Back to Journals » Drug Design, Development and Therapy » Volume 14
Miconazole Contributes to NRF2 Activation by Noncanonical P62-KEAP1 Pathway in Bladder Cancer Cells
Authors Tsai TF , Chen PC , Lin YC, Chou KY, Chen HE, Ho CY, Lin JF , Hwang TIS
Received 19 August 2019
Accepted for publication 18 January 2020
Published 24 March 2020 Volume 2020:14 Pages 1209—1218
DOI https://doi.org/10.2147/DDDT.S227892
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jianbo Sun
Te-Fu Tsai,1,2 Po-Chun Chen,3,4 Yi-Chia Lin,1,2 Kuang-Yu Chou,1,2 Hung-En Chen,1 Chao-Yen Ho,1,5 Ji-Fan Lin,3 Thomas I-Sheng Hwang1,2,6
1Division of Urology, Department of Surgery, Shin-Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan; 2Division of Urology, School of Medicine, Fu-Jen Catholic University, New Taipei, Taiwan; 3Central Laboratory, Shin-Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan; 4Department of Biotechnology, College of Health Science, Asia University, Taichung, Taiwan; 5Institute of Traditional Medicine, School of Medicine, National Yang-Ming University, Taipei, Taiwan; 6Department of Urology, Taipei Medical University, Taipei, Taiwan
Correspondence: Thomas I-Sheng Hwang
Division of Urology, Department of Surgery, Shin Kong Wu Ho-Su Memorial Hospital, Taipei 11102, Taiwan
Tel +886-2-28332211 Ext 2065
Fax +886-2-28389404
Email [email protected]
Po-Chun Chen Email [email protected]
Purpose: Nuclear factor (erythroid-derived 2)-like 2, also known as NFE2L2 or NRF2, a transcription factor capable of upregulating antioxidant response element (ARE)-mediated expression and cytoprotective proteins, plays critical roles in chemoprevention, inflammation and aging. NRF2 has recently been proposed as a novel target for cancer chemoprevention. The fungicide miconazole has shown promising antiproliferative effects in cancer cells.
Materials and Methods: After miconazole treatment, the p62-KEAP1-NRF2 activation was analyzed by qPCR and Western blot. The nuclear translocation indicating NRF2 activation was further confirmed by immunofluorescence. Finally, the ROS production was detected by CM-H2DCFDA staining.
Results: We demonstrate in this study that miconazole dramatically increases NRF2 activation in bladder cancer cells, in a dose- and time-dependent manner. Interestingly, levels of expression of p62, a noncanonical pathway that mediates NRF2 activation, appeared to increase in accordance with NRF2. We also investigated levels of the negative regulator kelch-like ECH-associated protein 1 (KEAP1), which is involved in NRF2 activation. As expected, a decrease in KEAP1 expression was found after miconazole exposure. Confirmation of NRF2 nuclear translocation was monitored by immunofluorescence. Miconazole-induced generation of reactive oxygen species (ROS) promoted NRF2 activation. Pretreatment of bladder cancer cells with ROS scavengers abolished NRF2 expression and nuclear translocation, indicating that miconazole activates the noncanonical p62-KEAP1-NRF2 pathway, which is regulated by ROS production.
Conclusion: Our study elucidates the mechanisms through which miconazole stimulates NRF2 which may contribute to cancer chemopreventive effects.
