Back to Journals » International Journal of Nanomedicine » Volume 18
Metal-Based Nanoparticles: A Prospective Strategy for Helicobacter pylori Treatment
Authors Yin X, Lai Y, Du Y , Zhang T, Gao J, Li Z
Received 3 February 2023
Accepted for publication 24 March 2023
Published 9 May 2023 Volume 2023:18 Pages 2413—2429
DOI https://doi.org/10.2147/IJN.S405052
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Xiaojing Yin,1,* Yongkang Lai,1,2,* Yiqi Du,1,* Tinglin Zhang,3,* Jie Gao,3 Zhaoshen Li1
1Department of Gastroenterology, Shanghai Changhai Hospital, Naval Medical University, Shanghai, 200433, People’s Republic of China; 2Department of Gastroenterology, Ganzhou People’s Hospital Affiliated to Nanchang University, Ganzhou, Jiangxi, 341000, People’s Republic of China; 3Changhai Clinical Research Unit, Shanghai Changhai Hospital, Naval Medical University, Shanghai, 200433, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhaoshen Li; Jie Gao, Email [email protected]; [email protected]
Abstract: Helicobacter pylori (H. pylori) is an infectious pathogen and the leading cause of gastrointestinal diseases, including gastric adenocarcinoma. Currently, bismuth quadruple therapy is the recommended first-line treatment, and it is reported to be highly effective, with > 90% eradication rates on a consistent basis. However, the overuse of antibiotics causes H. pylori to become increasingly resistant to antibiotics, making its eradication unlikely in the foreseeable future. Besides, the effect of antibiotic treatments on the gut microbiota also needs to be considered. Therefore, effective, selective, antibiotic-free antibacterial strategies are urgently required. Due to their unique physiochemical properties, such as the release of metal ions, the generation of reactive oxygen species, and photothermal/photodynamic effects, metal-based nanoparticles have attracted a great deal of interest. In this article, we review recent advances in the design, antimicrobial mechanisms and applications of metal-based nanoparticles for the eradication of H. pylori. Additionally, we discuss current challenges in this field and future perspectives that may be used in anti-H. pylori strategies.
Keywords: H. pylori, antimicrobial applications, metal based nanoparticles, antibiotic-free, antimicrobial mechanisms
Introduction
Helicobacter pylori (H. pylori) is a Gram-negative microaerobic bacterium that colonizes the human stomach.1–5 Accordingly, it has been associated with gastrointestinal diseases including chronic gastritis, peptic ulcer, gastric carcinoma, and MALT lymphomas.1–5 In addition, numerous studies have confirmed that H. pylori is closely related to the occurrence and development of such extra-intestinal diseases as idiopathic thrombocytopenic purpura, unexplained iron deficiency anemia and vitamin B12 deficiency.6 Moreover, there is growing evidence that H. pylori infection is associated with other diseases, particularly relating to cardiovascular, metabolic, and neurological functions.6 Correspondingly, H. pylori was classified as a Group I carcinogen by the International Agency for Research on Cancer as early as 1994. By the 15th edition of the US Carcinogen Report in 2021, H. pylori was classified as a definite carcinogen.7 Xie et al indicated that H. pylori can contribute to gastric cancer by inhibiting autophagy and promoting DNA damage.8–10 In addition, several recent long-term follow-up studies with large samples in regions with a high prevalence of gastric cancer have confirmed the long-term benefits of H. pylori eradication in preventing gastric cancer.11–13 Although a recent meta-analysis showed that the prevalence of H. pylori in China appears to be on a decreasing trend compared to the past (from 58.3% during 1983–1994 to 40.0% during 2015–2019),14 it is inevitable that this pathogen will remain increasingly resistant to antibiotics and its infection burden will remain high.15,16 This necessitates the development of antibiotic alternatives or antibiotic loading systems to mitigate the problem of antibiotic resistance.
