Back to Journals » Drug Design, Development and Therapy » Volume 12
miR-708-5p promotes fibroblast–like synoviocytes' cell apoptosis and ameliorates rheumatoid arthritis by inhibition of Wnt3a/β-catenin pathway
Authors Wu J, Fan W, Ma L, Geng X
Received 13 June 2018
Accepted for publication 20 July 2018
Published 10 October 2018 Volume 2018:12 Pages 3439—3447
DOI https://doi.org/10.2147/DDDT.S177128
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Jie Wu,1 Wenqiang Fan,1 Ling Ma,1 Xiuqin Geng2
1Department of Rheumatology and Immunology, Central Hospital of Xinxiang, Henan 453000, People’s Republic of China; 2Department of Endocrinology, Central Hospital of Xinxiang, Henan 453000, People’s Republic of China
Aim: MicroRNAs (miRNA) are a class of small, highly conserved noncoding RNA molecules, which contain 18–28 nucleotides and are involved in the regulation of gene expression. It has been proved that microRNAs play a very important role in several key cellular processes, such as cell differentiation, cell cycle progression, and apoptosis, as well as in autoimmune disease. One recently identified miRNA, miR-708-5p, has been demonstrated to have profound roles in suppressing oncogenesis in different types of tumors. However, the role of miR-708-5p in rheumatoid arthritis (RA) remains to be fully elucidated. Therefore, in this study, we are aiming to identify the role of miR-708-5p in RA.
Methods: The expression level of miR-708-5p in synovial tissues of patients with RA is much lower than in non-RA controls. The effects of miR-708-5p on cell apoptosis, colony formation, and migration in fibroblast-like synoviocytes were assessed in MH7A cells.
Results: Results showed that delivery of miR-708-5p mimics into synovial fibroblasts MH7A could induce cell apoptosis and inhibit colony formation and migration. In addition, miR-708-5p mimics significantly inhibit Wnt3a/β-catenin pathway activity both in transcription and protein level, which could be reversed by the addition of R-spondin 1, an activator of Wnt pathway. R-spondin 1 could also reverse the inhibition of cell survival and proliferation, which was induced by miR-708-5p mimics in MH7A. Moreover, injection of miR-708-5p mimics into collagen-induced rat RA model could ameliorate the RA index and decrease Wnt3a/β-catenin expression in rat joint tissues.
Conclusion: Therefore, we concluded that miR-708-5p is likely to be involved in RA pathogenesis via inhibition of Wnt3a/β-catenin pathway.
Keywords: miR-708-5p, rheumatoid arthritis, cell proliferation, Wnt
Introduction
Rheumatoid arthritis (RA) is a systemic disease that is mainly based on joint diseases. It is an intractable disease characterized by chronic, systemic, and autoimmune diseases. It mainly manifests as joint pain, swelling, rigidity, deformity, and functions’ obstacles. The development of the disease to the late stage can also invade other organs and tissues of the body, including the heart, blood vessels, eyes, lungs, subcutaneous tissue, spleen, lymph nodes, and serosa.1 Current clinically used drugs include nonsteroidal anti-inflammatory drugs, immunosuppressive agents, glucocorticoids, and biological agents developed in recent years to achieve the purpose of relieving pain, reducing inflammation, and slowing or ending joint destruction.2 However, various drugs have their own limitations in the clinical treatment of RA.