Keywords: miconazole, NRF2, p62, KEAP1, bladder cancer
Introduction
Nuclear factor erythroid 2-related factor 2 (NRF2) is a 66-kDa transcription factor that coordinates cellular stress response thus against oxidative stress.1 Considerable evidence shows that NRF2 activators can prevent not only tumor progression but also various chronic diseases in which oxidative and inflammatory stress are crucial for pathogenesis.2–4 Ongoing clinical trials are investigating the antitumor activity of several NRF2 activators, including sulforaphane,5 curcumin,6 dimethyl fumarate,7 bardoxolone methyl8 and resveratrol.9 These agents may offer new a strategy for preventing tumor progression.10
It is well established that kelch-like ECH-associated protein 1 (KEAP1)-NRF2 system activation is the major oxidative stress response pathway.11 Analyses of the molecular components involved in NRF2 activation have demonstrated that its transcriptional activity is inhibited by KEAP1, a cytoplasmic, cysteine-rich, actin-bound protein that sequesters NRF2 in the cytoplasm. Under normal conditions, the transcription factor NRF2 is constitutively inactivated by ubiquitin-proteasome degradation. KEAP1, an adaptor of the ubiquitin ligase complex that targets NRF2, plays a major role in NRF2 degradation.12 When cells encounter oxidative stress or NRF2 activators, NRF2 quickly disassociates from KEAP1 and inhibits NRF2 degradation.13 Subsequently, the NRF2 translocates to the nucleus and regulates anti-oxidative stress genes by binding to antioxidant response elements (AREs).14 The noncanonical pathway by which p62/sequestosome 1 (SQSTM1), a key autophagy adaptor, promotes NRF2 nuclear translocation and activates ARE-related genes was reported in 2007.15 Subsequent investigations found that the interaction between p62 and KEAP1 enables p62 to sequester KEAP1 into autophagosomes and abrogate the ubiquitin-proteasome degradation of NRF2, preventing NRF2 activation.16,17 Previous findings have indicated that overexpression of p62 increases the degradation of KEAP1, suggesting that p62 plays a critical role in regulating KEAP1 turnover.18
Miconazole has long been used as an effective antifungal drug in humans.19 Systemic fungal infection is the major cause of morbidity and mortality in cancer patients, especially those with neutropenia. Antifungals, including miconazole, have been used as adjuvant therapy to decrease mortality in cancer patients with neutropenia.20,21 Miconazole exhibits antiproliferative effects in cancer cells.22 In an antitumor drug screening study, miconazole inhibited cell proliferation in breast cancer and bladder cancer.23 In preclinical testing, miconazole demonstrated antitumor effects through promoting cell apoptosis and G0/G1 cell cycle arrest in colon cancer.24 Miconazole has an inhibitory effect on melanogenesis in B16 melanoma cells25 and its antioxidative effects have already been investigated.26 However, the mechanisms involved in NRF2 activation of miconazole have not been well described. This study describes how miconazole promotes NRF2 activation and the key role played by the p62-KEAP noncanonical pathway in this activation.
Materials and Methods
Materials
Anti-mouse and anti-rabbit IgG-conjugated horseradish peroxidase, rabbit polyclonal antibodies specific for p62 (Abcam; Cambridge, UK; cat. No. ab109012), KEAP1 (Cell Signaling Technology, Inc.; Danvers, MA, USA; cat. No. #8047), NRF2 (GeneTex, Inc.; San Diego, CA, USA; cat. No. GTX103322) and β-Tubulin (Cell Signaling Technology, Inc.; Danvers, MA, USA; cat. No. #2146) were purchased from commercial companies. All other chemicals were obtained from Sigma-Aldrich (St Louis, MO, USA).
Cell Culture
Human bladder cancer cell lines (5637 and T24) were obtained from Bioresource Collection and Research Center (BCRC; Hsinchu, Taiwan) and maintained at 37°C under 5% CO2. 5637 and T24 cells were cultured in RPMI-1640 and McCoy’s 5a media, respectively. Culture media were supplemented with 10% FBS, 2 mM GlutaMAX-1, 100 units/mL penicillin and 100 μg/mL streptomycin. Cells were seeded on 96-well plates (5 x 103/well) or 6-well plates (5 x 105/well) or 10-cm dishes, 2 x 106/dish for cell viability assays and collection of RNA or protein samples.
Western Blot Analysis
The cellular lysates were prepared and proteins were resolved on SDS-PAGE and transferred to Immobilon polyvinyldifluoride (PVDF) membranes. The blots were blocked with 4% BSA for 1 hr at room temperature and then probed with mouse or rabbit primary antibodies (1:1000) overnight at 4°C. After undergoing three TBST washes, the blots were incubated with anti-rabbit or -mouse peroxidase-conjugated secondary antibody (1:1000) for 1 hr at room temperature. After another three washes with TBST, the blots were visualized with enhanced chemiluminescence using a ChemiDoc-It® Imaging System (UVP Inc., Upland, CA, USA). ImageJ software (National Institutes of Health, USA) was used to quantify Western blot results.
