Back to Journals » Clinical Ophthalmology » Volume 14
Mid-Term Evaluation of iStent Inject® Trabecular Micro-Bypass Stent Implantation with or without Phacoemulsification: A Retrospective Study
Authors Silveira Seixas RC , Balbino M , Basile Neto A , de Alcantara Almeida Costa A, Jordão MLS, Russ HHA
Received 15 October 2020
Accepted for publication 13 November 2020
Published 16 December 2020 Volume 2020:14 Pages 4403—4413
DOI https://doi.org/10.2147/OPTH.S283587
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Regina Cele Silveira Seixas,1 Marcos Balbino,2 Alberto Basile Neto,3 Amanda de Alcantara Almeida Costa,4 Marcelo Lopes da Silva Jordão,5 Heloisa Helena Abil Russ6
1HCloe Oftalmologia Especializada, Universidade Federal de São Paulo, São Paulo, SP, Brazil; 2HCloe Oftalmologia Especializada, Centro Universitário São Camilo, São Paulo, SP, Brazil; 3Clínica Oftalmológica do Complexo Hospitalar Padre Bento de Guarulhos, Guarulhos, SP, Brazil; 4Clínica Oftalmológica do Complexo Hospitalar Padre Bento de Guarulhos, Guarulhos, Universidade Estadual de Campinas, Campinas, SP, Brazil; 5Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto Medical School, Universidade de São Paulo, Ribeirão Preto, Brazil; 6HR Oftalmologia, Universidade Federal do Paraná, Curitiba, PR, Brazil
Correspondence: Marcos Balbino
HCloe Oftalmologia Especializada, Centro Universitário São Camilo, Cipriano Barata 1869 Ap. 93, São Paulo, SP, Brazil
Tel +55 11983462354
Email [email protected]
Introduction: This study evaluated the 6-month performance and safety of micro-invasive glaucoma surgery (MIGS) with iStent inject either with or without cataract surgery.
Material and Methods: Longitudinal retrospective study of 86 surgeries in 49 patients with inadequately controlled open-angle glaucoma (OAG) or ocular hypertension who underwent iStent inject trabecular micro-bypass implantation either alone (isolated group) or combined with cataract surgery (combined group). The two primary outcomes included an intraocular pressure (IOP) drop of ≥ 20% versus preoperative values (adequate drop) and IOP maintenance between 6 and 18 mmHg (adequate Range). For both outcomes, we determined “complete” and “qualified” success if patients did not require or did require glaucoma medications, respectively, at the end of follow-up. Safety outcomes included best-corrected visual acuity, adverse events, and secondary surgeries.
Results: In the adequate drop analysis, 30.2% achieved “complete success,” and 37.2% achieved “qualified success.” For adequate range, 40.7% achieved “complete success” and 39.5% achieved “qualified success.” There was no difference in medication decrease (p=0.77) nor IOP reduction (p=0.46) between the isolated and combined groups. Safety was generally favorable and similar between groups, with mild transient adverse events that resulted in no sequelae.
Discussion/Conclusion: iStent inject implantation either with or without cataract surgery was able to safely decrease IOP and medication requirements through 6 months after surgery.