Some studies suggest that genetic approach may offer new therapeutic methods; although several genes have been identified as genetic factors that contribute to RA, including CTLA-4 and PADI4, but there is still no clear mechanism illustrating that these genetic factors induce the appearance of RA.3 Recently, researchers have found several microRNAs having potential role in regulating the pathogenesis of RA, such as miR-23 and miR-223, which may serve both as predictor and biomarker of response to anti-TNFα therapies in RA.4 miRNAs are short with 22 nucleotides in length and control the post-transcriptional regulation up to 30% of all human genes.5 Notably, there are increasing reports revealing that deregulation of miRNA expression contributes to human autoimmune diseases.6 Several types of microRNAs have been found upregulated in peripheral blood mononuclear cells (PBMCs) in RA patients, such as miR-146a, miR-155, miR-132, and miR-16.7 Recently, miR-223 was also identified to be overexpressed in peripheral T cells of RA patients.8
One of the microRNAs, miR-708-5p, was found to have a metastasis-suppressive role by targeting endoplasmic reticulum (ER) protein neuronatin in breast cancer.9 Moreover, miR-708-5p expression was proved to be associated with metastatic status of non-small-cell lung cancer (NSCLC) regulating cell apoptosis and metastasis, indicating its potential as a novel and effective therapeutic agent against metastatic malignancy of NSCLC.10 Here, we found there is close interaction between miR-708-5p and RA. In addition, the effects of miR-708-5p on RA was investigated as well. We also indicated a new therapeutic approach using miR-708-5p mimics for RA treatment.
Patients and methods
Patients and non-RA controls
Fresh synovial tissue specimens were obtained from non-RA controls (n=20) with no history of autoimmune diseases or patients with RA (n=22) at the Central Hospital of Xinxiang. Written informed consent was obtained from all study participants. Experiments involving human tissues were approved by the ethics committee of Xinxiang Hospital.
Quantification of miRNA
Total RNA was isolated using the TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA), and complementary DNA was synthesized using a Reverse-iT First-Strand Synthesis kit (ABgene, Thermo Fisher Scientific). Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) was performed to quantify miR-708-5p.
Cell lines and antibodies
Cell line MH7A was obtained from RIKEN CELL BANK and cultured at 37°C under 5% CO2 in RPMI 1640 (Thermo Fisher Scientific) supplemented with 10% FBS (Thermo Fisher Scientific). Antibodies used in the present study were as follows: Wnt3a (Abcam, Cambridge, MA, USA), phospho-LRP6 ((Cell Signaling Technology, Danvers, MA, USA)), Axin1 (Cell Signaling Technology), β-catenin (Cell Signaling Technology), c-myc (Novus, Littleton, CO, USA), and GAPDH (Abcam).
miRNA mimics
miR-708-5p mimics are chemically synthesized in GenePharma Company (Shanghai, China). miRNA mimics are small, chemically modified double-stranded RNAs designed to mimic endogenous mature miRNA molecules. The sequences of miR-708-5p mimics were 5′-AAGGAGCUUACAAUCUAGCUGGG-3′ for sense and 5′-CAGCUAGAUUGUAAGCUCCUUUU-3′ for antisense.
Cell proliferation assay
Cell proliferation was carried out based on a colorimetric assay using cell counting kit 8 (CCK-8; Dojindo Molecular Technologies , Kumamoto, Japan) as described by the manufacturer. A total of 100 μL of cell suspension incubated for 0, 24, 48, and 72 h were mixed with CCK-8 solution and incubated for 1 h, and absorbance at OD450 was measured. Image-based proliferation assay was performed based on 5-ethynyl-2′-deoxyuridine (EdU) incorporation. Cells were incubated with 10 μM EdU (EMD Millipore, Billerica, MA, USA) for 3 days and fixed by formaldehyde. After PBS washing, EdU-detection cocktail was added to the cells for 15 min and images were collected by fluorescent microscopy.
Colony formation assay
Soft agar colony formation assay was established as described previously with minor changes.11 In brief, in a 30 mm petri dish, bottom layer contained 0.75% soft agar in 3 mL of culture medium. When the layer became solid, 1.5 mL of medium containing 1.5×104 cells and 0.36% agar was carefully added. The dish was incubated for 2 weeks and stained with 0.04% crystal violet −2% ethanol in PBS.
