Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Modified Non-Cultured Cell Spray Induced Epithelization in LAMB3 Mutation Epidermolysis Bullosa
Authors Widhiati S , Dewi ST , Yefta, Danarti R , Soebono H , Irmawati YE, Puspitasari M, Trisnowati N , Wibawa T , Purnomosari D , Wirohadidjojo YW
Received 27 June 2022
Accepted for publication 7 September 2022
Published 14 October 2022 Volume 2022:15 Pages 2197—2202
DOI https://doi.org/10.2147/CCID.S377753
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Suci Widhiati,1,2 Shinta Trilaksmi Dewi,3 Yefta,3 Retno Danarti,3 Hardyanto Soebono,3 Yulia Eka Irmawati,3 Monika Puspitasari,3 Niken Trisnowati,3 Tri Wibawa,4 Dewajani Purnomosari,5 Yohanes Widodo Wirohadidjojo3
1Department of Dermatology and Venereology, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia; 2Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia; 3Department of Dermatology and Venereology, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia; 4Department of Microbiology, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia; 5Department of Histology and Cell Biology, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia
Correspondence: Yohanes Widodo Wirohadidjojo, Department of Dermatology and Venereology, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Gedung Radiopoetro, lt. 3 Sekip, Yogyakarta, 55281, Indonesia, Tel +62 274 560700, Email [email protected]
Background: Autologous non-cultured cell (ANCC) spray has been used to treat burns, chronic wounds, and vitiligo, but its use in junctional epidermolysis bullosa (JEB) has not been published previously. Chronic wounds in JEB are caused by mutations of laminin 332 (L322), whose function is to attach and act as a glue in the basal membrane. It is proposed that ANCC applications can provide keratinocytes and fibroblasts required to improve epithelization and spontaneously correct revertant keratinocytes in the wound area.
Purpose: To develop a modified procedure of ANCC spray and improve epithelization using silver sulfadiazine covered with plastic wrap to treat chronic wounds of JEB.
Patients and Methods: Shave excision of the donor site was performed on a 19-year-old girl with JEB. The ANCC spray was prepared and applied to the chronic wound, which was then covered with silver sulfadiazine occluded with plastic wrap.
Results: Following the ANCC spray application, epithelization was successfully initiated. Unfortunately, the wounds recurred after four months of follow-up.
Conclusion: The modified application method of ANCC spray provides a good alternative to treat chronic wounds in JEB.
Keywords: revertant mosaicism, silver-sulfadiazine, plastic wrap, chronic wound, junctional epidermolysis bullosa
Introduction
Junctional epidermolysis bullosa (JEB) is a severe hereditary bullous disease caused by reduced dermal-epidermal adhesion due to mutations in LAMB3, LAMA3, LAMC2, or COL17A1.1 According to reported cases, a mutation in LAMB3 occurred in about 80% of patients with generalized severe JEB.2–5 The clinical manifestations of JEB are usually severe, with mucocutaneous blistering at birth and early mortality. Blistering may be severe, and exuberant granulation tissue can form on the skin around the oral and nasal cavities, fingers and toes, and internally around the upper airway,1 leading to chronic wounds.6 Chronic wounds in JEB remain a major concern to patients and caregivers since they can cause anxiety, fear, loss of power or control, and poor body image.
Until recently, the treatment of EB was focused on maintaining the patient’s condition by providing symptomatic treatment to reduce itch or pain and optimizing wound care.7 However, therapies with curative intentions are currently being developed, such as gene therapy, cell therapy, protein therapy, and antisense oligonucleotides, which appear promising to cure EB.8
Cell-based therapy for EB has been established in multiple early-phase clinical trials, which involve keratinocytes, fibroblasts, bone marrow transplantation (BMT), mesenchymal stem/ stromal cells (MSCs), and induced pluripotent stem cells (iPSCs).7 Intradermal injection of allogeneic fibroblasts to chronic wounds of patients with recessive dystrophic epidermolysis bullosa (RDEB) can promote wound healing by as much as 30–80%. Unfortunately, the results have been varied and not consistently superior to placebo injections. Also, the injection of fibroblasts into wounded skin can be very painful, which limits its clinical application.9,10 Based on these findings, we aimed to modify our cell-therapy,11 using a non-cultured cell suspension spray applied to JEB chronic wounds to improve the adherence of keratinocytes. In addition, the occlusion of 10% silver sulfadiazine using plastic wrap was applied afterwards to induce epithelization.