Keywords: glaucoma, medical treatment, trabecular micro-bypass, glaucoma surgery combined with cataract surgery, micro-invasive glaucoma surgery
Introduction
Glaucoma is a disease characterized by cupping of the optic nerve head and visual field damage.1 It is the third most frequent cause of blindness worldwide, and the first of irreversible blindness.2 In 2013, researchers estimated the prevalence of glaucoma in the global elderly population to be 64.3 million, increasing to 76.0 million in 2020 and 111.8 million in 2040.3 Reducing intraocular pressure (IOP) is the only treatment proven to slow the progression of glaucomatous optic neuropathy.4,5 The Early Manifest Glaucoma Trial estimated a 10% lower risk of progression for each 1 mmHg reduction in IOP.5 Likewise, the Advanced Glaucoma Intervention Study (AGIS) demonstrated that eyes with IOP <18 mmHg in 100% of visits during follow-up demonstrated no average change on visual fields, as measured by standard automated perimetry (SAP).6
Medical treatment and laser therapy can reduce IOP; however, when these treatments are insufficient in reducing IOP to target pressures according to disease severity, ab externo filtering procedures are frequently utilized to provide greater IOP reduction. Unfortunately, filtering procedures are higher-risk options that may result in bleb-related complications, choroidal hemorrhage, hyphema, hypotony, infection, inflammation, loss of vision, or reoperation.7
There are currently a growing number of surgical procedures designed to lower IOP8 via an ab interno approach involving minimal trauma to ocular tissues; these procedures are classified as micro-invasive glaucoma surgery (MIGS).9,10 The iStent trabecular micro-bypass (the first US FDA-approved MIGS device) and the second-generation iStent inject (both from Glaukos Corporation, San Clemente, CA, USA) are manufactured from heparin-coated titanium and can be implanted alone or combined with cataract removal.11 A wealth of evidence has proven these stents to be safe and effective in decreasing IOP and medication burden.12,13 The present study aims to examine the six-month performance and safety outcomes of iStent inject implantation either alone or in combination with cataract surgery in eyes with mild to moderate open-angle glaucoma (OAG).
Materials and Methods
Ethics
The Institutional Review Board of HCLOE Clinicas de Oftalmologia Especializada approved the study, and all data accessed complied with relevant data protection and privacy regulations. The research methods fulfilled the Helsinki Declaration. The study did not require informed consent due to its retrospective design and non-interventional review of medical records.
Data Collection and Eligibility
This longitudinal retrospective multicenter study evaluated the six-month outcomes of eyes that underwent implantation of iStent inject with or without cataract surgery from August 2017 to August 2019. All surgeries were performed in one of three ophthalmic surgery centers by three surgeons (located in the cities of São Paulo and Ribeirão Preto, in São Paulo state; and Curitiba, in Paraná state, Brazil). The surgeons included in the study were well experienced in this procedure, having implanted more than 50 iStent devices. Complete data were included from 86 eyes of 49 patients (both eyes in 37 patients and one eye in 12 patients).
Patients were eligible for inclusion if they were above 18 years of age and had indications of antiglaucoma surgery (per Brazilian Society of Glaucoma guidelines) and cataract surgery. Diagnoses could include primary open-angle glaucoma (POAG) or ocular hypertension that was not controlled by drugs and laser trabeculoplasty.
Outcomes
There were two primary outcomes: an IOP drop of ≥20% versus preoperative values (adequate drop), and maintenance of IOP from 6 to 18 mmHg throughout the study (adequate range). In both outcomes, we determined “complete” and “qualified” success if the patients did not require or did require glaucoma medications, respectively, at the end of the study. Therefore, in the corresponding survival analyses, failure was defined as an IOP reduction less than 20% versus preoperative in two consecutive appointments (failure related to suboptimal drop); or an IOP value outside the range of 6–18 mmHg at any appointment (failure related to suboptimal range).
Other outcomes include IOP decrease, visual acuity improvement, and reduction in glaucoma medications. The data also included surgical complications, adverse events, and secondary surgeries. Given the retrospective design, no patients underwent medication washout prior to surgery.
Statistical Analysis
The identification of the eye (right or left) was treated as a unit of measurement in analyses due to the dependence between eyes and in order to prevent losing data from specific surgical procedures. A test for skewness and kurtosis verified the normal distribution of the data. We applied a t-test (if normal distribution) and Wilcoxon rank-sum test (if non-normal distribution) for comparison of means. The one-way ANOVA was applied for analysis of variance of several means, and Chi-square test for categorical variables. The McNemar test was used to compare pre and postoperative proportions of eyes on zero medications or eyes on ≥1 medication.
We calculated survival estimates using a Kaplan–Meier analysis and assessed possible differences with a Log rank test. For the outcome of IOP drop, failure was set at IOP reduction less than 20% versus baseline in two consecutive appointments. For the outcome of IOP range, failure was considered an IOP outside the range of 6–18 mmHg at any appointment. We set the p-value <0.05 as the threshold for statistical significance. For statistical analysis, Stata software (StataCorp. 2013 Stata Statistical Software: Release 13, College Station, TX: StataCorp LP) was used for calculation, and Minitab 17 (Minitab 17 Statistical Software. State College, PA: Minitab, Inc.) was used for some figures and graphs.
