Back to Journals » ClinicoEconomics and Outcomes Research » Volume 7
Modeling the budget impact of long-acting injectable paliperidone palmitate in the treatment of schizophrenia in Japan
Authors Mahlich J , Nishi M, Saito Y
Received 26 March 2015
Accepted for publication 22 April 2015
Published 22 May 2015 Volume 2015:7 Pages 267—272
DOI https://doi.org/10.2147/CEOR.S85514
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio L Colombo
Jörg Mahlich,1,2 Masamichi Nishi,3 Yoshimichi Saito1
1Health Economics, Janssen Pharmaceutical KK, Tokyo, Japan; 2Düsseldorf Institute for Competition Economics, University of Düsseldorf, Düsseldorf, Germany; 3Pricing, Janssen Pharmaceutical KK, Tokyo, Japan
Background: The cost of schizophrenia in Japan is high and new long-acting injectable (LAI) antipsychotics might be able to reduce costs by causing a reduction of hospital stays. We aim to estimate budget effects of the introduction of a new 1-month LAI, paliperidone palmitate, in Japan.
Methods: A budget impact analysis was conducted from a payer perspective. The model took direct costs of illness into account (ie, costs for inpatient and outpatient services, as well as drug costs). The robustness of the model was checked using a sensitivity analysis.
Results: According to our calculations, direct total costs of schizophrenia reach 710,500 million yen a year (US$6 billion). These costs decrease to 691,000 million yen (US$5.9 billion) 3 years after the introduction of paliperidone palmitate.
Conclusion: From a payer point of view, the introduction of a new treatment for schizophrenia in Japan helps to save resources and is not associated with a higher financial burden.
Keywords: budget impact, schizophrenia, long-acting injectables, paliperidone, Japan
Introduction
Schizophrenia is a mental illness that causes considerable costs to society.1 A recent Japanese study estimates the annual burden of disease to exceed 3.5 million yen per patient (approximately US$30,000).2 The high costs are mainly due to the early age of onset of schizophrenia as symptoms typically begin already in young adulthood.3 Many patients are affected during their entire lifetime with negative effects on their working ability and limited opportunities with regard to the labor market. Relapses and subsequent hospitalizations have been identified as another significant cost driver.4–6 Hospitalizations in turn are caused by a poor adherence to antipsychotic medication.7 In this context, it has been discussed whether the treatment with long-acting injectables (LAIs) can be a cost-effective strategy to reduce overall health care costs.8 LAIs are intramuscularly injected every 2 weeks or 4 weeks and are usually more expensive than oral antipsychotics. On the other hand, patient adherence can be improved and, eventually, the number of relapses that lead to hospitalizations can be reduced.9–16 Accordingly, several cost-effectiveness studies demonstrated economic superiority of LAIs over oral antipsychotics.17–24
In Japan, the long-acting atypical antipsychotic paliperidone long-acting injectable (PLAI) (Xeplion®), a once-monthly LAI antipsychotic, was approved for treatment of schizophrenia and was launched in November 2013. Paliperidone is the primary active metabolite of risperidone, which has been available since the early 1990s as an oral second-generation antipsychotic and, later, in an LAI formulation (Risperdal Consta®). To our knowledge, there have been no health economic studies conducted on the use of LAIs in Japan. We therefore aim to estimate the budget impact of PLAI in the Japanese health care delivery system.
Methods
We use a budget impact model that has been published for Austria25 and populate it with Japanese data. The model takes a 3-year horizon and is built from a health insurance perspective in that it only includes direct medical costs and abstracts from indirect costs. In schizophrenia, direct costs constitute only a small fraction of the societal burden of schizophrenia as most of the total costs are indirect productivity costs that are due to unemployment and early retirement. Included direct costs are acquisition costs of medication and hospitalization costs. Costs for outpatient treatment are included but are not related to the medication in our model. Therefore, the major drivers of the budget impact are 1) number of treated patients, 2) drug prices and respective market shares, 3) drug-specific relapse probabilities, and 4) costs and utilization of hospitals. We chose 2013 as the baseline year (t=0) that served as a benchmark for our projected economic costs and benefits.