Quantitative Real-Time PCR
Quantitative real-time PCR (qPCR) analysis was carried out using Taqman® one-step PCR Master Mix (Applied Biosystems, Foster City, CA, USA). One hundred ng of total cDNA was added per 20-µL reaction with sequence-specific primers and Taqman probes. Sequences for all target gene primers and probes were purchased commercially (β-actin served as the internal control) from Applied Biosystems. Quantitative RT-PCR assays were carried out in triplicate on a StepOnePlus sequence detection system (Applied Biosystems). Cycling conditions consisted of 10 min of polymerase activation at 95°C followed by 40 cycles at 95°C for 15 sec and 60°C for 60 sec. The threshold was set above the non-template control background and within the linear phase of target gene amplification to calculate the cycle number at which the transcript was detected (denoted as CT).
Immunofluorescence Staining
BC cells were seeded on chamber slides (5 x 103 cells/well) for 24 hrs before the experiment. After treatment with miconazole alone or in the presence with ROS inhibitors (catalase, DPI and NAC) for 24 hrs. Cells were washed three times with PBS and fixed in 3.7% formaldehyde for 10 mins at room temperature. Cells were washed three times with PBS and blocked with 4% BSA for 15 mins. Cells were then incubated with the p62 (Abcam; Cambridge, UK; cat. No. ab109012) or NRF2 (Genetex; cat. No. GTX103322) primary antibodies (1:100) overnight at 4°C. Twenty-four hour later, the cells were washed again by PBS for three times and incubated with Alexa Fluor 488 secondary antibody (1:100) for 1 hr. Finally, cells were washed, mounted with DAPI-containing solution, and photographed with a Nikon Ti2 microscopy System.
ROS Detection
Molecular Probes™ CM-H2DCFDA was purchased from Thermo Fisher Scientific (Waltham, MA, USA) to examine the production of ROS in bladder cancer cells. The cells were incubated with different concentrations of miconazole (0, 12.5, 25, or 50 μM) for 24 hrs, were washed twice with PBS, trypsinized into PBS and centrifuged at 5000 g for 3 mins at 4°C. Then, collected cells were incubated with 25 μM CM-H2DCFDA for 45 mins at 37°C. The cells were washed, and CM-H2DCFDA fluorescence in the cells was monitored using a flow cytometer.
Statistics
All experiments were performed at least three times, each time in triplicate. Data from two experimental groups were analyzed using the Student’s t test and multiple group comparisons were performed using one-way analysis of variance (one-way ANOVA) with Bonferroni’s post hoc tests. Statistical significance was expressed as the mean ± standard deviation (S.D.). A p-value of <0.05 was considered statistically significant.
Results
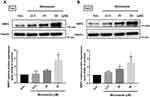
Miconazole Treatment Contributes to NRF2 Protein Expression in Bladder Cancer Cells
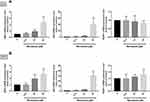
To examine the effects of miconazole on NRF2 expression, T24 and 5637 bladder cancer cells were incubated with miconazole (24 hrs) in different concentrations (0, 6.25, 12.5, or 50 μM). Levels of NRF2 protein expression were investigated by Western blot. As shown in Figure 1, the treatment of bladder cancer cells with miconazole increased NRF2 protein expression in a dose-dependent manner.
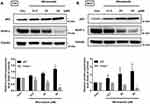
Miconazole Treatment Activates a P62-KEAP1 Noncanonical Pathway Responsible for NRF2 Activation
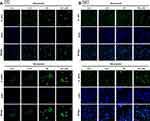
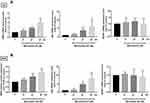
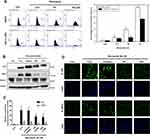
To explore the mechanism that regulates NRF2 activation, we investigated levels of KEAP1 and p62 expression, which lead to noncanonical activation of NRF2 after miconazole incubation. We found that miconazole treatment inhibited KEAP1 protein expression and increased p62 expression, suggesting the activation of the p62-KEAP1 noncanonical pathway (Figure 2). When we examined the effect of miconazole on NRF2 expression in bladder cancer cells incubated with miconazole (25 μM) for different time intervals (0, 12, 24, or 48 h), miconazole promoted NRF2 protein expression level in a time-dependent manner (Figure 3A and B), via the p62-KEAP1 noncanonical pathway examined by p62 siRNA (Figure 3C). In order to confirm NRF2 activation after miconazole treatment, we investigated NRF2 nuclear translocation by cell immunofluorescence. The data revealed dose-dependent increases in NRF2 nuclear translocation and p62 protein expression in the presence of miconazole in bladder cancer cells (Figure 4).
