Back to Journals » Clinical Ophthalmology » Volume 14
Methylene Blue Inhibits Acute Hemorrhagic Conjunctivitis Virus Production and Induction of Caspase-3 Mediated Human Corneal Cell Cytopathy
Authors Langford MP , Sebren AR, Burch MA, Redens TB
Received 7 August 2020
Accepted for publication 4 December 2020
Published 23 December 2020 Volume 2020:14 Pages 4483—4492
DOI https://doi.org/10.2147/OPTH.S275762
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Marlyn P Langford, Alexandra R Sebren, Maxwell A Burch, Thomas B Redens
Department of Ophthalmology, Louisiana State University Health Sciences Center, Shreveport, LA 71130, USA
Correspondence: Marlyn P Langford
Department of Ophthalmology, Louisiana State University Health Sciences Center, 1501 Kings Hwy/PO Box 33932, Shreveport, LA 71130-3932, USA
Tel +1 (318) 675-5018
Fax +1 (318) 675-6000
Email [email protected]
Background: Acute hemorrhagic conjunctivitis (AHC) is a highly contagious eye disease caused by enterovirus type 70 (E70) and Coxsackievirus A24 variant (CA24v) with no clinically approved treatment. The antiviral activity of methylene blue (MB; a WHO essential medicine) against AHC viruses was investigated using human corneal epithelial cells (HCEC).
Methods: Time and concentration-dependent MB accumulation by HCEC was determined colorimetrically and MB inhibition of virus production of 5 E70 and 3 CA24v AHC epidemic isolates in HCEC was determined by micro-plaque assay. AHC virus cytopathy inhibition by MB was detected by reductions in virus-induced caspase-3 activity and polymeric DNA fragments.
Results: MB uptake by HCEC was rapid and concentration dependent. MB inhibition of E70 and CA24v production was concentration dependent. AHC virus yields were significantly lower (50 to > 10,000 fold) in HCEC pre-treated with 0.25– 1% MB than in placebo controls (p’s ≤ 0.01). MB pre-treatment significantly inhibited virus-induced caspase-3 activation and DNA fragmentation (p’s< 0.01). Virus-infected cells accumulate oxidized MB and MB application up to 6 h after infection inhibited virus production and virus-induced HCEC cytopathy.
Conclusion: The results suggest MB treatment prior to and shortly after infection can inhibit AHC virus production and caspase-mediated HCEC cytopathy. The results support the therapeutic potential of ophthalmic solutions containing MB against AHC virus infection during epidemics.
Keywords: Coxsackievirus A24, eye, infection, enterovirus 70, epidemic, caspase, apoptosis
Introduction
Acute hemorrhagic conjunctivitis (AHC) is a highly contagious picornaviral eye infection characterized by a short incubation period (6–12 h) followed by an acute onset of symptoms (over 3–5h).1 Bilateral AHC is common and characterized by foreign body sensation, profuse tearing, itching, lid edema, and erythema, but may also include chemosis (subconjunctival fluid accumulation), photophobia, and subconjunctival hemorrhage.2 Infection of the corneal epithelium occurs in up to 60% of epidemic AHC cases.3–5 The acute ocular symptoms are caused by enterovirus 70 (E70) and Coxsackievirus A24 variant (CA24v)-induced caspase-mediated cell death of conjunctival and corneal epithelial cells.1,2,6 AHC usually resolves in 10–14 days as serum antibody titers rise.2,7
Outbreaks due to sporadic cases or spread by infected travelers can quickly become explosive reaching epidemic proportions over a few weeks. Acute hemorrhagic conjunctivitis (AHC) is transmitted primarily through hand-to-eye-to-hand contact. Overcrowded housing, poor sanitation, high humidity, and school/factory exposure facilitate spread during outbreaks with high transmission rates in families.2 Epidemics and pandemics have occurred worldwide since the first epidemics reported in 1969–1971 in Accra, Indonesia, Singapore, and Hong Kong.8–10 Within the past 5 years, epidemics have occurred in China, Central and South America.11–14
Vaccine or therapeutic clinical trials have not been reported. Precautionary actions are employed currently to inhibit transmission, mass hysteria, and economic loss during epidemics. Home remedies and topical corticosteroids are discouraged as they can predispose an already compromised cornea to secondary infections and toxicity.15,16
Based upon the inhibition of poliovirus by methylene blue (MB),17 the effect of MB on E70 and CA24v infection of human corneal epithelial cells (HCEC) was investigated in vitro. MB was rapidly taken up by HCEC and intracellular MB inhibited E70 and CA24v virus production. Moreover, MB inhibited HCEC cytopathy mediated by AHC virus activation of caspase-3 and endonuclease (DNA fragmentation). The results suggest that low MB concentrations exhibit antiviral activity and advocate short-term judicious use of ophthalmic solutions containing MB to inhibit AHC during epidemics.