Results
We included charts from 86 eyes of 49 patients in this retrospective study (11 patients had unilateral surgery and 38 patients had bilateral surgery). One patient with bilateral surgery underwent standalone stent implantation in one eye and stent-cataract surgery in the other eye; however, all other bilateral cases (37) received the same procedure in both eyes (standalone or combination).
We collected data from the preoperative period to 6-months postoperative. We present results according to the type of procedure: iStent inject implantation as an isolated procedure or combined with phacoemulsification. Table 1 shows the baseline characteristics of the patients in the study.
 |
Table 1 Baseline Demographic and Ocular Characteristics of the Two Surgery Groups |
Intraocular Pressure
In the six-month period of study, the mean IOP of the overall cohort decreased from 18.24 mmHg (95% CI 17.1–19.4) to 12.9 mmHg (95% CI 12.2–13.7), p = 0.0001 (Figure 1). There was no difference in the 6-month IOP variation among the two groups (isolated and combined surgery), ANOVA Prob > F = 0.46 (Figure 2).
 |
Figure 1 Intraocular pressure (mean and 95% confidence interval) from baseline to 6 months postoperative, all eyes. |
 |
Figure 2 Intraocular pressure from baseline to 6 months postoperative, combined and isolated surgery groups. Notes: Graph shows line plots of the means. ANOVA prob > F = 0.46. |
Number of Glaucoma Medications
Preoperatively, eyes in the standalone group were on more glaucoma medications preoperatively than those in the combined group (2.98 ± 0.89 versus2.51 ± 1.03, respectively; p=0.014). However, both groups had statistically comparable medication burden at 6 months (0.70 ± 0.83 versus 0.95 ± 1.02 medications in the two groups, respectively; p=0.10). The mean number of medications in the overall cohort decreased from 2.74 (95% CI 2.53–2.96) preoperatively to 0.83 (95% CI 0.63–1.03) at 6 months (p=0.0001). The medication decrease is similar between groups (reduction of 1.81 ± 1.07 in the combined group and 2.02 ± 1.14 in the isolated group, p = 0.77, Mc Nemar’s Chi-square test), Figure 3.
Almost half of the eyes (47.67%, n=41) were free of glaucoma medication at the end of the study. During the 6 months of follow-up, glaucoma medication burden reduced by 0–4 medications versus preoperative; 100% of the eyes were able to maintain or decrease their medication burden versus baseline. There was no difference between groups in the percent of eyes becoming medication-free (p=0.52) or the proportions of eyes achieving different levels of drop reduction (p=0.8) at 6 months postoperative, as observed in Table 2.
 |
Table 2 Proportional Analysis at 6 Months in Combined and Isolated Surgery Groups: Eyes on Zero Medications, and Eyes Experiencing Different Levels of Medication Reduction vs Baseline |
Survival analyses showed that 67% of the eyes achieved an adequate drop (IOP reduction ≥20% versus baseline), and 81% achieved an adequate range (6–18 mmHg at all visits). The Log rank test did not find a between-group difference in the survival estimates for each endpoint (p=0.056 for adequate drop outcome and p=0.82 for adequate range outcome). Figure 4 presents the Kaplan–Meier survival estimates for all eyes by surgery type for the two primary outcomes.