Number of patients
The number of Japanese patients with a schizophrenia diagnosis is available from publicly available sources published by the Ministry of Health, Labour and Welfare. According to these estimates, the number of patients is 713,000 or 0.56% of the total Japanese population (Table 1).
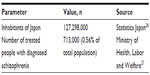  | Table 1 Treated patients with schizophrenia |
Drug prices and market shares
Table 2 provides an overview of the antipsychotic medication that is available in Japan and considered in our model. The prices were taken from the official reimbursement price list.28,29 In the case of generic drugs, we calculated the weighted average price. Additionally, we report the lowest price of any available generic in Table 2 for information only. Due to the large number of conventionals in the Japanese market, we report only the price of haloperidol, which is taken as an estimate for all conventionals.
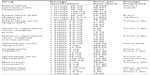  | Table 2 Prices of antipsychotics in the Japanese market (as of April 2014) |
With regard to the prescribed dosages, we assumed that patients are treated with the dosage as specified on the label. In cases where the dosage is not specified and only a range is recommended on the label, we took the mean value. For paliperidone palmitate, we were able to draw on market research data that provided us with the respective maintenance dosage distribution (IMS, unpublished data, 2014) (Table 3).
  | Table 3 Dosage distribution of paliperidone palmitate |
An important building block of a budget impact model is the assumptions on future market shares of the drugs. Market shares (in terms of treatment days) were calculated by combining sales figures and dosage information according to the Japanese label. Future market shares and future prices are based on unpublished pharmaceutical market research data. Table 4 reveals significant expected price decreases for drugs that face generic competition. Quetiapine, for instance, is expected to experience a price decline of >26% within 3 years. This is in stark contrast to other markets, such as the US, where older branded drugs even increase their prices after generic entry. Economists refer to this observation as the “generic paradox.”30 While the use of generics in Japan is still low in comparison with that in other countries, it has increased substantially during the past years and is expected to rise further.31,32 Among the nonconventional generic drugs, the market share of generics is expected to grow from around 15% to 34% within 3 years. Note that paliperidone palmitate had been on the market in 2013 for 1 month; therefore, its market share is slightly >0 at t=0.
  | Table 4 Future market shares and prices (based on defined daily dose) |
It can also be seen from the table that conventional antipsychotics are expected to lose market share primarily because of/on account of Quetiapine and blonanserin. The LAIs have a combined market share of roughly 1%. Probably, LAIs continue to have an image problem among patients and mental health staff despite their clinical and economic benefits. Thus, it is felt that they are primarily used as a “last resort” for the most stigmatized individuals, as argued by Waddell and Taylor.33 Another factor that has caused a low prescription rate of LAIs is a sufficiently good estimated compliance with the oral formulation.34 A recent analysis of Japanese claims data, however, came to the conclusion that only 25% of Japanese schizophrenia patients are adherent to their medication, while the vast majority are either under- or overadherent.35
Probabilities of hospitalizations and associated costs
As noted in the Introduction section, one major cost driver of schizophrenia is the direct costs of schizophrenia-caused hospitalizations. The model uses annual medication-specific probabilities for hospitalization. For risperidone, olanzapine, aripiprazole, and conventionals (haloperidol), we used the annualized relapse probabilities published by the UK National Institute for Health and Care Excellence, which were based on a systematic review.36 Drawing on a meta-analysis by Leucht et al,37 we assumed the rate for quetiapine to be equal to that of haloperidol. The rate for risperidone LAI (RLAI) was taken from the risperidone long-acting injectable versus quetiapine relapse prevention trial (ConstaTRE) clinical trial, which compared RLAI with quetiapine.14 For PLAI, we assumed the same rate as for RLAI. This assumption is backed by a recent long-term randomized clinical trial of PLAI, where the relapse rate of PLAI monotherapy was 11.5%.38 The Austrian model included olanzapine LAI and ziprasidone (Zeldox®); however, those two drugs are not available on the Japanese market. Instead, blonanserin (Lonasen®), marketed by Dainippon Sumitomo Pharma, is a local drug only available in Japan and Korea. According to a recent meta-analysis by Kishi et al39 and a review by Tenjin et al,40 blonanserin has a comparable profile as risperidone oral. For this reason, we took the same hospitalization rate as for risperidone. Table 5 summarizes the rehospitalization rates that were used in the model.
