filaments with calcofluor-white produced negative results, demonstrating that filaments are not cellulose as found in cotton, linen, or other plant-based textile fibers, or chitin as found in fungal cells and insect exoskeletons (unpublished data).
A preliminary study using scanning electron microscopy (SEM) showed hairlike scales on a blue filament, suggesting that at least some MD fibers are hairs.10 The blue coloration of some fibers was first determined to be the result of melanin pigmentation as shown by positive Fontana Masson staining.11 An independent study concurred that embedded blue fibers in an MD specimen (supplied by the authors of this paper) were not textile fibers. SEM revealed that the blue fibers were microscopic hairs with cuticular scaling, and transmission electron microscopy (TEM) revealed darkly stained melanosomes that were not organized, a finding consistent with human hairs (Shawkey MD, unpublished data, 2013).
Microspectrophotometry reflectance data on fibers were consistent with patterns of pigmented tissues. Raman spectroscopy14 on two separate blue fibers showed relevant peaks that were indicative of carbamate compounds and melanin aromatic rings (Shawkey MD, unpublished observation, 2016). Hence, independent studies using different methodologies provided evidence that Morgellons fibers are hairlike extrusions and that the blue coloration is the result of melanin pigmentation. Although the mechanism for coloration of red fibers is not yet understood, there are no known textile fibers colored by blue melanin pigmentation.11
Association of MD with Borrelia infection
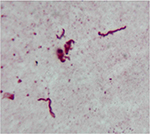
Borrelia spirochetes have repeatedly been detected in MD skin and tissue samples (Figures 3 and 4). Initial studies confirmed the presence of Borrelia burgdorferi sensu stricto (Bb ss) spirochetes within dermatological tissue removed from MD lesions of four North American patients.11,12 A subsequent study reported the detection and identification of Borrelia garinii in Morgellons skin samples obtained from an Australian patient.15 A larger study subsequently reported the detection of Borrelia spirochetes in 25 MD subjects.13 Detection of Borrelia DNA by polymerase chain reaction (PCR) followed by Sanger sequencing in two independent laboratories determined that the Borrelia spirochetes detected in these studies were predominantly Bb ss, but B. garinii and Borrelia miyamotoi were also reported. More recently, studies of MD specimens in two additional laboratories have detected Borrelia DNA of three Borrelia spp., Bb ss, B. garinii, and Borrelia hermsii.16,17 The fact that four different laboratories have been able to detect Borrelia DNA in Morgellons specimens shows that these findings are reproducible.
  | Figure 3 Spirochete detected with Dieterle silver stain in culture of skin sample from Morgellons disease patient. Notes: 1000× original magnification. Figure courtesy of Marianne J Middelveen. |
Motile spirochetes identified as Borrelia spp. have been cultured in Barbour-Stoenner-Kelly (BSK)-H medium inoculated with MD dermatological tissue. This demonstrated that the spirochetes present in MD tissue are alive and viable.12,13 The culture of Borrelia spirochetes can be challenging because of fastidious growth requirements and pleomorphism.18,19 Therefore, PCR amplification of cultured spirochetes has been used to confirm the presence and provide molecular identification of live Borrelia spirochetes in MD dermatological tissue.13 The combination of Borrelia culture and PCR lends strong support to the clinical association of spirochetal infection with MD.
Although the common denominator in the evolution of MD lesions seems to be infection with Borrelia spp., the etiology of MD is presumed to be multifactorial. Secondary etiologic factors such as genetic background, hormonal influences, immune status, and the presence of other coinvolved infections appear to play a role in the development of this phenomenon.2–6 Other pathogens have been detected in Morgellons tissue samples. Strains of Helicobacter pylori and closely related bacteria were detected along with Borrelia spp. in tissue samples from MD lesions.20 One study detected Treponema denticola along with Borrelia spp. in some Morgellons specimens,12 while another study found H. pylori, T. denticola, and Bartonella henselae in Morgellons specimens.17 A putative role of Agrobacterium in MD has not been confirmed.