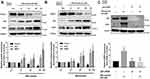
Miconazole Increases P62 and NRF2 Transcription Levels in Bladder Cancer Cells
We next evaluated the transcription levels of NRF2 and its corresponding regulators, p62 and KEAP1, after miconazole treatment in bladder cancer cells. The cells were treated with miconazole in dose- and time-course experiments as described in Figures 1 and 3, and real-time qPCR determined NRF2, p62 and KEAP1 mRNA expression. Miconazole promoted increases in mRNA expression levels of NRF2 and p62 (Figures 5 and 6), although no significant changes in KEAP1 transcription levels were observed following miconazole exposure. This lack of response suggests that miconazole may suppress KEAP1 protein expression through post-translation regulation such as p62-dependent proteasomal degradation.
ROS Generation Is Required for Activation of the P62-KEAP1-NRF2 Noncanonical Pathway After Miconazole Exposure
NRF2 activation is the major regulator of cytoprotective responses to ROS-induced oxidative stress and restores redox balance.27 We, therefore, evaluated ROS production after miconazole incubation. We found that miconazole increased ROS levels in bladder cancer cells, and pretreatment with the ROS scavenger N-acetylcysteine (NAC) reversed ROS production (Figure 7A). To ascertain whether ROS production is responsible for the p62-KEAP1 noncanonical pathway that activates NRF2, we pretreated bladder cancer cells with ROS scavengers including catalase, diphenyleneiodonium (DPI) and NAC, followed by miconazole exposure. Levels of p62 and NRF2 expression were assessed by Western blot and real-time qPCR. We found that NAC and DPI, but not catalase, dramatically abrogated p62 and NRF2 up-regulation after miconazole treatment, suggesting that miconazole-induced NRF2 activation is mediated through the superoxide-generating NADPH oxidase (NOX) (Figure 7B–D).
Discussion
It is well established that NRF2 plays a critical role in adaptive cellular responses to oxidative stress.1 The discovery of novel NRF2 activators has attracted attention for their therapeutic potential in chronic inflammation and cancer. Our present study shows that miconazole, a widely used antifungal agent, promotes NRF2 expression through the noncanonical p62-KEAP1 pathway in bladder cancer cells. Miconazole also promotes ROS generation, which is responsible for p62-KEAP1-NRF2 expression in bladder cancer. Many natural and synthetic compounds have been identified as NRF2 activators and implicated in cancer therapy including sulforaphane, curcumin, resveratrol, lycopene, oleanane triterpenoid.28 Our findings conclusively support the use of miconazole in a novel application, as an NRF2 activator in the treatment of bladder cancer.
Typical NRF2 activation is accompanied by modification of KEAP1 cysteine residues that protect NRF2 from ubiquitylation, allowing NRF2 accumulation and subsequently nuclear translocation. Finally, NRF2 binds to its responsible DNA element AREs and exerts cytoprotective functions.29,30 Here, we found that miconazole improved levels of p62 protein expression, whereas miconazole suppressed KEAP1 in bladder cancer cells. qPCR results indicated a close alignment between p62 mRNA expression and protein levels. However, transcription levels of KEAP1 were not decreased after treatment with miconazole. This finding does not match with our investigations in protein expression levels, revealing that KEAP1 down-regulation is caused by translational or post-translational regulation. A previous report indicates that p62 accumulation in the noncanonical pathway interacts with KEAP1, sequestering KEAP1 into the autophagosomes, abrogating the ubiquitin-proteasomal degradation of NRF2 and leading to activation of the NRF2 response genes.31 Moreover, a previous study has indicated that KEAP1 serves as a p62-regulated substrate for autophagy-mediated degradation.18 Our results suggest that miconazole-induced inhibition of KEAP1 protein expression may be controlled by p62-mediated degradation. The detailed mechanisms need to be explored in future research.
