Back to Journals » Clinical Ophthalmology » Volume 15
Microscope Integrated Optical Coherence Tomography Guided Descemet Stripping Automated Endothelial Keratoplasty in Congenital Hereditary Endothelial Dystrophy
Authors Asif MI, Bafna RK , Sharma N , Kaginalkar A, Sinha R, Agarwal T, Maharana PK, Kaur M, Taank P , Titiyal JS
Received 19 January 2021
Accepted for publication 8 April 2021
Published 27 July 2021 Volume 2021:15 Pages 3173—3181
DOI https://doi.org/10.2147/OPTH.S300286
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Supplementary video of "Mi-OCT guided DSAEK in CHED" [ID 300286].
Views: 391
Mohamed Ibrahime Asif,1 Rahul Kumar Bafna,1 Namrata Sharma,1 Ananya Kaginalkar,1 Rajesh Sinha,1 Tushar Agarwal,1 Prafulla Kumar Maharana,1 Manpreet Kaur,1 Priya Taank,2 Jeewan S Titiyal1
1Dr Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, India; 2Department of Ophthalmology, Command Hospital, Pune, 411040, India
Correspondence: Namrata Sharma
Department of Ophthalmology, Dr Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, 110029, India
Tel +91 9810856988
Fax +91 11-26588919
Email [email protected]
Purpose: To describe the outcomes of descemet stripping automated endothelial keratoplasty (DSAEK) in congenital hereditary endothelial dystrophy (CHED) and to evaluate the role of microscope integrated optical coherence tomography (Mi-OCT) during the surgery.
Design: Retrospective data analysis.
Methods: A retrospective study from the medical records of all those patients who were diagnosed with CHED and underwent DSAEK at our centre from 2015 were evaluated. All patients underwent Mi-OCT-guided standard DSAEK procedure. Intra-operative difficulties, visual outcomes and graft survival were recorded.
Results: A total of 48 eyes of 29 patients with a mean age of 9.87 ± 8.2 years and mean follow-up of 17.3 months were evaluated. Thirty-nine eyes underwent primary DSAEK and 9 eyes underwent PKP. Three eyes who underwent PKP had failed graft for which they underwent DSAEK. The mean preoperative Snellen’s visual acuity was 1.71 ± 0.66 and the mean preoperative central corneal thickness was 1.10 ± 0.174 mm. Intraoperatively, all the grafts were attached which was confirmed using Mi-OCT. Graft detachment was seen in the immediate postoperative period in 10.4% (4 eyes) of primary DSAEK, out of which DM scoring was not performed in 2 eyes. Following DSAEK, cornea cleared at four-week follow-up in 89.7% eyes and in all the eyes the cornea cleared at six-week follow-up.
Conclusion: Primary DSAEK could be a preferred option over PKP for CHED with early presentation and in those eyes with failed primary PKP. Mi-OCT is a very useful tool in these eyes for various intraoperative procedures, thereby improving the outcomes of the procedure.
Keywords: DSAEK, CHED, Mi-OCT
Introduction
Congenital hereditary endothelial dystrophy (CHED) is a rare genetic disorder which is characterized by bilateral corneal clouding due to dysfunctional and degenerative corneal endothelium.1 Though the pathology is in the corneal endothelium and Descemet membrane (DM), penetrating keratoplasty (PKP) had been the standard treatment till the time when Busin et al2 suggested that descemet stripping automated endothelial keratoplasty (DSAEK) allowed the rapid restoration of corneal clarity with minimal intra-operative and post-operative complications.
Microscope Integrated Optical Coherence Tomography (Mi-OCT) has been recently introduced which has helped to refine various surgeries.3,4 Mi-OCT provides real-time OCT images of the eye during the entire procedure which aids in various surgical steps during the entire procedure. There are few studies which have focused on the role of Mi-OCT in DSAEK.5–7 In the current study, we have evaluated its role during the surgery and also evaluated the postoperative outcomes in cases where DSAEK was done for CHED.