  | Table 5 Rehospitalization rates per annum |
The associated costs of schizophrenia-related hospitalizations were taken from the study by Sado et al.2 Their estimate of the total inpatient costs of schizophrenia is 602,771 million yen for 2008. Adjusting this value for price inflation using the official Japanese consumer price index41 and dividing it by the number of schizophrenia-related hospital admissions of 187,400,27 we estimate the costs of hospitalization to be 3.15 million yen per case (around US$26,700).
We also include costs for outpatient services, although this cost component is not related to the choice of medication in our model. The cost is based on the study of Sado et al,42 wherein they estimate outpatient costs (without drug costs) to sum up to 80,525 million yen in the year 2008. Again, all values were adjusted using the Japanese consumer price index. There was no need to discount future costs as the 3-year interest rate in Japan is 0% according to the Japanese Ministry of Finance.43
Results and discussion
The direct medical costs of schizophrenia before and after the launch of PLAI in Japan are reported in Table 6. Costs are categorized into drug and nondrug costs.
  | Table 6 Costs per annum of schizophrenia with and without PLAI (million yen) |
Drug costs are expected to increase in the short run; however, due to the downward price spiral and an increase of generic drug use, the long-term drug costs are projected to fall below the baseline level. When it comes to nondrug costs, we also expect a decline in this cost component due to the shift toward more effective medical treatment opportunities that prevent relapses and hospital stays. If average values are calculated over the 3 years’ time horizon, our model estimates a decrease of both drug costs and nondrug costs, which results in an overall annual cost reduction of 8,000 million yen (US$68 million). Even in the first year, cost offsets in terms of lower hospitalization rates are higher than the increase in drug costs. We therefore estimate a positive budget impact for each of the 3 years of our observation period.
Sensitivity analysis
Due to a degree of uncertainty with regard to the cost per hospitalization, we ran a deterministic sensitivity analysis for this parameter. In addition to the baseline case of 3.15 million yen for the per capita cost of hospitalization, we assumed hospitalization costs to be 2.15 million yen and 4.15 million yen respectively. The results of this sensitivity analysis are reported in Table 7. Even with lower cost of hospitalization, the results remain robust in the sense that cost offsets prevail, which lead to an overall reduction of annual direct medical costs in the range of 6,200 million yen to 9,800 million yen.
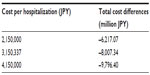  | Table 7 Deterministic sensitivity analysis of cost per hospitalization |
The 3.15 million yen per case (around US$26,700) that were used for the baseline case are considerably lower than estimates for the US (US$38,100)44 or England (£25,000 or US$37,500).45 However, this amount is notably higher than the €7,182 (US$8,100) that was used in the Austrian model. This difference can be attributed to the differences in the length of stay in comparison to other countries.46 While it is only 16 days in Austria, the majority of Japanese patients are hospitalized for >1 year.47 A recent investigation of >8,000 Japanese schizophrenia patients even reported an average length of stay of 3,242 days,48 although it has been argued that 40% of the hospitalized patients could easily be discharged sooner.49 Despite the long hospital stays, the cost of hospitalization is low in Japan. To validate this assertion, we calculated the daily cost of schizophrenia-related hospital stays based on a claims database provided by Japan Medical Data Center Co, Ltd (JMDC). The JMDC database is an employer-based database of health insurance claims with approximately 2.5 million beneficiaries. On the basis of this analysis, mean daily costs were only 6,312 yen (US$54) per inpatient. This compares, eg, to €220 (US$250) in Germany.18 In our view, Japanese health policy makers should address the issue of long hospital stays in combination with low daily-reimbursed costs.