Materials and Methods
Cell Culture and Reagents
Transformed human corneal epithelial cells (HCEC) were a gift from Dr. Zan Pan (Department of Ophthalmology, Dyson Vision Research Institute, Weill Cornell Medical College, New York, NY).18 [As the HCEC line used was not purchased from an accredited commercial source, the use of the human cells was sought and approved by our institutional ethics committee.] The HCEC were maintained in Dulbecco’s minimum essential medium (DMEM) (Corning Mediatech, Inc., Manassas, VA) supplemented with 10% newborn bovine calf serum (GE Healthcare Life Sciences, Hyclone Laboratories, Logan, UT). Experiments were performed in confluent HCEC in Costar® 6-well culture plates or 96-well cell culture cluster plates (Corning Incorporated, Corning, NY) utilizing DMEM supplemented with 2% newborn calf serum and antibiotics (Penicillin-Streptomycin-Neomycin Solution, Sigma-Aldrich, St. Louis, MO).
AHC Viruses (E70 and CA24v Isolates)
Five E70 and 3 CA24v epidemic isolates were evaluated in this study. The E70 prototype J670/71 isolated in Hokkaido, Japan was provided by Dr. Reisaku Kono (Central Virus Diagnostic Laboratory, National Institutes of Health, Tokyo, Japan).9 Epidemic isolates of E70 from Morocco (R20/71), Karachi, Pakistan (1604/81), Honduras (V1250/81), and Key West, Florida (KW97) were obtained from Dr. Kenneth Dimock (Department of Biochemistry, Microbiology and Immunology, University of Ottawa, Ottawa, Ontario, Canada).19–22 Prototype CA24v (SEC24/70), Brunei (3751/75), and Singapore (75,308/75) CA24v isolates were generously supplied by Dr. Margaret Yin-Murphy (Department of Bacteriology, University of Singapore, Singapore).23–25 AHC virus stocks were propagated in HCEC, the culture media harvested into Eppendorf centrifuge tubes, the cell debris pelleted by centrifugation (5,000×g for 5 min), the supernatant harvested, aliquoted, and stored in an ultra-low freezer (−80°C; Revco, Kendro Laboratory Products, Asheville, NC).
MB Solution
Stock MB solution was made by adding 0.3 g MB (Sigma) to 30 mL of 95% ethanol diluted up to 100 mL in distilled water containing 0.01% KOH (BAM R45: Loeffler’s MB; https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm062250.htm) and stored in the dark. Dilutions (v/v) of the stock MB solution were made in DMEM.
MB Accumulation by Confluent HCEC
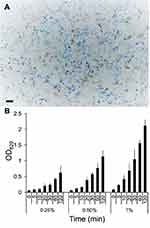
Triplicate 48 h confluent HCEC cultures were treated with 1.0 mL DMEM containing 0.25%, 0.5%, or 1% MB. The media was removed at times up to 120 min. Unabsorbed MB was removed by rinsing cell monolayers 2X with 1.0 mL volumes of phosphate-buffered saline (PBS; pH 7.4). Digital images of MB-treated cells were captured using a Dell personal computer using CellSens Standard microscopy imaging software interfaced with an Olympus BX43 light microscope equipped with a DP73 microscope digital camera (Olympus Life Science, Olympus America, Inc. Center Valley, PA; www.olympus-lifescience.com/en/software/cellsens/). The cell-associated oxidized MB levels were determined by adding 300 µL of cell lysis reagent [1.0% sodium dodecyl sulfate; 1.0% Triton X-100] (Sigma) to the MB treated HCEC cultures. The cells were detached with a plastic spatula, and the suspension harvested into a labeled Eppendorf tubes and centrifuged at 5,000×g for 5 min. Duplicate volumes of each clarified lysate (100 µL) were added to replicate 96-well microtiter plate wells. The mean optical densities (OD620nm) and standard errors from duplicate experiments were determined by Multiskan Ascent spectrophotometer (Thermo-Fischer, Rockford, IL).