Migration assay
In vitro cell migration assays were performed as described previously using Trans-well chambers (8 μM pore size; CoStar, New York, NY, USA). When cells reached subconfluency (∼75%–80%), cells were serum-starved for 24 h. After detachment with trypsin, cells were washed with PBS and resuspended in serum-free medium. Next, 100 μL of cell suspension (2×106 cells/mL) was added to the upper chamber while complete medium was added to the bottom wells. 24 h later, cells that had not migrated were removed from the upper surface of the filters using cotton swabs. Cells that had migrated were fixed with 5% glutaraldehyde solution to determine the number of migratory cells. The lower surface of the filters was stained with 0.25% trypan blue. Images of six fields were captured from each membrane, and the number of migratory cells were counted. The mean of triplicate assays for each experimental condition was used.
Induction of RA rat model and gene therapy
A total of 60 male SD rats (190–210 g, 6–7 weeks old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. They were housed under specific pathogen-free conditions (22°C, 12 h/12 h light/dark, 50%–55% humidity) and given free access to food and water. All animal experiments were performed based on the guidelines approved by the Laboratory Animal Care and Use Committee of National Institutes of Health for the care and use of laboratory animals. All animal experiments were approved by the ethics committee of Xinxiang Hospital.
SD rats were immunized intradermally at the tail base with 0.2 mL of bovine collagen type II (1 mg/mL in 0.1 M acetic acid; Chondrex) emulsified in an equal volume of Freund’s complete adjuvant (Chondrex, Chondres, Redmond, WA, USA) on day 0 and boosted intradermally with 0.1 mL of bovine collagen type II emulsified in Freund’s incomplete adjuvant on day 7. When immunized rats developed collagen-induced arthritis (CIA) (arthritis score >8) on day 14, rats with CIA were then randomly divided into CIA group and miRNA treated group (n=7 per group). miRNA mimics was injected via tail vein in a volume of 200 μL at a concentration of 1 μM. On day 21, arthritis scores (0–4 for each paw; maximum possible score 16) were determined. And blood was collected for C-reactive protein (CRP) and rheumatoid factor (RF) measurement.
Histochemistry and immunohistochemistry
HE-stained paraffin-embedded ankle joint sections were evaluated for synovial hyperplasia, cartilage erosion, and inflammatory cell infiltration. Immunohistochemistry was used to detect Wnt3a and β-catenin.
Statistical analysis
Values are expressed as the mean ± SD of data from at least three independent experiments. Student’s t-test or ANOVA was used. All data were analyzed using the GraphPad Prism Version 5 (GraphPad Software, Inc., La Jolla, CA, USA) for Windows®. P<0.05 was considered to indicate a statistically significant difference between values.
Results
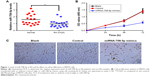
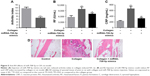
Decreased expression of miR708-5p in RA patients and its proapoptotic role in fibroblast cell lines
Using the RT-qPCR analysis, miR-708-5p expression levels in synovial tissues of patients with RA and non-RA controls were determined. It was found that miR-708-5p expression in synovial tissues was significantly decreased in RA patients compared with those in non-RA controls (P<0.05; Figure 1A). We hypothesized that miR-708-5p may exert a negative effect on cell proliferation; thus, we further examined the effect of miR-708-5p in MH7A, which is a fibroblast-like synoviocytes (FLS) cell line. Upon transfection with high efficiency using lentivirus, MH7A with miR-708-5p overexpression showed decreased proliferative properties compared with their controls (Figure 1B). A total of 72 h post-transfection, cell numbers were significantly less by microscopy observation, while transfection of scrambled oligos had no obvious change compared with blank control (Figure 1C).
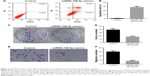
MiR-708-5p increases cell apoptosis and suppresses cell migration
To further determine whether miR-708-5p has any effect on cell apoptosis, MH7A transfected with miR-708-5p or control miRNA was stained with Annexin V and propidium iodide (PI) for flow cytometric analysis. Results showed that after miR-708-5p introduction, ~30% cells were positive for apoptosis markers and almost no cells were positive in the control group (Figure 2A and B). Similar to CCK-8 results (Figure 1B), cells that were transfected with miR-708-5p mimics formed less colonies compared with the control group. Quantification results showed that there is 50% decrease in colonies formed (Figure 2C and D). In order to further illustrate the effects of miR-708-5p on synoviocytes, cell migration was evaluated in Transwell assay. A total of 24 h after serum starvation, MH7A cells transfected with miR-708-5p mimics showed attenuated migration property compared to corresponding negative control (Figure 2E and F). In summary, abovementioned results consistently suggested that miR-708-5p exhibits a negative effect on cell survival, proliferation, and migration.