Materials and Methods
This study was conducted at Dr Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi. This is a retrospective study carried out at Dr Rajendra Prasad Centre for Ophthalmic Sciences, AIIMS, New Delhi, India. Ethical clearance was obtained from the institutional review board (Ref No. IECPG-291/07.09.2017, RT-37/ 29.11.2017; Institute Ethics Committee, All India Institute of Medical Sciences). Case records of all patients who were diagnosed with CHED and underwent DSAEK at our centre since 2015 were evaluated. Corneas for all our cases were donated voluntarily with written informed consent which was conducted in accordance with the Declaration of Istanbul. A written informed consent statement was obtained from all our patients. All cases were performed by experienced surgeons using Mi-OCT (OPMI Lumera 700 and RESCAN 700, Carl Zeiss, Meditec, AG, Jena, Germany). The Mi-OCT device has a commercially available platform which is fully integrated with the operating microscope and real-time OCT images are projected in a heads-up fashion on the screen as well as in the oculars. RESCAN 700 captures 27,000 A-scans/ second with an axial resolution of 5 microns. The intraoperative details of the surgery were recorded in all cases. Surgical video recordings were evaluated.
The following parameters were noted from the case records: patient demographic details, indication for surgery, preoperative parameters (best-corrected visual acuity (BCVA), intraocular pressure (IOP), central corneal thickness (CCT), associated ocular findings), intraoperative surgical details (DM scoring, lenticule thickness, lenticule diameter, complications), postoperative complications (graft detachment/ displacement and need for re-bubbling, raised IOP), BCVA, IOP and CCT at follow-ups, need for re-surgery based on graft survival].
Surgical Technique
All surgeries were performed under general anaesthesia. The surgical steps were as follows:
Donor preparation: The donor cornea was mounted on an artificial chamber (Moria ALTK; Moria, Doylestown, PA) and the donor lenticule was prepared. Mi-OCT guided donor corneal thickness was measured, and the microkeratome head was chosen accordingly. In our study, microkeratome heads ranged from 350 to 450 microns were used. The residual lenticule thickness was measured using Mi-OCT intraoperatively. Though Mi-OCT does not have a measuring scale for direct measurement, a 9 mm cube was used for analysis and the measurements were done as described.8 The lenticule was then cut using handheld disposable trephines depending on the host corneal diameter.
Recipient bed preparation: Epithelial trephination mark using disposable handheld trephine was made with the same trephine that was used to cut the donor lenticule. Two side port incisions were made using a 20-gauge microvitreoretinal blade. These incisions are moved towards the surgeon by 1–2 mm rather than the centre to avoid the pupillary region, thereby preventing inadvertent lens damage during subsequent manoeuvres. Pupils were constricted using pilocarpine 2% to avoid lens touch during instrumentation. The anterior chamber was formed with ophthalmic viscosurgical devices (OVD). Scoring of the DM was done using reverse Sinskey hook in few cases whereas in rest this step was avoided. A 23-gauge infusion cannula was used for anterior chamber maintenance during the procedure. The main incision was made either clear corneal incision using a 3.2 mm keratome blade or through the scleral tunnel using a crescent blade (Supplementary Video S1).
The prepared lenticule was loaded on the Busin glide (Moria USA, Doylestown, Pennsylvania, USA) and the lenticule was pulled inside the anterior chamber using 23-gauge Internal Limiting Membrane (ILM) peeling forceps (Grieshaber® DSP). This was followed by air injection with a 27-gauge blunt cannula through one of the side port incision after removing the anterior chamber maintainer. The entire process was monitored through Mi-OCT. Peripheral iridectomy was done in all cases.
Mi-OCT was used at various steps during the surgery right from the assessment of the posterior stroma, DM, iris and anterior chamber before the start of the procedure, to various intraoperative manoeuvres. Any area of peripheral anterior synechiae could be assessed which could help in planning the incisions. Mi-OCT was useful during the scoring of DM to ensure complete scoring without any Descemet’s or stromal tags. In hazy corneas, it was also useful to ensure the orientation and proper unfolding of extremely thin lenticules. The most important advantage of Mi-OCT was to ensure complete graft adhesion at the end of the procedure as most eyes of CHED have hazy corneas which could hamper visualization of the graft.