MB Concentration-Dependent Inhibition of Infection by AHC Virus Isolates
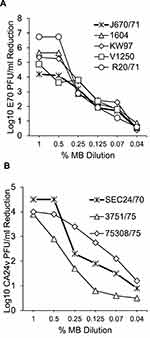
To determine an effective anti-AHC virus inhibitory concentration of MB replicate 6-well HCEC cultures were pre-treated for 30 min at 37°C with DMEM alone or with 2-fold serial dilutions of MB in DMEM. The media was removed and the cell monolayers washed 2X with pre-warmed fresh DMEM. A low multiplicity infection was performed by pipetting 1.0 mL of fresh DMEM containing 1000–3000 plaque forming units (PFU)/mL of E70 and CA24v onto treated and untreated cell HCEC cultures. After 20 h incubation at 37°C, the DMEM was harvested, clarified by desktop centrifugation (10,000×g for 10 min), and frozen (−20°C). Virus quantification was performed by duplicate virus micro-plaque assays as previously described.26 MB’s inhibitory effect was determined from the differences in mean PFU/mL produced in replicate treated and untreated cultures in at least 3 experiments. All viral experiments were performed in dim light or complete darkness to circumvent the possibility of photo-inactivation.
Inhibition of AHC Virus Production and Caspase-3-Mediated Cell Death
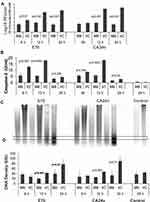
To investigate MB inhibition of AHC virus-induced cytopathogenic activity,6 replicate HCEC cultures were pre-treated with 0.5% MB (1.0 mL/culture) for 30 min at 37°C, the unabsorbed MB was removed, the cells were washed twice with 1.0 mL volumes of PBS. Pre-warmed fresh DMEM containing 104−5 PFU E70 (J670/71) or CA24v (SEC24/70)/mL was pipetted onto MB treated and untreated cells and incubated for 6, 12 and 24 h at 37°C. The DMEM was removed, clarified, and frozen (−20°C) for virus quantification. PBS (300 µL) was added to each culture and the adherent cells were detached using a plastic spatula, cells were pooled with the pelleted cells from the harvested DMEM. Following desktop centrifugation (3,000×g for 3 min), the pooled cell pellet was processed for caspase-3 activity as previously described.6 Briefly, the pelleted cells were lysed in 300 µL cell lysis buffer (50 mM HEPES, 10 mM CHAPS, 5 mM DTT, pH 7.4) on ice for 20 min, and cell extract produced by centrifugation at 15,000×g at 4°C for 10 min. Caspase-3 activity in 150 µL of the cell extracts was determined in triplicate colorimetric caspase-3 assays (CASP-3-C, Sigma, St. Louis, MO) according to the manufacturer’s instruction. Polymeric DNA fragmentation analysis was performed on the remaining cell pellet and cell extract (150 µL) as previously described.6 Accordingly, 0.25 mL of DNA extraction buffer (50 mM Tris X Cl, 10 mM EDTA, 0.5% Triton X-100, 0.5% NP-40, pH 7.6) was added to the remaining cell lysate. The cell lysate was phenol-extracted and nucleic acids precipitated by ethanol and recovered by centrifugation at 12,000×g for 10–15 min. The DNA precipitates were washed with 70% ethanol and 100% ethanol in succession and air-dried for 5 min. The DNA fraction was dissolved in RNase A (Thermo Scientific) digestion buffer (50 mM Tris-HCl, 5 mM EDTA, 60 μg/mL RNase A, pH 7.5). Samples were incubated at 37°C for 60 min to allow complete RNA digestion. Following electrophoresis through 1.2% agarose gel, the DNA bands were visualized with ethidium bromide under ultraviolet light, and digital images were obtained using a Bio-Rad Gel Documentation System and Quantity One software (www.bio.rad.com). Parallel caspase-3 levels (units/mL) and DNA densities were determined from 2 cultures in replicate experiments. The density of the ~300bp DNA band under ultra-violet light was determined using a digital scanner (Epson Perfection 4490 digital ICE Technology, Seiko Epson Corp., Japan). The DNA band density profiles were plotted from grey scale unit (GSU) density data obtained using free download ImageJ software (http://rsb.info.nih.gov/ij/download.html).