Conclusion
According to our estimates, the market introduction of PLAI is associated with overall annual savings for the Japanese health care system of >8,000 million yen (US$68 million) in the baseline case. This result is robust to variations in the hospitalization costs. In our view, this estimate is rather conservative as we have not modeled any effects on the utilization of outpatient services, although there might be some additional cost savings in this sector as well.50 We also abstracted some intangible benefits of LAIs. In a recent publication for instance, patients reported their preference for an injectable because of increased convenience when compared with oral antipsychotics.51 As direct costs of schizophrenia constitute only 28% of the total costs for society in Japan,2 the overall effect on the Japanese economy at large would be substantially higher.
Disclosure
JM, MN, and YS are employed at Janssen KK. The authors report no other conflicts of interest in this work.
References
Montgomery W, Liu L, Stensland MD, Xue HB, Treuer T, Ascher-Svanum H. The personal, societal, and economic burden of schizophrenia in the People’s Republic of China: implications for antipsychotic therapy. Clinicoecon Outcomes Res. 2013;14(5):407–418. | |
Sado M, Inagaki A, Koreki A, et al. The cost of schizophrenia in Japan. Neuropsychiatr Dis Treat. 2013;9:787–798. | |
van Os J, Kapur S. Schizophrenia. Lancet. 2009;374(9690):635–645. | |
Lin I, Muser E, Munsell M, Benson C, Menzin J. Economic impact of psychiatric relapse and recidivism among adults with schizophrenia recently released from incarceration: a Markov model analysis. J Med Econ. 2014;26:1–11. | |
Fitch K, Iwasaki K, Villa K. Resource utilization and cost in a commercially insured population with schizophrenia. Am Health Drug Benefits. 2014;7(1):18–26. | |
Frey S. The economic burden of schizophrenia in Germany: a population-based retrospective cohort study using genetic matching. Eur Psychiatry. 2014;29(8):479–489. | |
Higashi K, Medic G, Littlewood KJ, Diez T, Granström O, De Hert M. Medication adherence in schizophrenia: factors influencing adherence and consequences of nonadherence, a systematic literature review. Ther Adv Psychopharmacol. 2013;3(4):200–218. | |
Peng X, Ascher-Svanum H, Faries D, Conley R, Schuh K. Decline in hospitalization risk and health care cost after initiation of depot antipsychotics in the treatment of schizophrenia. Clinicoecon Outcomes Res. 2011;3:9–14. | |
Acosta FJ, Bosch E, Sarmiento G, Juanes N, Caballero-Hidalgo A, Mayans T. Evaluation of noncompliance in schizophrenia patients using electronic monitoring (MEMS) and its relationship to sociodemographic, clinical and psychopathological variables. Schizophr Res. 2009;107(2):213–217. | |
Willis M, Svensson M, Löthgren M, Eriksson B, Berntsson A, Persson U. The impact of schizophrenia-related hospital utilization and cost of switching to long-acting risperidone injections in Sweden. Eur J Health Econ. 2010;11(6):585–594. | |
Tiihonen J, Haukka J, Taylor M, Haddad PM, Patel MX, Korhonen P. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168(6):603–609. | |
Grimaldi-Bensouda L, Rouillon F, Astruc B, et al; CGS Study Group. Does longacting injectable risperidone make a difference to the real-life treatment of schizophrenia? Results of the cohort for the general study of schizophrenia (CGS). Schizophr Res. 2012;134(2–3):187–194. | |
Leucht C, Heres S, Kane JM, Kissling W, Davis JM, Leucht S. Oral versus depot antipsychotic drugs for schizophrenia – a critical systematic review and meta-analysis of randomised long-term trials. Schizophr Res. 2011;127(1–3):83–92. | |