Results
Total of 48 eyes of 29 patients (55% female and 45% male) who had been diagnosed with CHED and had undergone Mi-OCT guided DSAEK at our institution in the past 5 years were evaluated. Diagnosis of CHED was made clinically and was confirmed with the histopathological report in the post-operative period wherever available. All the patients were phakic with a clear crystalline lens at the time of presentation.
The mean age at which surgery was performed for the patients was 9.87 ± 8.2 years (8 months to 42 years). The mean follow-up of our patients was 17.3 months (8–58 months). Most eyes with CHED had nystagmus as the most common ocular association (19/48 eyes; 39.5%). Other ocular associations that were observed were glaucoma (14/48 eyes; 29.1%), squint (9/48 eyes; 18.75%), microcornea (2/48 eyes; 4.16%) and myopia (2/48 eyes; 4.16%). Out of 14 eyes who had associated glaucoma, 13 eyes had undergone trabeculectomy and one eye underwent Diode Laser Cyclophotocoagulation (DLCP). Systemic associations were seen in 5 patients (three had hearing loss, one had hypothyroidism and one had juvenile diabetes).
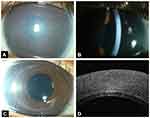
The mean preoperative Snellen’s visual acuity was 1.71 ± 0.66 (0.6–2.7) and the mean preoperative intraocular pressure was 15.5 ± 3.5 mm of Hg (8–28 mm of Hg). The mean preoperative central corneal thickness was 1.10 ± 0.174 mm (0.85–1.63 mm). The mean white to white corneal diameter in our patients was 11.5 ± 0.6 mm (10–13 mm) and mean anterior chamber depth was 2.84 ± 0.34 mm (2.24–3.66). The mean thickness of the donor cornea was 525.71 ± 40.21 microns (450–641 microns). Microkeratome head used to cut the donor tissue ranged from 350 to 450 microns. The mean lenticule thickness was 119.39 ± 39.90 microns (70–225 microns). The mean lenticule diameter was 8 mm in diameter. Thirty-nine eyes underwent DSAEK and 9 eyes underwent PKP. Three out of 39 eyes who underwent DSAEK had repeat DSAEK for failed graft. Three out of 9 patients who had PKP as their initial surgery, subsequently underwent DSAEK for failed graft. Graft failure was due to graft rejection in all our cases. Intraoperatively DM scoring was performed in 18 eyes whereas, scoring was not done in 21 eyes who underwent primary DSAEK and 3 eyes in whom a repeat DSAEK was done for failed PKP. In the primary DSAEK, scoring was not done in eyes with poor visualization due to thick and hazy corneas and in infants’ eyes. Intraoperatively, all the grafts were attached which was confirmed using Mi-OCT. Graft detachment was seen in the immediate postoperative period in 4 eyes of primary DSAEK (4/39 eyes; 10.4%) out of which scoring was not performed in 2 eyes. In all the cases the donor tissue remained attached following re-bubbling. 89.7% eyes (35/39 eyes) had clear cornea at four-week follow-up and all the eyes had clear cornea at six-week follow-up period. (Figure 1) Pupillary block resulting in a rise in IOP was noted in one eye. Mean final Snellen’s best-corrected visual acuity of our patients at the last follow-up was 0.76 ± 0.19 (0.6–2.7) and the mean intraocular pressure was 15.91 ± 2.76 mm of Hg. In our series, following DSAEK, none of the patients had newly developed glaucoma or required additional anti-glaucoma medications to their preoperative medications. The mean refraction (spherical equivalent) was 2.01 ± 2.13 (−1 to +6.25D). One patient had cataract for which lens aspiration with posterior chamber intraocular lens implantation was done. This patient had undergone DSAEK for failed PKP. Endothelial cell density could be determined for 20 eyes at the final follow-up (mean, 17.3 months; range, 8–58 months), mean endothelial loss was 36.2% (range, 14.7 to 49.3%).