Post-Infection Treatment Experiments
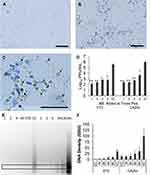
To investigate the uptake of oxidized MB by virus-infected cells, 24 h confluent HCEC monolayers in 6-well plate cultures were challenged with 100 PFU of E70 and incubated for 18 h to allow the formation of virus niduses (foci of <200 cells at all stages of active virus replication). The media was removed and fresh media containing 0.5% MB was added. After incubation at 37°C for 30 min, digital images were captured using an Olympus microscope, digital camera, and CellSens Standard Microscopy Imaging Software (as above). To assess the effects of MB application at times post infection, 24 h confluent HCEC cultures were challenged with 104−4.5 PFU of E70 (J670/71) and CA24v (SEC24/70) in 1.0 mL of DMEM. After incubation for 30 min at 37°C, the DMEM was removed, the cells rinsed with PBS (2X) to remove unabsorbed virus, and 1.0 mL of fresh DMEM added to the culture. At times through 6 h post virus infection, the DMEM was removed, pre-warmed DMEM containing 1% BM was added to each culture and incubated for 30 min, the media was removed, rinsed with PBS (2X), 1.0 mL of fresh DMEM was pipetted onto each culture, and cultures were incubated at 37°C in a humidified 5% CO2 atmosphere. After 24 h incubation, the adherent cells were detached with a sterile plastic spatula, the DMEM was harvested into centrifuge tubes, clarified by centrifugation (3,000×g for 3 min), and the supernatant frozen for virus quantification. DNA extraction buffer (150 μL) was added to the cell pellet and processed for polymeric DNA fragment analysis as described above.6
Statistical Analysis
Significance differences between mean virus yields (PFU/mL) from independent experiments were estimated by Student’s t-test performed on a Dell OptiPlex 7040 PC equipped with Excel software. The ANOVA test was used to analyze differences between caspase-3 levels and DNA densities and were performed on an IBM PC XT equipped with Epistat statistical software. P-values <0.05 were considered significant.
Results
MB Accumulation by HCEC
The efficacy of a topical ophthalmic antiviral against AHC would depends upon rapidly achieving and maintaining therapeutic levels in the conjunctival and corneal epithelial cells. Cells containing oxidized MB are blue because the positively charged dye binds to negatively charged particles such as polyphosphates, DNAs, and RNAs.27 The interaction of MB at different concentrations with 48 h confluent HCEC monolayers was monitored in replicate 6-well HCEC cultures incubated in media containing 0.25%, 0.5%, or 1.0% MB for different times through 120 min (Figure 1). Variable intracellular levels of oxidized MB were detected in confluent HCEC monolayers after 30 min incubation with 1% MB, but most of the cells were colorless indicative of cell viability/vitality27 (Figure 1A). The time-dependent uptake of different concentrations of MB was calculated based on the optical density of the HCEC lysates (Figure 1B). The increasing concentrations of MB through 120 min suggest that MB was rapidly taken up by HCEC. The results suggest the increase in cell-associated oxidized MB was concentration and time-dependent through the short adsorption period.
Concentration-Dependent Inhibition of E70 and CA24v Isolates
The following experiments were performed to assess MB concentration dependent inhibition of AHC virus production. Serial MB dilutions or DMEM alone were added to replicate HCEC cultures and incubated for 30 min. The media was removed, the cells rinsed and the cells infected with 5 E70 and 3 CA24v AHC epidemic isolates. The mean reductions in AHC virus yields after 24 h incubation suggest that 30 min incubation of HCEC with 0.07–1% MB can inhibit E70 and CA24v virus production by 90% (≥1.0 Log10 PFU/mL; p’s<0.01) (Figure 2). Notably, a 30-min incubation of HCEC with 0.5% MB prior to infection significantly reduced E70 production by >99.9% (mean Log10 reduction of 103.7 to 106.8 PFU/mL) (Figure 2A). Likewise, 30 min pre-incubation with 0.5% MB significantly reduced virus production by the 3 CA24v isolates (reductions of 102.8 to 104.5 PFU/mL) (Figure 2B). By comparison, a 30-min pre-incubation with 1% MB resulted in a greater inhibitory effect than 0.5% for only for 2 isolates; E70 V1250 (Figure 2A) and CA24v 3751/75 (Figure 2B). While AHC virus yield reductions were MB concentration-dependent for all E70 and CA24v virus isolates, the results suggest a 30-min pre-treatment of HCEC with 0.5% or 1.0% MB resulted in maximal inhibition of E70 and CA24v virus production.