The terms “complete success” and “qualified success” express the need for glaucoma medication at 6 months postoperative. An eye achieving a primary outcome (adequate drop or adequate range) without medication was classified as a “complete success,” while eyes achieving a primary outcome with one or more glaucoma medications were classified as a “qualified success” (see Table 3). Figure 5 shows the Kaplan–Meier survival estimates for the overall cohort for both primary outcomes in terms of “complete success” (no medication at Month 6) and “qualified success” (medication at Month 6); p=0.52 and p=0.81 for adequate drop and adequate range analysis, respectively.
 |
Table 3 Proportional Analysis: Eyes with Adequate Drop (IOP Reduction ≥20% vs Baseline), and Adequate Range (IOP of 6–18 mmHg) |
Visual Acuity and Safety
Overall visual acuity (decimal) improved from 0.65 (95% CI 0.59–0.71) to 0.81 (95% CI 0.75–0.87), p < 0.0001, Figure 6. When analyzed separately, the combined group improved VA significantly [from 0.55 (95% CI 0.48–0.62) to 0.85 (95% CI 0.77–0.93), p = 0.0001], consistent with expectations following cataract surgery. The isolated group VA remained stable ([0.75 (95% CI 0.66–0.85) preoperatively and 0.77 (95% CI 0.67–0.86) postoperatively, p = 0.085].
Successful implantation of two second-generation trabecular micro-bypass stents was completed in all but two eyes from the same patient, who was in the isolated group and had floppy iris syndrome. In these two surgeries, there was a failure of the placement of the second iStent, after correct placement of the first one. In one of those cases, the stent remained inside the injector, and in the second case, the stent fell inside the anterior chamber and was removed immediately without further complications. The patient did not have elevation or decrease in IOP in either eye throughout follow-up.
Three eyes (all in the combined group) had a mild hyphema on Day 1. One eye had hyphema with associated IOP elevation (in the isolated group). These resolved by Week 1 after treatment with ocular hypotensive drops (in the high-IOP eye) or careful observation (in the normotensive eyes). IOP peaks were more prevalent in patients that underwent combined surgery, and occurred almost exclusively on Day 1 postoperative; these were presumed to be due to retained viscoelastic (Healon®, usually removed with Centurion® Vision System). All but one patient had no IOP peaks during the rest of the follow-up. In that one patient, an IOP peak was noted at Month 2 and Month 6 and was attributed to withdrawal of glaucoma medications, with no relation to the stents. All the IOP pikes in isolated implant occurred in pseudophakic eyes. Table 4 summarizes the complication rates in each group; there was no difference between the groups (p=0.066).
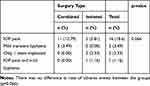 |
Table 4 Incidence of Adverse Events (n (%)) in Isolated and Combined Surgery Groups |
Discussion
Results from this retrospective study suggest iStent inject implantation is a suitable treatment option for patients with glaucoma, either as a standalone procedure or in combination with cataract surgery. In our series, the IOP decreased from 18.2 mmHg to 12.9 at 6 months postoperative, and the number of glaucoma medications decreased from 2.6 to 0.7, which are clinically and statistically significant improvements. Nearly one-third (30.2%) of eyes achieved the “ideal result,” defined as 20% IOP reduction and no need for glaucoma medication; another 37.2% achieved this level of IOP reduction with medication (“qualified success”). The amount of IOP reduction did not differ between groups.
The visual acuity improved significantly in the combined group, as would be expected following cataract extraction. The addition of stent implantation at the end of the procedure did not appear to have any negative impact on this post-cataract visual improvement. Meanwhile, visual acuity remained stable in the isolated group, suggesting that iStent inject is a vision-preserving or acuity-neutral procedure, as expected since no measure was taken to improve acuity in this group.
The degree of IOP and medication reduction was similar between the combined and isolated groups, thereby confirming the findings of numerous other studies in combined and standalone settings.13–23 For example, Hengerer et al published two papers; in the first, they included 81 eyes undergoing iStent inject implantation with cataract removal. The IOP dropped from 22.6 ± 6.2 mmHg to 14.8 ± 2.2 mmHg after 12-month follow-up, a 34.5% reduction.13 The second study evaluated 44 eyes undergoing standalone iStent inject implantation. They reported an IOP reduction from 25.3 ± 6.0 mmHg to 15.2 ± 3.1 mmHg after 12 months of follow-up, a 39.9% reduction.20 In the 36-month follow-up, the IOP reductions were similar in the isolated and combined groups.20
The present study evaluated isolated stent implantation and stent implantation combined with cataract surgery. Both groups demonstrated similar IOP reduction, suggesting that the cataract surgery did not enhance the hypotensive effect caused by isolated stent implant; in other words, the iStent inject device, rather than cataract surgery, appears to be responsible for the IOP reduction. In addition, the IOP decreased by over 4.1 mmHg (24.7% reduction), greater than the <2 mmHg average reduction observed after cataract surgery.25–28 Furthermore, Shrivastava and Singh showed that anterior chamber angle aperture has a large influence on the IOP-lowering effect of isolated cataract extraction, with narrower angles having greater post-phacoemulsification IOP reductions.28 Since the present study included only open-angle eyes, cataract extraction would be expected to play a small role in IOP reduction, so the reduction can be attributed to stent implantation.