The precise mechanism of Morgellons filament formation has not been elucidated. In MD lesions, collagen and keratin filaments arise from proliferative keratinocytes and fibroblasts in human epithelial tissue.10,11 Borrelia has the capability to invade fibroblasts and keratinocytes and to replicate inside these cells.21–23 We speculate that infection and replication of Borrelia within keratinocytes and fibroblasts alter keratin and collagen gene regulation. Furthermore, intracellular sequestration of Borrelia may be a factor contributing to the development of refractory infection and MD. Borrelia spirochetes have been isolated in vitro from monolayers of keratinocytes and fibroblasts that were treated with antibiotics.21,22 Spirochetes were detected in MD dermatological tissue taken from patients who had been given aggressive antibiotic therapy.13 Persistent infection and resistance to antibiotic treatment may therefore result from sequestration of Borrelia spirochetes within keratinocytes and fibroblasts in MD patients.
The diagnosis of LD is controversial, and research showing a connection between MD and LD has been challenged. A study from the Centers for Disease Control and Prevention (CDC) concluded that MD was not caused by a pathogen.24 However, in that study the search for spirochetal pathogens was limited to nonspecific silver nitrate staining on a small number of tissue samples and commercial two-tiered serological LD testing as recommended by the CDC.24,25 Although Borrelia spirochetes can be readily detected in MD tissue, the use of sensitive and specific molecular methods is required.13 Two-tiered serological testing for LD lacks sensitivity for a number of reasons, and seronegativity in positive cases occurs.26,27 Detection of LD is complicated by the genetic diversity of Borrelia spp.28,29,30 Other methods such as PCR detection of Borrelia DNA and antigen detection are not standardized and vary in sensitivity and specificity.31,32 Although these tests can be effective means of detecting Borrelia spirochetes in human fluids and tissues, they are not accepted as diagnostic by the CDC.13,25,32 Thus, the relationship between MD and LD shown in clinical, histopathological and molecular studies remains controversial.
Historical view of MD
While the first reports of LD in the USA date back to the 1970s,33 filamentous dermatological manifestations were not reported to be associated with Borrelia infection until 2005.2 Although this temporal disparity may seem anomalous to some, there are explanations. Sir Thomas Browne’s description of dermal hairlike extrusions coupled with movement sensations dates back to 1674.1 In 1938, Ekbom published a seminal account of delusional parasitosis with sensations of insects crawling on or under skin, and it is notable that many of Ekbom’s study subjects had syphilis, a known spirochetal infection.34 LD is not a new disease: a 5,300-year-old mummy is the oldest known case.35 Thus, Morgellons cases may have occurred sporadically in parallel with LD, and the association between LD and MD may have gone unrecognized and unreported.
Misdiagnosis of MD is likely to be common as the filaments are microscopic and invisible without sufficient magnification or, if observed under magnification, may be miscategorized as textile fibers. One could also argue that because serological testing for LD lacks sensitivity,26,27 patients frequently go undiagnosed. The few patients who do get a positive two-tiered test for LD are likely to be treated early for Bb infection and therefore may fail to develop MD. We see many MD patients who are diagnosed with other dermatological conditions (prurigo nodularis, eczema, etc) or mental illnesses, and thus were not tested for LD. Although MD is usually associated with chronic symptoms of LD, some patients develop MD lesions in conjunction with a documented tick bite and/or erythema migrans rash.
MD and psychiatric diagnoses
There are over 250 peer-reviewed articles linking LD and associated tickborne diseases to mental illness.36–38 A spectrum of psychiatric illnesses occurs in patients with neuroborreliosis, including paranoia, schizophrenia, bipolar disorder, delusions, sensory hallucinations, major depression, and mania.39 Neurological symptoms associated with LD range in severity from marginal to critical, and can include cognitive impairment, dementia, insomnia, irritability, anxiety, depression, personality disorders, psychosis, and suicidal or homicidal tendencies.37 The effects of microbial infection can impact neuronal functioning, and as a result of infection a prolonged inflammatory process can drive the chronic progression of neurodegenerative diseases.37,40
Levels of certain proinflammatory cytokines have been reported to increase in the cerebrospinal fluid of patients with neurological manifestations of LD.41 Similarly, elevated inflammatory markers that correlate with excess of immune cytokines have been described in MD patients.42 The outer membrane lipoproteins of Borrelia are proinflammatory and can mimic host antigens, theoretically resulting in autoimmune reactivity.37 The fact that elevated anti-nerve antibody reactivity can be found in pretreatment LD patients and in posttreatment patients with persistent symptoms supports this theory.37
Although MD may result from an infectious process, there may be a psychiatric component as well, and some (but not all) MD patients exhibit neuropsychiatric symptoms. In a study of 25 Morgellons patients, 23 had prior psychiatric diagnoses including bipolar disorder, attention deficit disorder, obsessive-compulsive disorder (OCD), and schizophrenia.42 The fact that MD patients may show neuropsychiatric symptoms complicates the diagnosis and explains why some health care providers consider MD to be a delusional disorder. To further complicate matters, some patients with MD who do not exhibit psychiatric abnormalities have been misdiagnosed with other conditions such as lichen sclerosus or prurigo nodularis.