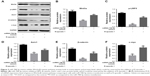
MiR-708-5p regulates Wnt3a/β-catenin signaling
It is well documented that Wnt3a/β-catenin is one of the major drivers, which control cell proliferation and migration. To further elucidate the underlying mechanism by which miR-708-5p mediated cell apoptosis, we examined whether canonical Wnt pathway was involved. To address this, we performed Western blot to check the expression level of several critical proteins on Wnt3a pathway. Results showed that after introduction of miR-708-5p, Wnt3a was decreased by 60%, while its downstream effector, including phospho-LRP5, Axin1, and β-catenin were also decreased to different extent (Figure 3A–E). And consistently, c-myc, which is the target of Wnt pathway, was found to decrease by 60% (Figure 3A and F). Conversely, after the addition of R-spondin1, which was known to be a positive modulator of Wnt pathway by antagonizing the internalization of LRPs,12 we found that the downstream effector of Wnt pathway was increased to a certain level, although not completely reversed (Figure 3A–F). This finding supported that Wnt pathway was at least partially if not all involved in miR-708-5p-mediated cell apoptosis.
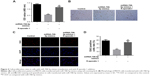
Involvement of Wnt pathway in miR-708-5p-mediated cell proliferation
To further strengthen the finding that miR-708-5p-induced cell proliferation was mediated by Wnt pathway, we evaluated the effect of R-spondin 1 in MH7A. A total of 72 h after the transfection of miR-708-5p, MH7A cells displayed a decrease in proliferation, which was showed by CCK-8 assay, while cells preincubated with R-spondin 1 could almost completely reverse the effect of miRNA (Figure 4A and B). Furthermore, EdU incorporation assay showed that miR-708-5p could dramatically decrease the proliferative potential of MH7A cells; however, this decrease was significantly reversed by R-spondin 1 (Figure 4C and D). Collectively, we confirmed that miR-708-5p could inhibit cell proliferation by Wnt pathway.
MiR-708-5p treatment suppressed synovial inflammation and joint damage
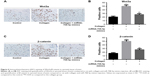
To investigate the effect of miR-708-5p in vivo, we established a CIA model on SD rat. As shown in Figure 5A, 7 days after miRNA delivery, we found that the arthritis index was significantly lower in the miR-708-5p-treated group compared with that in the scrambled oligo group. We also found that the serum level of RF and CRP was significantly decreased in the miR-708-5p-treated group (Figure 5B and C). To further assess the effect of miR-708-5p on developed arthritis, a histopathological evaluation was performed. The results suggested that the pathological features of RA were obviously observed in the ankle joints of vehicle-treated CIA rats including inflammatory cell infiltration, synovial hyperplasia, and cartilage destruction. In contrast, miR-708-5p significantly attenuated the above-described structural changes, further demonstrating the protective effect of miR-708-5p on RA (Figure 5D).
Moreover, we performed immunohistochemistry staining to evaluate the activity of Wnt3a pathway on CIA model. We found that after collagen stimulation, Wnt pathway was strongly activated and manifested by more Wnt3a- and β-catenin-positive staining. However, introduction of miR-708-5p reversed the activation of Wnt pathway, which was consistent with our finding in MH7A cell lines (Figure 6A–D). In all, we concluded that miR-708-5p could ameliorate RA symptoms on CIA models by downregulating Wnt pathway.
Discussion
RA is an autoimmune disease characterized by chronic inflammation of synovial tissue, which can lead to irreversible joint damage.13,14 RA is a chronic disease with severe complications and comorbidities, leading to lifelong disability and increased mortality.15 So far, the pathogenesis of RA and disease prediction were under intensive investigation.