MB Inhibition of AHC Virus Production and Caspase-Mediated Cell Death
AHC virus infection induces caspase-mediated conjunctival cell death6 and MB has been reported to inhibit caspases by oxidation of cysteine.28 Thus, the inhibitory effect of cell-associated MB on virus production, caspase-3 activation, and cell death was investigated by incubating replicate HCEC cultures with and without MB (1.0% for 30 min) before infection with E70 (J670/71; 3000 PFU/culture) or CA24v (SEC24/70; 10,000 PFU/culture). After infection, the cultures were incubated for 6, 12, and 24 h before the media was harvested for virus production and the cells harvested for caspase-3 activity and polymeric DNA extraction (Figure 3). Significantly less J670/71 (101.6−4.0 PFU/mL reduction) and SEC24/70 (≥104.0 PFU/mL reduction) were produced in MB-treated than in untreated HCEC at 12 h and 24 h (p’s<0.01) (Figure 3A). Concomitantly, E70-induced caspase-3 activity was significantly inhibited in MB treated versus untreated HCEC at 6 h (5.7±3 versus 12.9±0.3 U/mL; p=0.003) and 12 h (5.5±0.2 versus 17.8±1.6 u/mL; p<0.0001) (Figure 3B), but not at 24 h (p=0.206). CA24v-induced caspase-3 activity was not significantly inhibited in MB treated versus untreated HCEC at 6 h (5.7±0.74 versus 8.7±0.6 U/mL; p=0.764), but was significantly reduced at 12 h (5.7±1.3 versus 18.1±0.4 U/mL; p<0.0001) and 24 h (1.7±0.6 versus 2.9±0.5 U/mL; p=0.02). [Note: the lower caspase-3 levels at 24 h in the virus control cultures were due to the absence of active virus infection; >90% cell death.] MB treatment reduced polymeric DNA fragments (ie, virus-induced cytopathy) extracted from MB-treated E70 and CA24v-infected HCEC cultures (Figure 3C). Accordingly, significant differences in the 300bp polymeric DNA band density [grey scale density units (GSU)] were detected between MB treated and untreated E70 infected HCEC at 12 h (20±2 versus 42±5 GSU; p<0.001) and 24 h (39±18 versus 77±22 GSU; p=0.03). Similarly, lower levels of polymeric DNA fragments were extracted from MB treated than untreated CA24v infected HCEC at 12 h (27±2 versus 47±6 GSU; p=0.003) and 24 h (32±18 versus 90±22 GSU; p=0.01) (Figure 3D). [Note: DNA fragmentation occurs downstream of caspase activation, higher DNA levels are expected at 24 h with low or no caspase-3 activity.] Taken together, the results suggest MB pre treatment significantly inhibited AHC virus replication and caspase-mediated HCEC death through 24 h.
MB Applied Post Infection Inhibits AHC Virus Production and HCEC Death
The following studies were performed to evaluate the antiviral effect of MB applied during AHC virus infection. Only a few cells of a control 24 h confluent HCEC monolayer contained oxidized MB after 30 min incubation with 0.5% consistent with high vitality and viability (Figure 4A). In contrast, oxidized MB was associated with niduses of E70 infected HCEC after 30 min incubation (Figure 4B). Notably, oxidized (blue) and reduced (colorless) MB was detected in niduses of cells at different stages of the virus replication cycle (Figure 4C). In addition, the antiviral activity of MB applied at times up to 6 h post infection reduced E70 and CA24v production by 101.8−4.0 PFU/mL (p’s<0.01) (Figure 4D). Concomitantly, MB applied up to 6 h post infection reduced E70 and CA24v-induced cytopathy; ie, polymeric DNA formation (Figure 4E and F). The results suggest post infection application of low concentrations of MB can significantly inhibit virus production and corneal epithelial cell cytopathy.
Discussion
The rapid onset (3–5 h) of AHC symptoms 6–12 hr post exposure coincides with death of virus-infected conjunctival and corneal cells resolving in 3–5 days while the signs of inflammation resolve in 10–14 days.1,2 An effective topical antiviral ophthalmic solution should inhibit both E70 and CA24v and be non-toxic, rapidly active, and durable (providing protection for 12–24 h from endogenous and exogenous virus infection). A topical antiviral that also exerts anti-inflammatory activity could have the added benefit of decreasing AHC symptoms. Here we show that MB inhibits the AHC virus production and corneal cell cytopathy mediated by virus-induced caspase-3 and endonuclease activation. Accordingly, 0.5–1.0% MB pre-treatment maximally inhibits productive infection of HCEC by multiple E70 and CA24v isolates. Moreover, the results are consistent with the uptake of MB by corneal cells in vivo,27 demonstrate AHC virus-infected cells take up MB, and suggest MB applied before and up to 6 h post enteroviral infection effectively inhibits virus replication and enterovirus-induced cytopathy.