Gaebel W, Schreiner A, Bergmans P, et al. Relapse prevention in schizophrenia and schizoaffective disorder with risperidone long-acting injectable vs quetiapine: results of a long-term, open-label, randomized clinical trial. Neuropsychopharmacology. 2010;35:2367–2377. | |
Spill B, Konoppa S, Kissling W, Maino K, Messer T, Heres S. Long-term observation of patients successfully switched to risperidone long-acting injectable: a retrospective, naturalistic 18-month mirror-image study of hospitalization rates and therapy costs. Int J Psychiatry Clin Pract. 2010;14:53–62. | |
Laux G, Heeg B, van Hout B, Mehnert A. Costs and effects of long-acting risperidone compared with oral atypical and conventional depot formulations in Germany. Pharmacoeconomics. 2005;23(Suppl 1):49–61. | |
Olivares JM, Rodriguez-Morales A, Diels J; e-STAR Spanish Study Group, et al. Long-term outcomes in patients with schizophrenia treated with risperidone long-acting injection or oral antipsychotics in Spain: results from the electronic Schizophrenia Treatment Adherence Registry (e-STAR). Eur Psychiatry. 2009;24(5):287–296. | |
Zeidler J, Mahlich J, Greiner W, Heres S. Cost-effectiveness of paliperidone palmitate for the treatment of schizophrenia in Germany. Appl Health Econ Health Policy. 2013;11(5):509–521. | |
Mehnert A, Nicholl D, Pudas H, Martin M, McGuire A. Cost-effectiveness of paliperidone palmitate versus risperidone long-acting injectable and olanzapine pamoate for the treatment of patients with schizophrenia in Sweden. J Med Econ. 2012;15(5):844–861. | |
Olivares JM, Rodriguez-Martinez A, Burón JA, Alonso-Escolano D, Rodriguez-Morales A; e-STAR Study Group. Cost-effectiveness analysis of switching antipsychotic medication to longacting injectable risperidone in patients with schizophrenia: a 12- and 24-month follow-up from the e-STAR database in Spain. Appl Health Econ Health Policy. 2008;6(1):41–53. | |
Einarson TR, Vicente C, Zilbershtein R, et al. Pharmacoeconomic analysis of paliperidone palmitate versus olanzapine pamoate for chronic schizophrenia in Norway. Acta Neuropsychiatr. 2013;25(2):85–94. | |
Einarson TR, Geitona M, Chaidemenos A, et al. Pharmacoeconomic analysis of paliperidone palmitate for treating schizophrenia in Greece. Ann Gen Psychiatry. 2012;11:18. | |
Kim B, Lee TJ, Woo JM, et al. Cost-utility analysis of paliperidone palmitate long acting injection (PLAI) vs oral at ypical antipsychotics in non-adherent schizophrenia patients. Korean J Psychopharmacol. 2012;23:17–27. | |
Joshi K, Lin J, Lingohr-Smith M, Fu DJ. Estimated medical cost reductions for paliperidone palmitate versus placebo in a randomized, double-blind relapse-prevention trial of patients with schizoaffective disorder. J Med Econ. 2015;23:1–22. | |
Ransmeyr S, Mehnert A, Mahlich J. Budget Impact Analyse von Paliperidon Palmitat im österreichischen Versorgungskontext [Budget impact analysis of paliperdidone palmitate in the Austrian health care system]. PharmacoEconomics German Research Articles. 2013;11:25–32. | |
Statistics Japan. Current Population Estimates. Tokyo; 2013. Available from: http://www.stat.go.jp/english/data/jinsui/2013np/index.htm. Accessed December 15, 2014. | |
Ministry of Health, Labor and Welfare. Patient Survey (Kanja Chosa). Tokyo; 2011. Available from: http://www.mhlw.go.jp/toukei/saikin/hw/kanja/11/index.html. Accessed December 15, 2014. | |
Hokenyakujiten Plus+. The Edition of April 2013. Tokyo: Jiho; 2013. | |
Hokenyakujiten Plus+. The Edition of April 2014. Tokyo: Jiho; 2014. | |
Grabowski H, Vernon J. Longer patents for increased generic competition: the Waxman-Hatch act after on e decade. Pharmacoeconomics. 1996;10:110–123. | |
Jakovljevic MB, Nakazono S, Ogura S. Contemporary generic market in Japan – key conditions to successful evolution. Expert Rev Pharmacoecon Outcomes Res. 2014;14(2):181–194. | |