Furthermore, lack of scientific knowledge has led patients to interpret the physical presence of dermal filaments and symptoms of formication as parasitic infection. In these cases, the false belief of parasitic infestation is not genuinely delusional because patients are in fact misinterpreting symptoms caused by aberrant production of human biofibers. Furthermore, we find that electrostatic energy and mechanical energy can cause movement of filaments, interpreted by some patients as the movement of a living organism, and small insects such as fruit flies can adhere to open lesions, leading some patients to believe they are infested. If such patients do have a psychiatric condition such as OCD, then the false belief can be intensified or reinforced.
Hypothetically, some patients with delusional disorders could mistakenly believe they have MD. In addition, in our experience some LD patients exhibit crawling or stinging sensations, or can have ulcerative lesions – without developing associated dermal filaments. The overlap of mental illness, LD, and MD highlights the explicit need for a universally accepted clinical definition of MD. The following case definition for MD has been proposed: a somatic Lyme-like illness associated with spontaneously appearing, slowly healing, filamentous and ulcerative skin lesions. The key diagnostic criterion is colored, white, or black filaments protruding from or embedded in skin. However, because there is an overlap of LD, MD, and mental illness with a spectrum of different symptoms, we recommend that LD be considered in the differential diagnosis of patients with Lyme-like symptoms in conjunction with formication with or without ulcerations.
Clinical classification of MD
In a recent publication,13 we proposed a clinical classification scheme that reflects the duration and location of MD lesions:
- Early localized: lesions/fibers present for less than three months and localized to one area of the body (head, trunk, extremities).
- Early disseminated: lesions/fibers present for less than three months and involving more than one area of the body (head, trunk, extremities).
- Late localized: lesions/fibers present for more than six months and localized to one area of the body (head, trunk, extremities).
- Late disseminated: lesions/fibers present for more than six months and involving more than one area of the body (head, trunk, extremities).
As noted in that article, the classification scheme provides a medical framework that should help to validate and standardize the diagnosis of MD. Further studies are needed to determine whether this classification will have therapeutic and prognostic significance for MD patients.13
Treatment of MD
Since a clinical classification of MD has not been universally accepted, optimal treatment for the disease remains unsettled. Nevertheless, several therapeutic principles have emerged: 1) the earlier the treatment is initiated in the course of MD, the better the outcome appears to be; 2) treatment should be aimed at the underlying tickborne disease; 3) prolonged combination antibiotic therapy may be necessary to eradicate dermopathy; and 4) antiparasitic therapy may be useful in some patients with MD. At this point, the most logical treatment is supported by the guidelines of the International Lyme and Associated Diseases Society.43 Although treatment with antipsychotic agents has been proposed for patients with neuropsychiatric symptoms of MD, this treatment generally fails without concomitant therapy of the underlying tickborne disease.15 Additional approaches with agents such as dapsone merit further study.44
Conclusion
In summary, MD is an emerging dermopathy that is associated with Borrelia infection, and the growing number of MD cases reflects the increase in tickborne diseases around the world. Although some medical practitioners erroneously consider MD to be caused by a delusional disorder, studies have shown that MD is a somatic illness that appears to be triggered by Borrelia infection. The optimal treatment for MD remains to be determined.
Acknowledgments
The authors are grateful to Cindy Casey from the Charles E. Holman Morgellons Disease Foundation, Austin, TX, USA, for providing updated information about MD. Funding for open access publication was provided by the Charles E. Holman Morgellons Disease Foundation, Austin, TX, USA.
Disclosure
The authors report no conflicts of interest in this work.