In this study, eyes with higher baseline IOP experienced greater IOP reductions, confirming the findings of numerous prior studies in glaucomatous and nonglaucomatous eyes.26 A similar effect is also found in cataract surgery in addition to stent implantation, as described by Brown et al. They described a mean IOP reduction of 7.5 mmHg only on eyes with more than 21 mmHg; in less affected eyes (less than 15 mmHg), the reduction was minimal.24 These findings suggest that patients with lower preoperative IOP may derive benefit from the procedure in the form of medication reduction, rather than IOP reduction.
Applying Ohm’s Law o fluids, we can conclude that flow is directly proportional to the difference between the IOP and the episcleral pressure divided by the resistance to the Schlemm’s canal’s aqueous flow veins. The higher the IOP and lower the episcleral pressure and resistance to outflow, the more aqueous will flow through the stent. Following this principle, when the two pressures are equal, there will be little to no flow; accordingly, the IOP does not drop lower than the episcleral pressure (8–10 mmHg) in physiological conditions. With a trabecular bypass device, which acts upon the physiologic trabecular outflow system, it is improbable to have a hypotonic eye as a postoperative complication.13–24
The mechanism above underlies the occasional inflow of blood into the stent shortly after implantation. As the surgeon aspires the viscoelastic, the anterior chamber pressure briefly drops below the episcleral pressure, reversing the flow through the stent and causing a small amount of blood to flow backward from the episcleral system, producing a mild hyphema or microhyphema. Rather than being concerning, this effect confirms the proper positioning of the stent.
McEwen et al, in a mathematical analysis applying Poiseuille’s law to aqueous outflow, concluded that a single hole of 12 μm in the trabecular meshwork would be enough to overcome its resistance entirely.33 The iStent inject has a lumen of 80 μm and side flow outlets of 50 μm, making them more than sufficient to restore full aqueous outflow.
By 6 months, patients’ medication burden in our study decreased by nearly two full medications, a 72% reduction. Our data is similar to other studies, both with and without cataract surgery. For example, Neuhann reported a reduction of 85% in medication burden at 12 months following 164 eyes were implanted with the iStent inject device with concomitant cataract surgery.23 Guedes et al found a 77.8% reduction in the number of medications in 73 eyes that underwent cataract surgery combined with either iStent or iStent inject.32 Other studies have found similar medication usage reductions.13,16,18 Medication reduction decreases the need for patient compliance with the treatment,18 and plays a role in preventing drug toxicity due to preservatives.31,34–37
Quaranta et al, in a review of the literature published in 2016, showed that the complexity of the therapeutic regimen of eye drops could lower the patients’ satisfaction with their treatment and that satisfaction is an essential factor in their compliance with the treatment. Reducing the number of medications may lower the complexity of the drop regimen and improve the patient’s compliance.38 This was confirmed by Claxton et al in a 2001 study, in which compliance was inversely related to the number of doses per day.39
In the present study, Kaplan–Meier estimates show the success of over two-thirds (67.4%) of the patients at the 6-month follow-up. The result is following Gonnermann et al, who conducted a study with 25 patients who underwent cataract surgery plus iStent inject implantation. They found a survival proportion of approximately 75% using success criteria of intraocular pressure of less than 21 mmHg and a greater than 20% reduction from baseline without further surgery (topical medication allowed) in 6 months.40 Arriola-Villalobos et al, using IOP ≤18 mmHg success criteria without medication, found a survival rate of approximately 50% at 6 months and approximately 70% with medication.19
The safety profile was favorable, with events largely being transient and resulting in no sequelae. No subjects experienced endophthalmitis, hypotony, obstruction of the stent, or sight-threatening complications. These findings are in accord with other studies showing excellent safety of iStent and iStent inject in both combined and isolated procedures.12–23
As a retrospective study, the present paper did not have the power of randomized studies. It lacks randomization, power calculation, and net effect size for formal statistical calculations. Patients had predominantly mild to moderate glaucoma, which limits speculating the role of stents in more severe cases of the disease. Some glaucoma specialists may consider the 6-month follow-up a short time to define the device’s real therapeutic value to control glaucoma.