Discussion
Corneal transplantation in children could be challenging due to various ocular factors such as small eyeball, shallow anterior chamber, increased positive posterior pressure, low scleral rigidity, phakic status and decreased space for intraocular manoeuvres. These factors could lead to an increased chance of complications.9 In recent times, lamellar keratoplasties are being preferred over the full thickness grafts due to various advantages. In CHED, PKP was widely performed with favourable results.10–12 However, the trend is shifting towards endothelial keratoplasties over PKP due to various reasons such as suture-related complications, increased risk of graft rejection and failure, unstable refractive outcomes and need for multiple examinations under anaesthesia in post PKP children.13–15 A study by AlArrayedh et al16 demonstrated poor outcomes from PKP in CHED due to dense amblyopia and high risk of long-term graft failure. The outcomes of PKP were better when the surgery was done at an older age when compared to the early intervention.17 Busin et al2 reported successful DSAEK outcomes in terms of rapid restoration of corneal clarity with less intraoperative and postoperative complications. In a series of 18 eyes of 10 CHED patients with a median follow-up of 38 months demonstrated favourable outcomes of DSAEK in CHED patients.18 DMEK could be challenging especially in pediatric eyes, however, results are encouraging with favourable outcomes.19 Saad et al20 performed DMEK in 14 eyes of 8 CHED patients and reported good visual outcomes.
There are various intra-operative difficulties in performing EK in CHED patients which include poor visibility due to very severe corneal oedema in these cases13 and strong adherence of the Descemet membrane to the underlying stroma which could result in DM retention and graft failure.21 Stripping of DM is much easier in decompensated corneas due to other causes such as Fuch’s endothelial dystrophy (FECD) in contrast to the CHED eyes.22 This might result in residual DM remnants which would hinder the graft apposition. Mi-OCT is a very useful modality for continuous real-time visualization and complete removal of these remnants intraoperatively especially in cases of corneal clouding, which is not possible with a conventional microscope. The usefulness of this modality has been demonstrated in PIONEER study.4 Different techniques described such as the use of chandelier illumination22 or using crescent blade metal surface against stained DM23 could aid in the removal of DM due to poor visibility. During the surgery, continuous visualization of the graft dynamics helped to perform various intraoperative manoeuvres that resulted in graft adherence in minimal time. Busin et al2 performed DSAEK without Descemet stripping in infants less than one year as DM could not be identified. Donor tissue attached and the cornea cleared within a week although 4 eyes required re-bubbling which was attributed to various other factors such as poor compliance with postoperative posturing. Ashar et al24 compared DSEK with and without Descemet stripping and concluded similar outcomes although the surgical time and intraocular manipulations were less in the latter. Various other studies have also reported that the normal DM, neither does it affect the adherence of the donor graft, nor does it influence the visual outcomes.25–29 In our cases, DM stripping was done 18 eyes whereas it was not stripped in 21 eyes among primary DSAEK eyes. DM stripping was also not done in the 3 eyes which underwent DSAEK for failed graft. Re-detachment was seen in 4 eyes (2/18 eyes; 11.1% among eyes in which DM was stripped and 2/24 eyes; 8.3% among eyes in which DM was not stripped) in the immediate post-operative period for which re-bubbling was done. There was no significant difference in terms of DM detachment whether it was stripped or not in CHED eyes.
Conventionally, in DSAEK for FECD eyes, the corneal clarity improves on the table, whereas in cases of CHED, deturgescence of cornea takes much longer time affecting the visualization of the graft. Besides, the double ring sign30 that is useful in confirmation of the graft orientation, is not always possible in CHED eyes due to thick and hazy corneas. Mi-OCT is especially useful in these cases to visualize the real-time orientation of the graft. Acute-angled bevel sign could also be useful in confirmation of the graft orientation in these cases.31
The mean final visual acuity in our study group is poorer than the final acuity of other studies.2 This could be attributed to late presentation leading to thicker and hazier corneas and poorer presenting visual acuity compared to other studies.2,25,32 Amblyopia could be one other factor for poorer visual outcomes in our patients due to thick and hazy corneas at the time of presentation. Few studies had thicker corneas at presentation similar to our study with comparable visual outcomes.33–35 The comparative data with other studies published in literature has been compiled in Table 1. The endothelial loss at the final follow-up compared to the baseline was 36.2% (range, 14.7 to 49.3%), which was comparable to other studies in the literature.2,18,36,37 Thirty-five eyes (35/39; 89.7%) developed clear cornea at the four-week follow-up and all the eyes had clear cornea at six week follow-up period in our study which was X relatively long compared to other studies.2 This could again be attributed to the relatively thicker baseline corneas in our eyes compared to the other studies. Graft rejection, the leading cause for graft failure in children38 was observed in 33% (3/9 eyes) following PKP whereas in 7.7% (3/39 eyes) following DSAEK in our series. The rejection rate observed in our case series among PKP patients was similar to other studies in the literature.38–40 To conclude, primary DSAEK could be a preferred option over PKP for CHED with early presentation and in those eyes with failed primary PKP. Mi-OCT is a very useful tool in these eyes for various intraoperative procedures, thereby improving the outcomes of the procedure.