References
Kellett CE. Sir Thomas Browne and the disease called Morgellons. Ann Med Hist, n.s., VII. 1935;7:467–479. | ||
Savely G, Leitao MM. Skin lesions and crawling sensation: disease or delusion? Adv Nurse Pract. 2005;13(5):16–17. | ||
Savely VR, Leitao MM, Stricker RB. The mystery of Morgellons disease: infection or delusion? Am J Clin Dermatol. 2006;7(1):1–5. | ||
Savely VR, Stricker RB. Morgellons disease: the mystery unfolds. Expert Rev Dermatol. 2007;2(5):585–591. | ||
Savely VR, Stricker RB. Morgellons disease: analysis of a population with clinically confirmed microscopic subcutaneous fibers of unknown etiology. Clin Cosmet Investig Dermatol. 2010;3:67–78. | ||
Middelveen MJ, Stricker RB. Filament formation associated with spirochetal infection: a comparative approach to Morgellons disease. Clin Cosmet Investig Dermatol. 2011;4:167–177. | ||
Mayne PJ. Clinical determinants of Lyme borreliosis, babesiosis, bartonellosis, anaplasmosis, and ehrlichiosis in an Australian cohort. Int J Gen Med. 2015;8:15–26. | ||
Berry SL, Read DH, Famula TR, Mongini A, Dopfer D. Long-term observations on the dynamics of bovine digital dermatitis lesions on a California dairy after topical treatment with lincomycin HCl. Vet J. 2012;193(3):654–658. | ||
Gomez A, Cook NB, Bernardoni ND, et al. An experimental infection model to induce digital dermatitis infection in cattle. J Dairy Sci. 2012;95(4):1821–1830. | ||
Middelveen MJ, Rasmussen EH, Kahn DG, Stricker RB. Morgellons disease: a chemical and light microscopic study. J Clin Exp Dermatol Res. 2012;3:140. | ||
Middelveen MJ, Mayne PJ, Kahn DG, Stricker RB. Characterization and evolution of dermal filaments from patients with Morgellons disease. Clin Cosmet Investig Dermatol. 2013;6:1–21. | ||
Middelveen MJ, Burugu D, Poruri A, et al. Association of spirochetal infection with Morgellons disease. F1000Res. 2013;2:25. | ||
Middelveen MJ, Bandoski C, Burke J, et al. Exploring the association between Morgellons disease and Lyme disease: identification of Borrelia burgdorferi in Morgellons disease patients. BMC Dermatol. 2015;15:1. | ||
Galván I, Jorge A, Ito K, Tabuchi K, Solano F, Wakamatsu K. Raman spectroscopy as a non-invasive technique for the quantification of melanins in feathers and hairs. Pigment Cell Melanoma Res. 2013;26(6):917–923. | ||
Mayne P, English JS, Kilbane EJ, Burke JM, Middelveen MJ, Stricker RB. Morgellons: a novel dermatological perspective as the multisystem infective disease borreliosis. F1000Res. 2013;2:118. | ||
Shah JS. Morgellons disease – chronic form of Borrelia infection? Presented at: 9th Annual Medical-Scientific Conference on Morgellons Disease; April 30–May 1, 2016; Austin, TX. Available from: http://www.thecehf.org/jyotsna-s-shahphd.html. Accessed September 30, 2016. | ||
Allen L, Saylor-Hefley C. Morgellons under investigation: identification of associated microorganisms by molecular analysis of epithelial samples. Presented at: 7th Annual Medical-Scientific Conference on Morgellons Disease; March 29–30, 2014; Austin, TX. Available from: http://www.thecehf.org/resources/OSU%20_2015%20_Research.pdf. Accessed September 30, 2016. | ||
Mursic VP, Wanner G, Reinhardt S, Wilske B, Busch U, Marget W. Formation and cultivation of Borrelia burgdorferi spheroplast L-form variants. Infection. 1996;24(3):218–226. | ||
Brorson O, Brorson SH. An in vitro study of the susceptibility of mobile and cystic forms of Borrelia burgdorferi to tinidazole. Int Microbiol. 2004;7(2):139–142. | ||
Bandoski C. Evidence for the presence of human pathogens Borrelia and Helicobacter in Morgellons patients’ skin samples. Presented at: 7th Annual Medical-Scientific Conference on Morgellons Disease; March 29–30, 2014; Austin, TX. Available from: http://www.thecehf.org/cheryl-bandoski.html. Accessed September 30, 2016. | ||