Case
A 19-year-old girl presented to the clinic with chronic wounds on her philtrum, perinasal, and auricular regions for over two years. During this time, the wounds would decrease in size but never heal. Six months before admission, the wounds on her philtrum became bigger and tended to bleed easily. Since childhood, she had blisters and wounds on her soles, which gradually healed. However, they developed in other parts of her body, including her philtrum. She was diagnosed with generalized intermediate JEB and was confirmed to have a novel homozygote mutation of LAMB3 exon 962 A>C (p.H321P), as reported in our previous work.12
Materials and Methods
Donor Site
Written informed consent was obtained before the procedure was performed. Additionally, the patient also gave written consent to participate in this case report and to publish the images of her disease and the case details. Institutional approval was not required. After disinfection of the donor area on the left thigh, anesthesia was performed with tumescent infiltration. A simple shave excision of a 7×3 cm2 skin area was performed using a razor blade. The donor site was sutured afterward, and the sutures were removed on day 10.
Autologous Non-Cultured Cell Spray Preparation (ANCC)
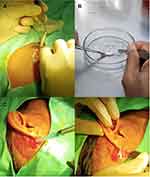
The skin tissue was immediately transferred to 4 mL of trypsin enzyme and incubated for one hour at 37° Celsius in order to release the skin cells from the extracellular matrix (ECM). The skin tissue was then rinsed with buffered solution to stop the trypsin digestion. The cells were mechanically removed by scraping them from the skin tissue with a scalpel. Eight mL of Dulbecco’s Modified Eagle Medium (DMEM) was added to the cell suspension as the nutrient medium, and the suspension was transferred to a sterile tube and centrifuged for 10 minutes at 2000 rpm. The supernatant was discarded to obtain the cell pellets, which were then resuspended in sterile phosphate-buffered saline (PBS), ready to be sprayed onto the recipient areas (Figure 1).
Recipient Sites
The recipient sites at the philtrum and auricular areas were disinfected with 1% povidone-iodine and rinsed with normal saline. Using a micropipette of 1000μm, the cell suspension was sprayed over the wounds and then covered with plastic wrap. The plastic wrap acted as an occlusion dressing from which a few small, punched holes were made earlier to improve aeration. Finally, the grafts were covered with sterile cotton gauges. After the procedure, the patient was advised to keep the dressing dry and to minimize manipulation. A broad-spectrum oral antibiotic treatment with 200mg cefixime was initiated for 5 days to prevent infection of the donor and grafted areas. Seven days after the procedure, during the follow-up visit, the dressing was substituted with 10% silver-sulfadiazine cream covered with the punched plastic wrap as the primary dressing and sterile cotton gauze as the secondary dressing. Improvement of the wounds was observed weekly.
Results
Follow-up was performed over 16 weeks. We utilized photographs to compare the weekly progress. The graft area developed epithelization after 3 weeks, and good improvement was evident after 4 weeks. Unfortunately, the graft area became eroded at week 5 due to the common cold experienced by the patient. The graft on her auricular regions showed good response after four weeks (Figure 2). After eight weeks from grafting, the responses were flattening and constant, although in week 16 there were still some areas of small erosion and hyper-granulation tissue in the philtrum and auricular region. The procedure helped to heal 70% of the wound area.
Discussion
In this case, chronic wounds on the facial region hallmark the presence of junctional EB (JEB). The mutation of LAMB3 causes detachment between dermis and epidermis, thus, giving exuberant granulation tissue leading to chronic wounds and prolonged inflammation.6 Treatments for the chronic wounds of EB have demonstrated varied results. All of the mutated proteins in the different types of EB are synthesized in keratinocytes except for collagen VII (C7); thus, autologous keratinocyte sheets from severe JEB were used to provide temporary improvement for wounds.13 Autografts for JEB were first introduced in 1987. The source of the graft was taken using a suction blister technique or punch biopsy, and the graft was then applied to the chronic denuded areas, from which 70% of cases completely healed and only 13% reoccurred after three months post-transplant.14 The use of autologous non-cultured cell (ANCC) suspension spray for JEB has never been published before. However, this procedure has been widely used for treating burns, chronic wounds, and even vitiligo.11,15,16
There is no standard for ANCC procedure; however, other studies use one or two ways to separate the cell from keratinocytes.17 The modification technique in our procedures involves the use of plastic wrap and 10% silver-sulfadiazine as the primary dressing.