While enterovirus photoinactivation (200 J/cm2) and disinfection by MB has been reported,29,30 the mechanism by which intracellular MB inhibits AHC enterovirus infection of HCEC is not clear. While the level of oxidized MB taken up by HCEC in vitro was time and concentration dependent, greater numbers of MB (blue) cells noted in older and infected HCEC monolayers. The greater numbers of MB observed in older cultures suggests lower levels of cell vitality/viability, since MB decolorization is directly proportional to cell vitality.27 The differential distribution of oxidized MB in epithelial cells within the nidus of virus-infected cells is consistent with MB binding to AHC enteroviral RNA as noted with other enteroviruses.29 The observation that some cells exhibiting viral cytopathogenicity were colorless (reduced MB) suggests inhibition of virus-induced oxidative stress (oxygen-free radicals). Accordingly, MB has been reported to bind to nitric oxide synthetase and oxygen-free radicals.31–33 As in these studies, MB’s inhibition of enterovirus-induced cytopathy likely involves inhibition of enterovirus-induced executioner caspases,28 as well as mitochondrial dysfunction,33 but other unidentified cellular or enteroviral proteins interactions cannot be ruled out.
Topical MB application stains conjunctival and corneal epithelial cells and has been used to test conjunctival and corneal epithelial cell viability.27 Nonprescription topical ophthalmic medications containing MB are commonly used internationally for various complaints of ocular discomfort and redness. Marchenko34 reported a clinically significant effect with an ophthalmic solution containing zinc sulfate, diphenhydramine hydrochloride, naphazoline hydrochloride, and MB (300 ng/mL) in patients with chronic allergic conjunctivitis, blepharo-conjunctivitis, or contact lens induced giant papillary conjunctivitis following treatment (3 times daily for 15–30 days). Brownstein et al35 reported 6 patients with a spectrum of adverse ocular reactions, following prolonged daily use (4 months to 8 years) of nonprescription topical ophthalmic medication composed of MB, naphazoline hydrochloride or nitrate, and amylocaine hydrochloride, that were presumably due to at least one of the ingredients. Intraocular application is highly discouraged due to iris epithelial and corneal endothelial cell toxicity.36
Based upon MB’s uptake by virus-infected HCEC, antiviral activity against AHC virus production, inhibition of virus-induced cytopathy, and neuroprotective potential,37 it is expected that topical MB will inhibit AHC virus infection and provide post infection symptom relief. Taken together, our results support the cautious use of ophthalmic solutions containing 0.25–1% MB. Further, a twice a day application during AHC infection may prove beneficial in reducing bilateral infections, virus-induced cell death/inflammation, and transmission to at risk persons during explosive AHC epidemics. However, unmanaged long-term prophylactic use of MB ophthalmic solutions against AHC infection by individuals unaware of the possible side effects (asymptomatic blue discoloration of the conjunctiva) due to improper or prolonged overdose application is strongly discouraged.
In conclusion, MB has potent in vitro antiviral activity against the enteroviruses that cause AHC. Moreover, post infection application of MB protected against virus production and induction of caspase-mediated corneal cell cytopathy suggesting it may decrease ocular symptoms and inflammation. Clinical investigations are needed to determine the therapeutic efficacy of ophthalmic solutions containing MB against AHC, but the current results may be taken to support the judicious use of ophthalmic solutions containing low MB concentrations to treat AHC during epidemics.
Ethical Approval
The use of the noncommercial human corneal epithelial cells was approved by the institutional review board of the LSU Health Sciences Center-Shreveport.
Acknowledgments
The authors gratefully acknowledge Dr. Zan Pan for generously providing the starter culture of human corneal epithelial cells and thank Christopher Duggan for his technical assistance. The authors recognize Dr. Reisaku Kono (Central Virus Diagnostic Laboratory, National Institutes of Health, Tokyo, Japan), Dr. Ken Dimock (Department of Biochemistry, Microbiology and Immunology, University of Ottawa, Ottawa, Ontario, Canada) and Dr. Margaret Yin-Murphy (Department of Bacteriology, University of Singapore, Singapore) for kindly providing E70 and CA24v isolates. The research was supported in part by an award from the LSU-Health Medical Student Research Program [ARS].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Langford MP, Anders EA, Burch MA. Acute hemorrhagic conjunctivitis: anti-coxsackievirus A24 variant secretory immunoglobulin A in acute and convalescent tear. Clin Ophthalmol. 2015;9:1665–1673. doi:10.2147/OPTH.S85358
2. Wright PW, Strauss GH, Langford MP. Acute hemorrhagic conjunctivitis. Am Fam Physician. 1992;45:173–178.
3. Babalola OE, Amoni SS, Samaila E, Thaker U, Darougar S. An outbreak of acute haemorrhagic conjunctivitis in Kaduna, Nigeria. Br J Ophthalmol. 1990;74:89–92. doi:10.1136/bjo.74.2.89
4. Kosrirukvongs P, Kanyok R, Sitritantikorn S, Wasi C. Acute hemorrhagic conjunctivitis outbreak in Thailand, 1992. Southeast Asian J Trop Med Public Health. 1996;27:244–249.