Georgilis K, Peacock M, Klempner MS. Fibroblasts protect the Lyme disease spirochete, Borrelia burgdorferi, from ceftriaxone in vitro. J Infect Dis. 1992;166(2):440–444. | ||
Klempner MS, Noring R, Rogers RA. Invasion of human skin fibroblasts by the Lyme disease spirochete Borrelia burgdorferi. J Infect Dis. 1993;167(5):1074–1081. | ||
Chmielewski T, Tylewska-Wierzbanowska S. Interactions between Borrelia burgdorferi and mouse fibroblasts. Pol J Microbiol. 2010;59(3):157–160. | ||
Pearson ML, Selby JV, Katz KA, et al; Unexplained Dermopathy Study Team. Clinical, epidemiologic, histopathologic and molecular features of an unexplained dermopathy. PLoS One. 2012;7(1):e29908. | ||
CDC 2016. Lyme disease: diagnosis, treatment and testing. Available from: http://www.cdc.gov/lyme/healthcare/. Accessed September 30, 2016. | ||
Dattwyler RJ, Volkman DJ, Luft BJ, Halperin JJ, Thomas J, Golightly MG. Seronegative Lyme disease. N Engl J Med. 1988;319:1441–1446. | ||
Stricker RB, Johnson L. Serologic tests for Lyme disease: more smoke and mirrors. Clin Infect Dis. 2008; 47(8):1111–1112. | ||
Girard YA, Federova N, Lane RS. Genetic diversity of Borrelia burgdorferi and detection of B. bissettii-like DNA in serum of north coastal California residents. J Clin Microbiol. 2011;49(3):945–954. | ||
Ogden NH, Margos G, Aanensen DM, et al. Investigation of genotypes of Borrelia burgdorferi in Ixodes scapularis ticks collected during surveillance in Canada. Appl Environ Microbiol. 2011;77(10):3244–3254. | ||
Péter O, Bretz AG, Bee D. Occurrence of different genospecies of Borrelia burgdorferi sensu lato in Ixodid ticks of Valais, Switzerland. Eur J Epidemiol. 1995;11(4):463–467. | ||
Lange R, Seyyedi S. Evidence of a Lyme borreliosis infection from the viewpoint of laboratory medicine. Int J Med Microbiol. 2002;291(33):120–124. | ||
Schmidt BL. PCR in laboratory diagnosis of human Borrelia burgdorferi infections. Clin Microbiol Rev. 1997;10(1):185–201. | ||
Steere AC, Malawista SE, Snydman DR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three connecticut communities. Arthritis Rheum. 1977;20(1):7–17. | ||
Ekbom KA. Praeseniler Dermat-zooenwahn. Acta Psychiatr Scand. 1938;13:227–259. | ||
Keller A, Graefen A, Ball M, et al. New insights into the Tyrolean Iceman’s origin and phenotype as inferred by whole-genome sequencing. Nat Commun. 2012;3:698. | ||
Fallon BA, Schwartzberg M, Bransfield R, et al. Late-stage neuropsychiatric Lyme borreliosis. Differential diagnosis and treatment. Psychosomatics. 1995;36(3):295–300. | ||
Bransfield RC. The psychoimmunology of Lyme/tick-borne diseases and its association with neuropsychatric symptoms. Open Neurol J. 2012;6:88–93. | ||
Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29(8):357–365. | ||
Mattinley DW, Koda MM. Association between Lyme disease and schizoaffective disorder, bipolar type: is it inflammation mediated? Indian J Psychol Med. 2015;37(2):243–246. | ||
Kristensson K. Microbes’ roadmap to neurons. Nat Rev Neurosci. 2011;12(6):345–357. | ||
Fallon BA, Levi ES, Schweitzer PJ, Hardesty D. Inflammation and central nervous system Lyme disease. Neurobiol Dis. 2010;37(3):534–541. | ||
Harvey WT, Bransfield RC, Mercer DE, Wright AJ, Ricchi RM, Leitao MM. Morgellons disease, illuminating an undefined illness: a case series. J Med Case Rep. 2009;3:8243. | ||
Cameron DJ, Johnson LB, Maloney EL. Evidence assessments and guideline recommendations in Lyme disease: the clinical management of known tick bites, erythema migrans rashes and persistent disease. Expert Rev Anti Infect Ther. 2014;12(9):1103–1135. | ||
Horowitz RI, Freeman PR. The use of dapsone as a novel “persister” drug in the treatment of chronic Lyme disease/post treatment Lyme disease syndrome. J Clin Exp Dermatol Res. 2016;7(3):345. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.