Chronic wounds in our patient occurred as a result of a prolonged inflammation phase and impaired wound closure. In tissue formation, during the second phase of wound healing, the new tissue is formed as the leading-edge keratinocytes along the periphery of the wound acquire epithelial-to-mesenchymal transition (EMT) characteristics to become migratory. This EMT process initiates proliferation and differentiation of adjacent keratinocytes, generating the cell mass necessary to seal the wound. These migratory keratinocytes deposit a scaffold of laminin 332 (LM332) and fibronectin to aid in wound closure. At the same time, the underlying dermal fibroblasts support these keratinocytes through the formation and remodeling of the ECM.14 In our patient, the mutation in LM332 is possibly the underlying cause of the non-healing wounds. The utilization of donor autologous keratinocytes and fibroblasts in the cell spray could potentially substitute for the loss of LM332, enhancing the migration of keratinocytes, and inducing epithelization.
Successful ANCC suspension relies on the availability of unaffected skin in JEB patients. The phenomenon of revertant mosaicism in which the underlying cause of a genetic disease is corrected by somatic mutational events, usually occurs during embryonic development. In our case, blisters never occurred on the left thigh, and after the procedure, the suture on this site recovered completely. Therefore, we considered the thigh to have revertant mosaicism and chose this area as the donor site. This is in line with Gostynski et al who reported effective results in one LM332-deficient patient treated with autologous transplants of punch biopsies from a revertant skin patch to multiple chronic ulcers.18 Grafted skin sites did not form blisters until 18 months post-transplant, and sequencing confirmed that the regenerated epidermis areas were comprised of revertant keratinocytes.18 Even though sequencing from the donor area was not performed in our case, we propose that this mechanism may underlie the efficacy of our ANCC spray application.
Modern wound dressing optimizes the time of wound healing and reduces the scar formation. However, the use of modern dressing in resource-constrained countries is restricted because of economic reasons. Alternative wound dressings such as using plastic wrap, banana leaves or cigarette rolling papers, have been introduced in these countries.19 Plastic wrap is typically used for sealing food items and keeping them fresh over an extended period of time. In medical situations, plastic wrap has been alternatively used for wrapping premature babies after birth to prevent low temperature,20 as well as a first aid dressing for burns. There was no significant growth of bacteria when plastic wrap was used for acute burn dressing.21 We developed the use of plastic wrap dressing as a modification to modern dressing for the chronic wounds of JEB. The dressing adhered to the wound and promoted the moist microenvironment needed for wound healing. During follow-up, 10% silver sulfadiazine cream was also applied before plastic wrap occlusion. This antibiotic application may help resolve the inflammation by decreasing bacterial contamination, thereby hastening epithelization by inhibiting matrix metalloproteinases, which might delay wound contraction.22
During follow-up, the ANCC application procedure helped to promote 70% wound closure in the philtrum and auricular areas. However, the wounds still tended to break open easily with minimal trauma. Despite this drawback, the procedure provides a new modified technique for limited-resources countries to enhance the process of wound closure. Further studies with well-designed methods need to be conducted in the future.
Conclusions
Autologous non-cultured cell spray in combination with silver-sulfadiazine on a plastic wrap dressing showed 70% improvement for healing chronic wounds in junctional epidermolysis bullosa with LAMB3 mutations.
Acknowledgments
Thank you for the staff at Pusat Bahasa FKKMK UGM for the English editing and special thanks to the nurses in the outpatient clinic for assisting with wound management.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pfendner EG, Lucky AW. Junctional epidermolysis bullosa. In: Adam MP, Ardinger HH, Pagon RA, editors. Genereviews((R)). Seattle (WA); 1993.