Despite these limitations, the study has many strengths. The surgical procedure was standardized across the three sites, since all three surgeons strictly followed the guidelines of the Brazilian Society of Glaucoma and had comparable results. The involvement of these different surgeons at different sites enhances the generalizability of the findings. The Brazilian patient population was ethnically and racially heterogeneous, further enhancing generalizability. The patients’ charts contained comprehensive clinical information with very little missing data. We believe the study brings valuable information to the experts involved in the surgical treatment of glaucoma.
Conclusions
Trabecular stents are still a relatively new technology, but iStent and iStent inject already have amassed a robust evidence base showing promising results. More studies, particularly long-term cohorts and metanalyses, are warranted to assess the impact of IOP reduction on glaucomatous neuropathy and long-term safety. iStent and iStent inject represent a new and safe alternative to the treatment of glaucoma. Alongside more conservative interventions such as laser trabeculoplasty and medications, trabecular micro-bypass stents have the potential to postpone or prevent more invasive filtering surgeries in the future. And, since they are micro-sized, conjunctival-preserving, tissue-sparing devices, they do not preclude future surgeries should they be needed. These recent papers bring to light the real effectiveness and safety of trabecular stents, but few cover miscegenated populations as the Brazilians with many ethnic backgrounds.
In conclusion, our data show that iStent inject offers a safe and less invasive way to decrease IOP, reducing glaucoma medication and costs in a period of 6 months. The substantial reduction in glaucoma medication improves patients’ quality of life and relieves adherence issues, crucial in developing countries. A new frontier of glaucoma therapeutics is ahead, bringing us closer to our ultimate goal: making glaucomatous blindness a thing of the past.
Disclosures
The authors received funding from Glaukos corporation to cover the Open Access Article Publishing Charge (APC) for the manuscript. Alberto Basile Neto reports medical residency scholarship from Clínica Oftalmológica do Complexo Hospitalar Padre Bento de Guarulhos, outside the submitted work. The authors report no other potential conflicts of interest for this work.
References
1. Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390(10108):2183–2193. doi:10.1016/S0140-6736(17)31469-1
2. Bourne RRA, Stevens GA, White RA, on behalf of the Vision Loss Expert Group, et al. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health. 1;2013:339–349. doi:10.1016/S2214-109X(13)70113-X
3. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi:10.1016/j.ophtha.2014.05.013
4. Mao LK, Stewart WC, Shields MB. Correlation between intraocular pressure control and progressive glaucomatous damage in primary open-angle glaucoma. Am J Ophthalmol. 1991;111:51–55. doi:10.1016/S0002-9394(14)76896-5
5. Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. doi:10.1001/archopht.120.10.1268
6. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000;130(4):429–440. doi:10.1016/S0002-9394(00)00538-9
7. Popovic M, Campos-Moller X, Saheb H, Ahmed IIK. Efficacy and adverse event profile of the iStent and iStent inject trabecular micro-bypass for open-angle glaucoma: a meta-analysis. J Curr Glaucoma Pract. 2018;12(2):67–84. doi:10.5005/jp-journals-10028-1248
8. Stefan C, Batras M, Iliescu Daniela A, Timaru Cristina M, De Simone A, Hosseini-Ramhormozi J. Current options for surgical treatment of glaucoma. Rom J Ophthalmol. 2015;59(3):194–201.