5. Harada K, Fujimoto T, Asato Y, Uchio E. Virological and epidemiological analysis of coxsackievirus A24 variant epidemic of acute hemorrhagic conjunctivitis in Okinawa, Japan, in 2011. Clin Ophthalmol. 2015;9:1085–1092. doi:10.2147/OPTH.S81386
6. Chen D, Texada DE, Duggan C, Deng Y, Redens TB, Langford MP. Caspase-3 and −7 mediate apoptosis of human Chang’s conjunctival cells induced by enterovirus 70. Virology. 2006;347:307–322. doi:10.1016/j.virol.2005.12.005
7. Langford MP, Stanton GJ, Barber JC, Baron S. Early-appearing antiviral activity in human tears during a case of picornavirus epidemic conjunctivitis. J Infect Dis. 1979;139:653–658. doi:10.1093/infdis/139.6.653
8. Chatterjee S, Quarcoopome CO, Apenteng A. Unusual type of epidemic conjunctivitis in Ghana. Br J Ophthalmol. 1970;54:628–630. doi:10.1136/bjo.54.9.628
9. Kono R, Sasagawa A, Ishii K, Sugiura S, Ochi M. Pandemic of new type of conjunctivitis. Lancet. 1972;i:1191–1194. doi:10.1016/s0140-6736(72)90921-x
10. Chang WK, Liu KC, Foo TC, Lam MW, Chan CF. Acute haemorrhagic conjunctivitis in Hong Kong 1971–1975. Southeast Asian J Trop Med Public Health. 1977;8:1–6.
11. Zhang S, Hu Q, Deng Z, et al. Transmissibility of acute haemorrhagic conjunctivitis in small-scale outbreaks in Hunan Province, China. Sci Rep. 2020;10:119. doi:10.1038/s41598-019-56850-9
12. Herriman R. Belize is the latest Central American country battling a ‘pink eye’ outbreak. Outbreak News Today, October 8, 2017. Available from: http://outbreaknewstoday.com/belize-latest-central-american-country-battling-pink-eye-outbreak-31171/. Accessed December 15, 2020.
13. Enfissi A, Joffret ML, Delaune D, Delpeyroux F, Rousset D, Bessaud M. Coxsackievirus A24 variant associated with acute haemorrhagic conjunctivitis cases, French Guiana, 2017. Intervirology. 2017;60:271–275. doi:10.1159/000489339
14. Sousa IP, Burlandy FM, Ferreira JL, et al. Re-emergence of a coxsackievirus A24 variant causing acute hemorrhagic conjunctivitis in Brazil from 2017 to 2018. Arch Virol. 2019;164:1181–1185. doi:10.1007/s00705-019-04157-5
15. Alfonso E, Friedland B, Hupp S, et al. Neisseria gonorrhoeae conjunctivitis. An outbreak during an epidemic of acute hemorrhagic conjunctivitis. JAMA. 1983;250:794–795. doi:10.1001/jama.250.6.794
16. Vajpayee RB, Sharma N, Chand M, Tabin GC, Vajpayee M, Anand JR. Corneal superinfection in acute hemorrhagic conjunctivitis. Cornea. 1998;17:614–617. doi:10.1097/00003226-199811000-00009
17. Kovacs E. Prevention of cytopathic effect and propagation of poliovirus by methylene blue. Z Naturforsch B. 1960;15B:588–592. doi:10.1515/znb-1960-0909
18. Pan Z, Wang Z, Yang H, Zhang F, Reinach PS. TRPV1 activation is required for hypertonicity-stimulated inflammatory cytokine release in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2011;52:485–493. doi:10.1167/iovs.10-5801
19. Nejmi S, Gaudin OG, Chomel JJ, Baaj AJ, Sohier R, Bosshard S. Isolation of a virus responsible for an outbreak of an acute conjunctivitis in Morocco. J Hyg. 1974;72:181–183. doi:10.1017/S002217240002338X
20. Ghafoor A, Burney ML, Iqbal J, Khan Z. Acute haemorrhagic conjunctivitis (AHC) epidemic of 1981. J Pak Med Assoc. 1984;34:245–246.