NRF2 is a major cellular defense regulator responsible for controlling those genes whose biological functions participate in the elimination of reactive oxidants. Through binding to its cis-acting element ARE, NRF2 can drive the expression of various antioxidant enzymes such as glutathione-s-transferase (GST), NAD(P)H quinone reductase 1 (NQO1) and heme oxygenase (HO-1).32 Alteration of the cellular redox balance elevates ROS and triggers the transcriptional activity of NRF2.1 Our results demonstrate that miconazole improved ROS production in bladder cancer cells, which in turn elicited NRF2 activation. Pretreatment with ROS scavengers abolished NRF2 expression and nuclear translocation, revealing the pivotal role of ROS in miconazole-induced NRF2 activation. Interestingly, only the ROS scavenger NAC and the NOX-specific inhibitor DPI reversed this response, whereas hydrogen peroxide (H2O2) scavenger catalase had no such effect, suggesting that miconazole triggered the NRF2 pathway via NOX-dependent regulation.
Conclusion
Miconazole is a commonly used antifungal agent in the treatment of fungal infection, and more recently, has been used in the treatment of different tumors.23–25 We have identified that miconazole is a novel NRF2 activator that may possess chemoprotective properties against antioxidant stress as well as chemopreventive properties. Our study confirms that miconazole promotes NRF2 activation through the p62-KEAP1 noncanonical pathway. In conclusion, our data add to the growing body of evidence indicating that NRF2 activators could be used as safe and effective agents for the chemoprevention of cancer and many other diseases.33 Preclinical testing of miconazole is warranted.
Acknowledgments
This work was supported by the Ministry of Science and Technology, Taiwan (MOST-106-2314-B-341-005-, MOST-107-2314-B-341-001-) and Shin Kong Wu Ho-Su Memorial Hospital (SKH-8302-105-0201, SKH-8302-106-0201, 2018SKHBDR001, 2019SKHBDR005).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284(20):13291–13295. doi:10.1074/jbc.R900010200
2. Calkins MJ, Johnson DA, Townsend JA, et al. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid Redox Signal. 2009;11(3):497–508. doi:10.1089/ars.2008.2242
3. Kwak MK, Kensler TW. Targeting NRF2 signaling for cancer chemoprevention. Toxicol Appl Pharmacol. 2010;244(1):66–76. doi:10.1016/j.taap.2009.08.028
4. Boutten A, Goven D, Artaud-Macari E, Boczkowski J, Bonay M. NRF2 targeting: a promising therapeutic strategy in chronic obstructive pulmonary disease. Trends Mol Med. 2011;17(7):363–371. doi:10.1016/j.molmed.2011.02.006
5. Kensler TW, Ng D, Carmella SG, et al. Modulation of the metabolism of airborne pollutants by glucoraphanin-rich and sulforaphane-rich broccoli sprout beverages in Qidong, China. Carcinogenesis. 2012;33(1):101–107. doi:10.1093/carcin/bgr229
6. Shureiqi I, Baron JA. Curcumin chemoprevention: the long road to clinical translation. Cancer Prev Res (Phila). 2011;4(3):296–298. doi:10.1158/1940-6207.CAPR-11-0060
7. Linker RA, Lee DH, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134(Pt 3):678–692. doi:10.1093/brain/awq386
8. Pergola PE, Raskin P, Toto RD, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365(4):327–336. doi:10.1056/NEJMoa1105351
9. Palsamy P, Subramanian S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochim Biophys Acta. 2011;1812(7):719–731. doi:10.1016/j.bbadis.2011.03.008
10. Zhao CR, Gao ZH, Qu XJ. Nrf2-ARE signaling pathway and natural products for cancer chemoprevention. Cancer Epidemiol. 2010;34(5):523–533. doi:10.1016/j.canep.2010.06.012
11. Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34(4):176–188. doi:10.1016/j.tibs.2008.12.008
12. Sporn MB, Liby KT. NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer. 2012;12(8):564–571. doi:10.1038/nrc3278
13. Dinkova-Kostova AT, Holtzclaw WD, Kensler TW. The role of Keap1 in cellular protective responses. Chem Res Toxicol. 2005;18(12):1779–1791. doi:10.1021/tx050217c
14. Itoh K, Chiba T, Takahashi S, et al. An Nrf2/small Maf heterodimer mediates the induction of Phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236(2):313–322. doi:10.1006/bbrc.1997.6943
15. Liu Y, Kern JT, Walker JR, Johnson JA, Schultz PG, Luesch H. A genomic screen for activators of the antioxidant response element. Proc Natl Acad Sci U S A. 2007;104(12):5205–5210. doi:10.1073/pnas.0700898104
16. Komatsu M, Kurokawa H, Waguri S, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12(3):213–223. doi:10.1038/ncb2021
17. Lau A, Wang XJ, Zhao F, et al. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol Cell Biol. 2010;30(13):3275–3285. doi:10.1128/MCB.00248-10
18. Copple IM, Lister A, Obeng AD, et al. Physical and functional interaction of sequestosome 1 with Keap1 regulates the Keap1-Nrf2 cell defense pathway. J Biol Chem. 2010;285(22):16782–16788. doi:10.1074/jbc.M109.096545
19. Garcia-Cuesta C, Sarrion-Perez MG, Bagan JV. Current treatment of oral candidiasis: a literature review. J Clin Exp Dent. 2014;6(5):e576–e582. doi:10.4317/jced.51798
20. Gotzsche PC, Johansen HK. Routine versus selective antifungal administration for control of fungal infections in patients with cancer. Cochrane Database Syst Rev. 2014;9:CD000026.