2. Riahi K, Ghanbari MF, Talebi F, Jasemi F, Mohammadi AJ. Digenic mutations in junctional epidermolysis bullosa in an Iranian family. Cell J. 2021;23(5):598–602. doi:10.22074/cellj.2021.7208
3. Khan FF, Khan N, Rehman S, et al. Identification and computational analysis of novel pathogenic variants in Pakistani families with diverse epidermolysis bullosa phenotypes. Biomolecules. 2021;11(5):620. doi:10.3390/biom11050620
4. Wang H, Yang Y, Zhou J, et al. Targeted next-generation sequencing identifies a novel mutation of LAMB3 in a Chinese neonatal patient presented with junctional epidermolysis bullosa. Medicine. 2018;97(49):e13225. doi:10.1097/MD.0000000000013225
5. Yenamandra VK, Vellarikkal SK, Kumar M, et al. Application of whole exome sequencing in elucidating the phenotype and genotype spectrum of junctional epidermolysis bullosa: a preliminary experience of a tertiary care centre in India. J Dermatol Sci. 2017;86(1):30–36. doi:10.1016/j.jdermsci.2016.12.020
6. El Hachem M, Fortugno P, Palmeri A, et al. Structural defects of laminin beta3 N-terminus underlie junctional epidermolysis bullosa with altered granulation tissue response. Acta Derm Venereol. 2016;96(7):954–958. doi:10.2340/00015555-2439
7. Hou PC, Wang HT, Abhee S, Tu WT, McGrath JA, Hsu CK. Investigational treatments for epidermolysis bullosa. Am J Clin Dermatol. 2021;22(6):801–817. doi:10.1007/s40257-021-00626-3
8. Bruckner-Tuderman L. Newer treatment modalities in epidermolysis bullosa. Indian Dermatol Online J. 2019;10(3):244–250. doi:10.4103/idoj.IDOJ_287_18
9. Moravvej H, Abdollahimajd F, Naseh MH, et al. Cultured allogeneic fibroblast injection vs. fibroblasts cultured on amniotic membrane scaffold for dystrophic epidermolysis bullosa treatment. Br J Dermatol. 2018;179(1):72–79. doi:10.1111/bjd.16338
10. Wong T, Gammon L, Liu L, et al. Potential of fibroblast cell therapy for recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 2008;128(9):2179–2189. doi:10.1038/jid.2008.78
11. Nareswari A, Zulfikar D, Julianto I, Widhiati S. Autologous non-cultured epidermal cell suspension combined with platelet rich fibrin for the treatment of stable vitiligo: a case series. Dermatol Rep. 2019;11(s1):21. doi:10.4081/dr.2019.8016
12. Widhiati S, Danarti R, Trisnowati N, Purnomosari D, Wibawa T, Soebono H. Novel mutations of epidermolysis bullosa identified using whole-exome sequencing in Indonesian Javanese patients. Intractable Rare Dis Res. 2021;10(2):88–94. doi:10.5582/irdr.2020.03150
13. Carter DM, Lin AN, Varghese MC, Caldwell D, Pratt LA, Eisinger M. Treatment of junctional epidermolysis bullosa with epidermal autografts. J Am Acad Dermatol. 1987;17(2 Pt 1):246–250. doi:10.1016/S0190-9622(87)70199-6
14. Keith AR, Twaroski K, Ebens CL, Tolar J. Leading edge: emerging drug, cell, and gene therapies for junctional epidermolysis bullosa. Expert Opin Biol Ther. 2020;20(8):911–923. doi:10.1080/14712598.2020.1740678
15. Rashid ST, Cavale N, Bowling FL. A pilot feasibility study of non-cultured autologous skin cell suspension for healing diabetic foot ulcers. Wound Repair Regen. 2020;28(6):719–727. doi:10.1111/wrr.12844
16. Barnwal S, Kant R, Yadav P. Autologous non-cultured keratinocyte cell suspension in non-healing diabetic ulcers: a preliminary study. J Family Med Prim Care. 2020;9(9):4686–4691. doi:10.4103/jfmpc.jfmpc_627_20
17. Hu X, Yu W, Sun H, Wang X, Han C. Epidermal cells delivered for cutaneous wound healing. J Dermatolog Treat. 2012;23(3):224–237. doi:10.3109/09546634.2010.495741
18. Gostynski A, Pasmooij AM, Jonkman MF. Successful therapeutic transplantation of revertant skin in epidermolysis bullosa. J Am Acad Dermatol. 2014;70(1):98–101. doi:10.1016/j.jaad.2013.08.052
19. Debra international. Healthy Body and Skin: Epidermolysis Bullosa Infographics; 2019. Available from: https://debraitalia.com/wp-content/uploads/2021/01/5B-Cura-Pelle-E-Ferite-Corpo-E-Pelle-Sano.pdf. Accessed October 6, 2022.
20. McCall EM, Alderdice F, Halliday HL, Jenkins JG, Vohra S. Interventions to prevent hypothermia at birth in preterm and/or low birthweight infants. Cochrane Database Syst Rev. 2010;2010(3):CD004210.
21. Liao AY, Andresen D, Martin HC, Harvey JG, Holland AJ. The infection risk of plastic wrap as an acute burns dressing. Burns. 2014;40(3):443–445. doi:10.1016/j.burns.2013.08.006
22. Arslan K, Karahan O, Okus A, et al. Comparison of topical zinc oxide and silver sulfadiazine in burn wounds: an experimental study. Ulus Travma Acil Cerrahi Derg. 2012;18(5):376–383. doi:10.5505/tjtes.2012.45381
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.