21. Kew OM, Notty BK, Hatch MH, Hierholzer JC, Obeijeski JF. Oligonucleotide fingerprinting analysis of enterovirus 70 isolates from the 1980 to 1981 pandemic of acute hemorrhagic conjunctivitis: evidence for a close genetic relationship among Asian and American strains. Infect Immun. 1983;41:631–635. doi:10.1128/IAI.41.2.631-635.1983
22. Hatch MH, Malison MD, Palmer EL. Isolation of enterovirus 70 from patients with acute hemorrhagic conjunctivitis in Key West, Florida. N Engl J Med. 1981;305:1648–1649.
23. Yin-Murphy M. Acute hemorrhagic conjunctivitis. Prog Med Virol. 1984;29:23–44.
24. Bahrin MA, Joshi ND, Yin-Murphy M. A coxsackievirus type A24 epidemic conjunctivitis in Brunei. Southeast Asian J Trop Med Public Health. 1976;7:355–358.
25. Yin-Murphy M. Epidemics of acute hemorrhagic conjunctivitis by coxsackievirus A24 variant. In: Uchida Y, Ishii K, Miyamura K, Yamazaki S, editors. Acute Hemorrhagic Conjunctivitis; Etiology, Epidemiology and Clinical Manifestations. New York: Karger; 1989:57–65.
26. Stanton GJ, Langford MP, Baron S. Effect of interferon, elevated temperature, and cell type on replication of acute hemorrhagic conjunctivitis viruses. Infect Immun. 1977;18:370–376. doi:10.1128/IAI.18.2.370-376.1977
27. De Ocampo G, Fojas MR. A new test for the viability and vitality of conjunctival and corneal epithelial cells. Methylene blue decolorization. Am J Ophthalmol. 1961;52:923–927. doi:10.1016/0002-9394(61)90338-5
28. Pakavathkumar P, Sharma G, Kaushal V, Foveau B, LeBlanc AC. Methylene blue inhibits caspases by oxidation of the catalytic cysteine. Sci Rep. 2015;5:13730. doi:10.1038/srep13730
29. Wong TW, Huang HJ, Wang YF, Lee YP, Huang CC, Yu CK. Methylene blue-mediated photodynamic inactivation as a novel disinfectant of enterovirus 71. J Antimicrob Chemother. 2010;65:2176–2182. doi:10.1093/jac/dkq301
30. Floyd RA, Schneider JE, Dittmer DP. Methylene blue photoinactivation of RNA viruses. Antiviral Res. 2004;61:141–151. doi:10.1016/j.antiviral.2003.11.004
31. Floyd RA. Serendipitous findings while researching oxygen free radicals. Free Radic Biol Med. 2009;46:1004–1013. doi:10.1016/j.freeradbiomed.2009.02.003
32. Lomniczi A, Cebral E, Canteros G, McCann SM, Rettori V. Methylene blue inhibits the increase of inducible nitric oxide synthase activity induced by stress and lipopolysaccharide in the medial basal hypothalamus of rats. Neuroimmunomodulation. 2000;8:122–127. doi:10.1159/000054271
33. Dang D, Zhang C, Zhang R, et al. Involvement of inducible nitric oxide synthase and mitochondrial dysfunction in the pathogenesis of enterovirus 71 infection. Oncotarget. 2017;8:81014–81026. doi:10.18632/oncotarget.21250
34. Marchenko NR. [Oсumethyl in the treatment of allergic diseases of eyelids and conjunctiva]. Vestn Oftalmol. 2016;132:81–85. doi:10.17116/oftalma2016132581-85. Russian.
35. Brownstein S, Liszauer AD, Jackson WB. Ocular complications of a topical methylene blue-vasoconstrictor-anesthetic preparation. Can J Ophthalmol. 1989;24:317–324.
36. Brouzas D, Droutsas D, Charakidas A, et al. Severe toxic effect of methylene blue 1% on iris epithelium and corneal endothelium. Cornea. 2006;25:470–471. doi:10.1097/01.ico.0000183488.78012.33
37. Tucker D, Lu Y, Zhang Q. From mitochondrial function to neuroprotection-an emerging role for methylene blue. Mol Neurobiol. 2018;55:5137–5153. doi:10.1007/s12035-017-0712-2
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.