Because of the critical role of miRNA as regulatory factors in many diseased cells, they have become potential targets for a variety of diseases.16,17 There have been increasing studies reported that several miRNAs expressed at abnormal level during the development of RA. miR-146a, miR-155, and miR-125 were reported to express in abnormal level in RA synovial fibroblasts, PBMCs, and T cells of RA patients.7,15,18,19 In our study, we observed that the expression level of miR-708-5p in synovial tissues of patients with RA is much lower than in non-RA controls. Therefore, inhibiting overexpressed miRNAs or restoring silenced miRNA is becoming a therapeutic direction for RA treatment.
The abnormal miRNA expression can lead to abnormalities of cell proliferation, apoptosis, migration, and inflammation. Nakamachi et al suggested that microRNA-124a is a key regulator of proliferation in FLS from patients with RA.20 Niederer et al reported that the downregulation of microRNA-34a in RA synovial fibroblasts promotes apoptosis resistance.21 Semman et al reported that miR-346 controlled the release of TNF-α (a major cytokine in RA) protein and the stability of its mRNA in RA via tristetraprolin stabilization.22 In this study, we evaluated the effects of miR-708-5p on cell apoptosis, colony formation, and migration in MH7A. Similar to previous studies about functions of other miRNAs, we found that delivery of miR-708-5p mimics into MH7A could induce cell apoptosis and inhibit colony formation and migration.
Accumulated evidence suggested that several signaling pathways are involved in the pathogenesis of RA, among which Wnt signaling pathway was also considered to play an important role.23 It was reported that the activation of Wnt signaling pathway can lead to the increase in β-catenin level in FLS from patients with RA, then contributing to the stable activation of FLS.24 Deeper understanding of the role of Wnt signaling pathway in RA could improve the knowledge of RA pathogenesis and therapeutic design. Moreover, increasing amount of in vitro and in vivo evidence suggested that epigenetic modifications by miRNA played an important role in Wnt pathway regulation.24 Thus, in our study, we interrogated whether Wnt3a/β-catenin pathway was involved in miR-708-5p-regulated RA. Our result revealed that miRNA-708-5p mimics significantly inhibit Wnt3a/β-catenin pathway activity both in transcription and protein level, which could be reversed by the addition of Wnt activator R-spondin 1. R-spondin 1 could also reverse the apoptosis and cell proliferation inhibition effects induced by miR-708-5p mimics in MH7A, indicating the involvement of Wnt3a/β-catenin pathway in the regulation of RA by miR-708-5p.
Taken together, we concluded that miR-708-5p was involved in RA pathogenesis via inhibition of Wnt3a/β-catenin pathway, both in vitro and in vivo, and addition of miR-708-5p mimics could ameliorate RA. Further investigations are warranted to interrogate the underlying mechanisms and associated molecules or pathways in RA pathogenesis, especially Wnt signaling pathway. This will provide guidance to the development of targeted therapy of RA. In addition, despite several miRNA-modulating agents have already entered clinical trials, treatment of RA with epigenetic drugs must overcome the barrier of potential off-target effects.
Disclosure
The authors report no conflicts of interest in this work.