 |  |  |
Table 1 Compilation of Various Studies of Endothelial Keratoplasty in CHED Patients |
The major limitation in our study is its retrospective nature without a control arm. A prospective randomized study with a control arm would add more value to the results. Intraoperative use of metallic instruments could result in shadowing beneath the instrument in the Mi-OCT which affected the visualization.
Funding
The authors have no funding to disclose.
Disclosure
No financial disclosures. None of the authors has any conflicts of interest for this work to disclose.
References
1. Weiss JS, Møller HU, Aldave AJ, et al. IC3D classification of corneal dystrophies–edition 2. Cornea. 2015;34(2):117–159. doi:10.1097/ICO.0000000000000307
2. Busin M, Beltz J, Scorcia V. Descemet-stripping automated endothelial keratoplasty for congenital hereditary endothelial dystrophy. Arch Ophthalmol Chic Ill 1960. 2011;129(9):1140–1146. doi:10.1001/archophthalmol.2011.114
3. Ehlers JP, Modi YS, Pecen PE, et al. The DISCOVER study 3-year results: feasibility and usefulness of microscope-integrated intraoperative OCT during ophthalmic surgery. Ophthalmology. 2018;125(7):1014–1027. doi:10.1016/j.ophtha.2017.12.037
4. Ehlers JP, Dupps WJ, Kaiser PK, et al. The prospective intraoperative and Perioperative Ophthalmic ImagiNg with Optical CoherEncE TomogRaphy (PIONEER) study: 2-year results. Am J Ophthalmol. 2014;158(5):999–1007. doi:10.1016/j.ajo.2014.07.034
5. Steverink JG, Wisse RPL. Intraoperative optical coherence tomography in descemet stripping automated endothelial keratoplasty: pilot experiences. Int Ophthalmol. 2017;37(4):939–944. doi:10.1007/s10792-016-0338-9
6. Sharma N, Priyadarshini K, Agarwal R, et al. Role of microscope-intraoperative optical coherence tomography in pediatric keratoplasty: a comparative study. Am J Ophthalmol. 2020. doi:10.1016/j.ajo.2020.07.048
7. Siebelmann S, Bachmann B, Lappas A, et al. [Intraoperative optical coherence tomography in corneal and glaucoma surgical procedures]. Ophthalmol Z Dtsch Ophthalmol Ges. 2016;113(8):646–650. doi:10.1007/s00347-016-0320-y
8. Titiyal JS, Kaur M, Sahu S, Sharma N, Sinha R. Real-time assessment of intraoperative vaulting in implantable collamer lens and correlation with postoperative vaulting. Eur J Ophthalmol. 2017;27(1):21–25. doi:10.5301/ejo.5000818
9. Vanathi M, Panda A, Vengayil S, Chaudhuri Z, Dada T. Pediatric keratoplasty. Surv Ophthalmol. 2009;54(2):245–271. doi:10.1016/j.survophthal.2008.12.011
10. Kirkness CM, McCartney A, Rice NS, Garner A, Steele AD. Congenital hereditary corneal oedema of Maumenee: its clinical features, management, and pathology. Br J Ophthalmol. 1987;71(2):130–144. doi:10.1136/bjo.71.2.130
11. Sajjadi H, Javadi MA, Hemmati R, Mirdeghan A, Parvin M, Nassiri N. Results of penetrating keratoplasty in CHED. Congenital hereditary endothelial dystrophy. Cornea. 1995;14(1):18–25.