21. Bensadoun RJ, Daoud J, El Gueddari B, et al. Comparison of the efficacy and safety of miconazole 50-mg mucoadhesive buccal tablets with miconazole 500-mg gel in the treatment of oropharyngeal candidiasis: a prospective, randomized, single-blind, multicenter, comparative, Phase III trial in patients treated with radiotherapy for head and neck cancer. Cancer. 2008;112(1):204–211. doi:10.1002/cncr.23152
22. Kaur R, Dwivedi AR, Kumar B, Kumar V. Recent developments on 1,2,4-triazole nucleus in anticancer compounds: a review. Anticancer Agents Med Chem. 2016;16(4):465–489. doi:10.2174/1871520615666150819121106
23. Shahbazfar AA, Zare P, Ranjbaran M, et al. A survey on anticancer effects of artemisinin, iron, miconazole, and butyric acid on 5637 (bladder cancer) and 4T1 (Breast cancer) cell lines. J Cancer Res Ther. 2014;10(4):1057–1062. doi:10.4103/0973-1482.137975
24. Wu CH, Jeng JH, Wang YJ, et al. Antitumor effects of miconazole on human colon carcinoma xenografts in nude mice through induction of apoptosis and G0/G1 cell cycle arrest. Toxicol Appl Pharmacol. 2002;180(1):22–35. doi:10.1006/taap.2002.9352
25. Mun YJ, Lee SW, Jeong HW, Lee KG, Kim JH, Woo WH. Inhibitory effect of miconazole on melanogenesis. Biol Pharm Bull. 2004;27(6):806–809. doi:10.1248/bpb.27.806
26. Wiseman H, Smith C, Arnstein HR, Halliwell B, Cannon M. The antioxidant action of ketoconazole and related azoles: comparison with tamoxifen and cholesterol. Chem Biol Interact. 1991;79(2):229–243. doi:10.1016/0009-2797(91)90085-L
27. Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal. 2005;7(3–4):385–394. doi:10.1089/ars.2005.7.385
28. Milkovic L, Zarkovic N, Saso L. Controversy about pharmacological modulation of Nrf2 for cancer therapy. Redox Biol. 2017;12:727–732. doi:10.1016/j.redox.2017.04.013
29. Itoh K, Ishii T, Wakabayashi N, Yamamoto M. Regulatory mechanisms of cellular response to oxidative stress. Free Radic Res. 1999;31(4):319–324. doi:10.1080/10715769900300881
30. Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. 2003;23(22):8137–8151. doi:10.1128/MCB.23.22.8137-8151.2003
31. Jiang T, Harder B, de la Vega MR, Wong PK, Chapman E, Zhang DD. p62 links autophagy and Nrf2 signaling. Free Radic Biol Med. 2015;88(Pt B):199–204. doi:10.1016/j.freeradbiomed.2015.06.014
32. Lee JS, Surh YJ. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 2005;224(2):171–184. doi:10.1016/j.canlet.2004.09.042
33. Giudice A, Arra C, Turco MC. Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Methods Mol Biol. 2010;647:37–74.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.