9. Prum BE, Rosenberg LF, Gedde SJ, et al. Primary open-angle glaucoma preferred practice pattern® guidelines. Ophthalmology. 2016;123(1):41–111. doi:10.1016/j.ophtha.2015.10.053
10. Saheb H, Ahmed IIK. Micro-invasive glaucoma surgery. Curr Opin Ophthalmol. 2012;23(2):96–104. doi:10.1097/ICU.0b013e32834ff1e7
11. Fea AM. Phacoemulsification versus phacoemulsification with micro-bypass stent implantation in primary open-angle glaucoma: randomized double-masked clinical trial. J Cataract Refract Surg. 2010;36(3):407–412. doi:10.1016/j.jcrs.2009.10.031
12. Neuhann TH, Hornbeak DM, Neuhann RT, Giamporcaro JE. Long-term effectiveness and safety of trabecular microbypass stent implantation with cataract surgery in patients with glaucoma or ocular hypertension: five-year outcomes. J Cataract Refract Surg. 2019;45(3):312–320. doi:10.1016/j.jcrs.2018.10.029
13. Hengerer FH, Auffarth GU, Riffel C, Conrad-Hengerer I. Second-generation trabecular micro-bypass stents as standalone treatment for glaucoma: a 36-month prospective study. Adv Ther. 2019;36(7):1606–1617. doi:10.1007/s12325-019-00984-9
14. Klamann MKJ, Gonnermann J, Pahlitzsch M, et al. iStent inject in phakic open angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2015;253:941–947. doi:10.1007/s00417-015-3014-2
15. Lindstrom R, Lewis R, Hornbeak H, et al. Outcomes following implantation of two second generation trabecular micro-bypass stents in patients with open-angle glaucoma on one medication: 18-month follow-up. Adv Ther. 2016;33:2082–2090. doi:10.1007/s12325-016-0420-8
16. Voskanyan L, Garcia-Feijoo J, Belda J, Fea A, Junemann A, Baudouin C, Synergy Study Group. Prospective, unmasked evaluation of the istent inject system for open-angle glaucoma: synergy trial. Adv Ther. 2014;31(2):189–201. doi:10.1007/s12325-014-0095-y
17. Berdahl J, Voskanyan L, Myers JS, et al. Implantation of two second-generation trabecular microbypass stents and topical travoprost in open-angle glaucoma not controlled on two preoperative medications: 18-month follow-up. Clin Exp Ophthalmol. 2017;45(8):797–802. doi:10.1111/ceo.12958
18. Fea AM, Belda JI, Rekas M, et al. Prospective unmasked randomized evaluation of the iStent inject versus two ocular hypotensive agents in patients with primary open-angle glaucoma. Clinical Ophthalmology. 2014;8:875–882.
19. Arriola-Villalobos P, Martinez-de-la-Casa J, Diaz-Valle M-FL, Fernandez-Perez C, Garcia-Feijoo J. Glaukos iStent inject trabecular micro-bypass implantation associated with cataract surgery in patients with co-existing cataract and open-angle glaucoma or ocular hypertension: A long-term study. J Ophthalmol. 2016;2016:1–7. doi:10.1155/2016/1056573
20. Hengerer FH, Auffarth GU, Riffel C, Conrad-Hengerer I. Prospective, non-randomized, 36-month study of second-generation trabecular micro-bypass stents with phacoemulsification in various types of glaucoma. Ophthalmol Ther. 2018;7(2):405–415. doi:10.1007/s40123-018-0152-8
21. Clement CI, Howes F, Ioannidis AS, Shiu M, Manning D. One-year outcomes following implantation of second-generation trabecular micro-bypass stents in conjunction with cataract surgery for various types of glaucoma or ocular hypertension: multicenter, multi-surgeon study. Clinical Ophthalmology. 2019;13:491–499. doi:10.2147/OPTH.S187272
22. Samuelson TW, Sarkisian SR
23. Neuhann R, Neuhann T. Second-generation trabecular micro-bypass stent implantation: retrospective analysis after 12- and 24-month follow-up. Eye Vis. 2020;7:1. doi:10.1186/s40662-019-0169-7