12. Javadi MA, Baradaran-Rafii AR, Zamani M, et al. Penetrating keratoplasty in young children with congenital hereditary endothelial dystrophy. Cornea. 2003;22(5):420–423. doi:10.1097/00003226-200307000-00006
13. Pineda R, Jain V, Shome D, Hunter DC, Natarajan S. Descemet’s stripping endothelial keratoplasty: is it an option for congenital hereditary endothelial dystrophy? Int Ophthalmol. 2010;30(3):307–310. doi:10.1007/s10792-009-9315-x
14. Mittal V, Mittal R, Sangwan VS. Successful Descemet stripping endothelial keratoplasty in congenital hereditary endothelial dystrophy. Cornea. 2011;30(3):354–356. doi:10.1097/ICO.0b013e3181e8441a
15. Ashar JN, Ramappa M, Vaddavalli PK. Paired-eye comparison of Descemet’s stripping endothelial keratoplasty and penetrating keratoplasty in children with congenital hereditary endothelial dystrophy. Br J Ophthalmol. 2013;97(10):1247–1249. doi:10.1136/bjophthalmol-2012-302602
16. AlArrayedh H, Collum L, Murphy CC. Outcomes of penetrating keratoplasty in congenital hereditary endothelial dystrophy. Br J Ophthalmol. 2018;102(1):19–25. doi:10.1136/bjophthalmol-2016-309565
17. Özdemir B, Kubaloğlu A, Koytak A, et al. Penetrating keratoplasty in congenital hereditary endothelial dystrophy. Cornea. 2012;31(4):359–365. doi:10.1097/ICO.0b013e31823d03af
18. Mohebbi M, Nabavi A, Fadakar K, Hashemi H. Outcomes of descemet-stripping automated endothelial keratoplasty in congenital hereditary endothelial dystrophy. Eye Contact Lens. 2020;46(1):57–62. doi:10.1097/ICL.0000000000000604
19. Srinivasan B, Agarwal M, Iyer G, Agarwal S, Padmanabhan P. Pediatric Descemet’s membrane endothelial keratoplasty. Am J Ophthalmol. 2021. doi:10.1016/j.ajo.2021.02.011
20. Saad A, Ghazzal W, Keaik M, Indumathy TR, Fogla R. Outcomes of Descemet’s membrane endothelial keratoplasty for congenital hereditary endothelial dystrophy. J AAPOS off Publ Am Assoc Pediatr Ophthalmol Strabismus. 2020. doi:10.1016/j.jaapos.2020.07.018
21. Nikolic L, Jovanovic V, Lackovic V, Todorovic V. Endothelial keratoplasty without descemet’s membrane stripping: histologic and ultrastructural findings. Ophthalmic Res. 2010;43(1):56–60. doi:10.1159/000246579
22. Ashar JN, Madhavi Latha K, Vaddavalli PK. Descemet’s stripping endothelial keratoplasty (DSEK) for children with congenital hereditary endothelial dystrophy: surgical challenges and 1-year outcomes. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2012;250(9):1341–1345. doi:10.1007/s00417-012-2014-8
23. Sharma N, Sharma VK, Arora T, Singh KR, Agarwal T, Vajpayee RB. Novel technique for descemet membrane remnant stripping in hazy cornea during DSAEK. Cornea. 2016;35(1):140–142. doi:10.1097/ICO.0000000000000659
24. Ashar JN, Ramappa M, Chaurasia S. Endothelial keratoplasty without Descemet’s stripping in congenital hereditary endothelial dystrophy. J AAPOS off Publ Am Assoc Pediatr Ophthalmol Strabismus. 2013;17(1):22–24. doi:10.1016/j.jaapos.2012.09.013
25. Bellucci R, Chierego C, Bellucci C. Endothelial keratoplasty in a newborn baby with CHED. Cornea. 2011;30(12):1488–1490. doi:10.1097/ICO.0b013e318221c2f3
26. Anwar HM, El Danasoury A, Hashem A. Descemet’s stripping automated endothelial keratoplasty for congenital hereditary endothelial dystrophy. Clin Ophthalmol Auckl NZ. 2012;6:159–163. doi:10.2147/OPTH.S28405