Iizuka T, Kubo K. The generic drug market in Japan: will it finally take off? Health Econ Policy Law. 2011;6(3):369–389. | |
Waddell L, Taylor M. Attitudes of patients and mental health staff to antipsychotic long-acting injections: systematic review. Br J Psychiatry. 2009;195:s43–s50. | |
Samalin L, Charpeaud T, Blanc O, Heres S, Llorca PM. Clinicians’ attitudes toward the use of long-acting injectable antipsychotics. J Nerv Ment Dis. 2013;201(7):553–559. | |
Kuwabara H, Saito Y, Mahlich J. Adherence and re-hospitalizations in patients with schizophrenia: evidence from Japanese claims data. Neuropsychiatr Dis Treat. 2015;11:935–940. | |
National Collaborating Centre for Mental Health. Schizophrenia. The NICE Guideline on Core Interventions in the Treatment and Management of Schizophrenia in Adults in Primary and Secondary Care. London: The British Psychological Society and The Royal College of Psychiatrists; 2010. | |
Leucht S, Corves C, Arbter D, Engel R, Li C, Davis J. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373:31–41. | |
Fu DJ, Turkoz I, Simonson BR, et al. Paliperidone palmitate once-monthly reduces risk of relapse of psychotic, depressive, and manic symptoms and maintains functioning in a double-blind, randomized study of schizoaffective disorder. J Clin Psychiatry. 2015;76(3):253–262. | |
Kishi T, Matsuda Y, Nakamura H, Iwata N. Blonanserin for schizophrenia: systematic review and meta-analysis of double-blind, randomized, controlled trials. J Psychiatr Res. 2013;47:149e154. | |
Tenjin T, Miyamoto S, Ninomiya Y, et al. Profile of blonanserin for the treatment of schizophrenia. Neuropsychiatr Dis Treat. 2013;9:587–594. | |
Statistics Bureau, Ministry of Internal affairs and Communications. Shouhishabukkashisu (Consumer Price Index). Tokyo; 2014. Available from: http://www.stat.go.jp/data/cpi/index.htm. Accessed December 15, 2014. | |
Sado M, Inagaki A, Koreki A. Seishin shikkan no syakaiteki cost(kosuto) no suikei. Estimation of Social Costs of Mental Diseases. Tokyo: Keio University; 2011. Available from: http://www.mhlw.go.jp/bunya/shougaihoken/cyousajigyou/dl/seikabutsu30-2.pdf#search=’%E7%B2%BE%E7%A5%9E%E7%96%BE%E6%82%A3%E3%81%AE%E7%A4%BE%E4%BC%9A%E7%9A%84%E3%82%B3%E3%82%B9%E3%83%88’. Accessed December 15, 2014. | |
Ministry of Finance. Interest Rate. Tokyo; 2015. Available from: http://www.mof.go.jp/english/jgbs/reference/interest_rate/index.htm. Accessed December 15, 2014. | |
Ascher-Svanum H, Zhu B, Faries D, et al. The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry. 2010;10:2. | |
Mangalore R, Knapp M. Cost of schizophrenia in England. J Ment Health Policy Econ. 2007;10(1):23–41. | |
Moriwaki K, Neuner T, Hübner-Liebermann B, et al. Acute psychiatric inpatient care: a cross-cultural comparison between two hospitals in Germany and Japan. Int J Soc Psychiatry. 2013;59(8):771–781. | |
Oshima I, Mino Y, Inomata Y. Institutionalisation and schizophrenia in Japan: social environments and negative symptoms nationwide survey of in-patients. Br J Psychiatry. 2003;183:50–56. | |
Sugibayashi Y, Yoshimura K, Yamauchi K, Inagaki A, Ikegami N. Influence of patient characteristics on care time in patients hospitalized with schizophrenia. Neuropsychiatr Dis Treat. 2014;10:1577–1584. | |
Oshima I, Mino Y, Inmata Y. How many long-stay schizophrenia patients can be discharged in Japan? Psychiatry Clin Neurosci. 2007;61:71–77. | |
Zeidler J, Heres S, Mahlich J, Greiner W. Treatment patterns and costs in patients with schizophrenia in Germany. Value Health. 2012;15(7):A336–A337. | |
Heres S, Schmitz FS, Leucht S, Pajonk FG. The attitude of patients towards antipsychotic depot treatment. Int Clin Psychopharmacol. 2007;22(5):275–282. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