References
Moran-Moguel MC, Petarra-Del Rio S, Mayorquin-Galvan EE, Zavala-Cerna MG. Rheumatoid Arthritis and miRNAs: A Critical review through a functional view. J Immunol Res. 2018;2018:2474529. | ||
Avouac J, Gossec L, Dougados M. Diagnostic and predictive value of anti-cyclic citrullinated protein antibodies in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis. 2006;65(7):845–851. | ||
Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275–2285. | ||
Chatzikyriakidou A, Voulgari PV, Georgiou I, Drosos AA. miRNAs and related polymorphisms in rheumatoid arthritis susceptibility. Autoimmun Rev. 2012;11(9):636–641. | ||
Castro-Villegas C, Perez-Sanchez C, Escudero A, Filipescu I, Verdu M, Ruiz-Limon P, et al. Circulating miRNAs as potential biomarkers of therapy effectiveness in rheumatoid arthritis patients treated with anti-TNFalpha. Arthritis Res Ther. 2015;17:49. | ||
Sand M, Gambichler T, Sand D, Skrygan M, Altmeyer P, Bechara FG. MicroRNAs and the skin: tiny players in the body’s largest organ. J Dermatol Sci. 2009;53(3):169–175. | ||
Tang Y, Luo X, Cui H, Ni X, Yuan M, Guo Y, et al. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009;60(4):1065–1075. | ||
Pauley KM, Satoh M, Chan AL, Bubb MR, Reeves WH, Chan EK. Upregulated miR-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res Ther. 2008;10(4):R101. | ||
Fulci V, Scappucci G, Sebastiani GD, Giannitti C, Franceschini D, Meloni F, et al. miR-223 is overexpressed in T-lymphocytes of patients affected by rheumatoid arthritis. Hum Immunol. 2010;71(2):206–211. | ||
Yang J, Wei J, Wu Y, Wang Z, Guo Y, Lee P, et al. Metformin induces ER stress-dependent apoptosis through miR-708-5p/NNAT pathway in prostate cancer. Oncogenesis. 2015;4:e158. | ||
Wu X, Liu T, Fang O, Dong W, Zhang F, Leach L, et al. MicroRNA-708-5p acts as a therapeutic agent against metastatic lung cancer. Oncotarget. 2016;7(3):2417–2432. | ||
Rafehi H, Orlowski C, Georgiadis GT, Ververis K, El-Osta A, Karagiannis TC. Clonogenic assay: adherent cells. J Vis Exp. 2011(49):pii: 2573. | ||
Binnerts ME, Kim KA, Bright JM, Patel SM, Tran K, Zhou M, et al. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc Natl Acad Sci U S A. 2007;104(37):14700–14705. | ||
Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–361. | ||
Pincus T, Sokka T, Wolfe F. Premature mortality in patients with rheumatoid arthritis: evolving concepts. Arthritis Rheum. 2001;44(6):1234–1236. | ||
Churov AV, Oleinik EK, Knip M. MicroRNAs in rheumatoid arthritis: altered expression and diagnostic potential. Autoimmun Rev. 2015;14(11):1029–1037. | ||
Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107(7):823–826. | ||
Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11(4):441–450. | ||
Zhou Q, Haupt S, Kreuzer JT, Hammitzsch A, Proft F, Neumann C, et al. Decreased expression of miR-146a and miR-155 contributes to an abnormal Treg phenotype in patients with rheumatoid arthritis. Ann Rheum Dis. 2015;74(6):1265–1274. | ||
Nakamachi Y, Kawano S, Takenokuchi M, Nishimura K, Sakai Y, Chin T, et al. MicroRNA-124a is a key regulator of proliferation and monocyte chemoattractant protein 1 secretion in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum. 2009;60(5):1294–1304. | ||
Niederer F, Trenkmann M, Ospelt C, Karouzakis E, Neidhart M, Stanczyk J, et al. Down-regulation of microRNA-34a* in rheumatoid arthritis synovial fibroblasts promotes apoptosis resistance. Arthritis Rheum. 2012;64(6):1771–1779. | ||
Semaan N, Frenzel L, Alsaleh G, Suffert G, Gottenberg JE, Sibilia J, et al. miR-346 controls release of TNF-alpha protein and stability of its mRNA in rheumatoid arthritis via tristetraprolin stabilization. PLoS One. 2011;6(5):e19827. | ||
Miao CG, Yang YY, He X, Li XF, Huang C, Huang Y, et al. Wnt signaling pathway in rheumatoid arthritis, with special emphasis on the different roles in synovial inflammation and bone remodeling. Cell Signal. 2013;25(10):2069–2078. | ||
Maeda K, Takahashi N, Kobayashi Y. Roles of Wnt signals in bone resorption during physiological and pathological states. J Mol Med (Berl). 2013;91(1):15–23. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.