24. Brown RH, Gibson Z, Zhong L, Lynch MG. Intraocular pressure reduction after cataract surgery with implantation of a trabecular microbypass device. J Cataract Refract Surg. 2015;41(6):1318–1319. doi:10.1016/j.jcrs.2015.01.015
25. Shingleton BJ, Gamell LS, O’Donoghue MW, Baylus SL, King R. Long-term changes in intraocular pressure after clear corneal phacoemulsification: normal patients versus glaucoma suspect and glaucoma patients. J Cataract Refract Surg. 1999;25(7):885–890. doi:10.1016/S0886-3350(99)00107-8
26. Poley BJ, Lindstrom RL, Samuelson TW. Long-term effects of phacoemulsification with intraocular lens implantation in normotensive and ocular hypertensive eyes. J Cataract Refract Surg. 2008;34:735–742. doi:10.1016/j.jcrs.2007.12.045
27. Mansberger SL, Gordon MO, Jampel H, et al. Reduction in intraocular pressure after cataract extraction: the ocular hypertension treatment study. Ophthalmology. 2012;119:1826–1831. doi:10.1016/j.ophtha.2012.02.050
28. Shrivastava A, Singh K. The effect of cataract extraction on intraocular pressure. Curr Opin Ophthalmol. 2010;21:118–122. doi:10.1097/ICU.0b013e3283360ac3
29. Rosenquist R, Epstein D, Melamed S, Johnson M, Grant WM. Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculotomy. Curr Eye Res. 1989;8:1233–1240. doi:10.3109/02713688909013902
30. Peterson WS, Jackson VL, Sears ML. Resistance to aqueous outflow in the rhesus monkey eye. Am J Ophthalmol. 1971;72:445–451. doi:10.1016/0002-9394(71)91317-1
31. Yee RW, Norcom EG, Zhao XC. Comparison of the relative toxicity of travoprost 0.004% without benzalkonium chloride and latanoprost 0.005% in an immortalized human cornea epithelial cell culture system. Adv Ther. 2006;23:511–519. doi:10.1007/BF02850039
32. Guedes RAP, Gravina DM, Lake JC, Guedes VMP, Chaoubah A. Intermediate results of istent or istent inject implantation combined with cataract surgery in a real-world setting: a longitudinal retrospective study. Ophthalmol Ther. 2019;8(1):87–100. doi:10.1007/s40123-019-0166-x
33. McEwen WK. Application of Poiseuille’s law to aqueous outflow. Arch Ophthalmol. 1958;60:290–307. doi:10.1001/archopht.1958.00940080306017
34. Ammar DA, Kahook MY. Effects of benzalkonium chloride- or polyquad-preserved fixed combination glaucoma medications on human trabecular meshwork cells. Mol Vis. 2011;17:1806–1813.
35. De Saint Jean M, Brignole F, Bringuier AF, Bauchet A, Feldmann G, Baudouin C. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest Ophthalmol Vis Sci. 1999;40:619–630.
36. Ammar DA, Kahook MY. The effects of combination glaucoma medications on ocular surface epithelial cells. Adv Ther. 2009;26:970–975. doi:10.1007/s12325-009-0076-8
37. Wu KY, Wang HZ, Hong SJ. Cellular cytotoxicity of antiglaucoma drugs in cultured corneal endothelial cells. Kaohsiung J Med Sci. 2007;23:105–111. doi:10.1016/S1607-551X(09)70384-5
38. Quaranta L, Riva I, Gerardi C, Oddone F, Floriani I, Konstas AG. Quality of life in glaucoma: a review of the literature [published correction appears in Adv Ther. 2016 Jun;33(6):982]. Adv Ther. 2016;33(6):959–981. doi:10.1007/s12325-016-0333-6
39. Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–1310. doi:10.1016/S0149-2918(01)80109-0
40. Gonnermann J, Bertelmann E, Pahlitzsch M, Maier-Wenzel AB, Torun N, Klamann MK. Contralateral eye comparison study in MICS & MIGS: trabectome® vs. iStent inject®. Graefes Arch Clin Exp Ophthalmol. 2017;255(2):359–365. doi:10.1007/s00417-016-3514-8
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