27. Price FW, Price MO. Endothelial keratoplasty to restore clarity to a failed penetrating graft. Cornea. 2006;25(8):895–899. doi:10.1097/01.ico.0000227888.03877.22
28. Caldwell MC, Afshari NA, Decroos FC, Proia AD. The histology of graft adhesion in descemet stripping with endothelial keratoplasty. Am J Ophthalmol. 2009;148(2):277–281. doi:10.1016/j.ajo.2009.03.025
29. Kobayashi A, Yokogawa H, Sugiyama K. Non-Descemet stripping automated endothelial keratoplasty for endothelial dysfunction secondary to argon laser iridotomy. Am J Ophthalmol. 2008;146(4):543–549. doi:10.1016/j.ajo.2008.05.028
30. Delfazayebaher S, Feizi S, Javadi MA, Baradaran-Rafii A, Sadoughi MM, Faramarzi A. Double-ring sign to confirm correct orientation of donor lenticules during descemet stripping automated endothelial keratoplasty. Cornea. 2015;34(8):980–984. doi:10.1097/ICO.0000000000000485
31. Titiyal JS, Kaur M, Shaikh F, Bari A. “Acute-angled bevel” sign to assess donor lenticule orientation in ultra-thin descemet stripping automated endothelial keratoplasty. BMJ Case Rep. 2019;12(2). doi:10.1136/bcr-2018-227927
32. Lenhart PD, Evans CT, Beck AD, Lee WB. Visual outcome after Descemet’s stripping automated endothelial keratoplasty in an 8-month-old with congenital hereditary endothelial dystrophy. J AAPOS off Publ Am Assoc Pediatr Ophthalmol Strabismus. 2013;17(6):637–639. doi:10.1016/j.jaapos.2013.08.005
33. Goshe JM, Li JY, Terry MA. Successful Descemet’s stripping automated endothelial keratoplasty for congenital hereditary endothelial dystrophy in a pediatric patient. Int Ophthalmol. 2012;32(1):61–66. doi:10.1007/s10792-011-9511-3
34. Vajpayee RB, Maharana PK, Jain S, Sharma N, Jhanji V. Thin lenticule Descemet’s stripping automated endothelial keratoplasty: single, slow pass technique. Clin Experiment Ophthalmol. 2014;42(5):411–416. doi:10.1111/ceo.12271
35. Panahi-Bazaz M, Sharifipour F, Malekahmadi M. Modified descemet’s stripping automated endothelial keratoplasty for congenital hereditary endothelial dystrophy. J Ophthalmic Vis Res. 2014;9(4):522–525. doi:10.4103/2008-322X.150836
36. Yang F, Hong J, Xiao G, et al. Descemet stripping endothelial keratoplasty in pediatric patients with congenital hereditary endothelial dystrophy. Am J Ophthalmol. 2020;209:132–140. doi:10.1016/j.ajo.2019.08.010
37. Madi S, Santorum P, Busin M. Descemet stripping automated endothelial keratoplasty in pediatric age group. Saudi J Ophthalmol off J Saudi Ophthalmol Soc. 2012;26(3):309–313. doi:10.1016/j.sjopt.2012.04.006
38. Yang LL, Lambert SR, Lynn MJ, Stulting RD. Long-term results of corneal graft survival in infants and children with peters anomaly. Ophthalmology. 1999;106(4):833–848. doi:10.1016/S0161-6420(99)90175-6
39. Comer RM, Daya SM, O’Keefe M. Penetrating keratoplasty in infants. J AAPOS off Publ Am Assoc Pediatr Ophthalmol Strabismus. 2001;5(5):285–290. doi:10.1067/mpa.2001.117568
40. Vajpayee RB, Ray M, Panda A, et al. Risk factors for pediatric presumed microbial keratitis: a case-control study. Cornea. 1999;18(5):565–569.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.