In recent years, the research and development of antibacterial materials that can replace antibiotics has become an increasingly popular topic.17–20 Accordingly, nanomaterials have garnered increasing attention in the medical field, particularly due to their significant advantages in drug delivery, as they can administer poorly soluble drugs, protect them from enzymatic reactions, target them to the specific organ to be treated, and diffuse through the biofilm matrix.21 Among these, metals and their metal oxide nanoparticles (NPs) have attracted increasing research interest due to their unique optical properties, such as surface plasmon resonance (SPR). Moreover, the use of metals as antibacterial agents dates back to the Edwin Smith Papyrus, an ancient Egyptian medical text from 1500 B.P., which describes the use of copper salts as astringents. In addition, over the past two centuries, doctors have also used metal oxides such as Te, Mg and As, as well as Cu and Hg salts, to treat tuberculosis, leprosy, syphilis, and gonorrhea.22 Although the advent of antibiotics complicated the use of antibacterial metals, but the emergence of antibiotic resistance has revived this strategy.22 With the advent of cutting-edge nanotechnology, the work spectrum of metal-based NPs has significantly expanded.23 Due to their optical, electrical, and chemical properties, metal-based NPs are widely used as contrast agents in imaging to diagnose diseases in diagnostic agents.21,24 In addition, due to their inherent antimicrobial activity, metal-based NPs are superior to other nanoparticles.17,18 Accordingly, they can be used as a drug carrier or to synthesize composites with related antibacterial agents such as antibiotics, to enhance antibacterial activity.25,26 As with most Gram-negative bacteria, metal-based NPs typically kill H. pylori synergistically, in a variety of ways, most notably via direct release of metal ions, generation of reactive oxygen species (ROS), and light-induced photothermal/photodynamic effects.22,27–29 For the emerging field of metal-based NPs, there is, to our knowledge, no comprehensive and systematic summary of metal-based NPs types, modification strategies, and mechanisms of action. However, there are numerous reviews discussing the application of one or more biofilm carriers (liposomes, extracellular vesicles, or cell membrane nanocarriers) encapsulated in metal nanocarriers (eg inorganic nanocarriers such as silica). Thus, in order to accelerate our understanding of the design, engineering, and application of advanced therapies for metal-based NPs, it is necessary to provide a comprehensive summary of research advances, application perspectives, and challenges. Accordingly, in this review, we discuss recent developments of metal-based NPs for the treatment of H. pylori infections, as metal-based NPs can provide solutions to antibiotic resistance. Additionally, we discuss briefly the limitations of the current methods for eradicating H. pylori. Moreover, we review the current research on metal and metal oxide NPs for the treatment of H. pylori based on the antibacterial mechanism of metal-based NPs in anticipation of their early clinical application. Furthermore, perspectives on the challenges and potential future directions of metal-based NPs in the treatment of H. pylori infection have also been provided in this review.
Current Clinical Strategies for Managing
In the 1990s, Correa proposed the evolution process of intestinal gastric cancer: chronic non-atrophic gastritis → atrophic gastritis → intestinal metaplasia → dysplasia → gastric cancer, and suggested that H. pylori played a significant role in the initiation of gastric cancer30,31 (Figure 1A). Correspondingly, H. pylori was classified as a class I carcinogen in 1994, and the eradication of H. pylori is closely associated with the prevention of gastric cancer.11 In 2015, the Kyoto Global Consensus Report defined H. pylori as an infectious pathogen and recommended treatment for all patients.32 Recently, from a 26.5-year follow-up of a total of 1630 H. pylori-infected patients in southern China, Yan et al concluded that the eradication of H. pylori reduced the incidence of gastric cancer by 43% in the overall population and even 63% in patients without precancerous gastric lesions (Figure 1B).13 Evidently, the eradication of H. pylori can significantly reduce the risk of gastric cancer, and hence, the eradication of H. pylori from the global population is of critical importance.
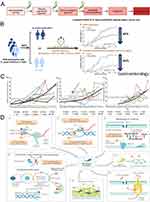 |
Figure 1 Schematic representation of H. pylori pathogenic mechanism and the biological attributes of resistance in H. pylori. (A) Role of H. pylori in the pathogenesis of enteric gastric cancer; (B) H. pylori eradication reduced the incidence of gastric cancer by 43% in the overall population and even 63% in patients without precancerous gastric lesions; (C) Global trend of resistance rates of clarithromycin (a), metronidazole (b) and levofloxacin (c) over time; (D) The diagram shows the biological characteristics of drug resistance in H. pylori (red asterisks indicate the potential for drug resistance mutations). It consists mainly of the formation of components that prevent cellular penetration and antibiotic molecular activity by altering drug targets (1–3), inhibiting intracellular drug activation (4), biofilm formation (5), increased drug efflux and reduced drug uptake (6), or producing ultrastructural and metabolic changes (7), all of which are not mutually exclusive but may co-exist in different strains and together lead to three resistance patterns. Single drug resistance (SDR), multidrug resistance (MDR) and heterogeneous resistance (HR). (B) Reprinted from Gastroenterology, 163, Yan L, Chen Y, Chen F, et al. Effect of Helicobacter pylori eradication on gastric cancer prevention: updated report from a randomized controlled trial with 26.5 years of follow-up. Copyright 2022, with permission from Elsevier.13 (C) Reproduced with permission from Thung I, Aramin H, Vavinskaya V, et al. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43(4):514–533. doi:10.1111/apt.13497. Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.103 (D) Reproduced with permission from Tshibangu-Kabamba E, Yamaoka Y. Helicobacter pylori infection and antibiotic resistance - from biology to clinical implications. Nat Rev Gastroenterol Hepatol. 18(9):613–629. doi:10.1038/s41575-021-00449-x. 2021, Springer Nature.1 |
Currently, the Maastricht VI/Florence consensus report recommends bismuth quadruple therapy (BQT) [proton pump inhibitors (PPIs) + bismuth + two types of antibiotics] as the first-line therapy for H. pylori in regions with high (>15%) or unknown rates of clarithromycin resistance; whereas in regions with low clarithromycin resistance (<15%), BQT or clarithromycin-containing triple therapy (PPI + clarithromycin + another antibiotic) are recommended as first-line empiric treatment.33 Following the failure of the first-line regimen, it is recommended that the patient receive a fluoroquinolone-based treatment or undergo susceptibility testing prior to taking further action.34 However, the long-term and excessive use of antibiotics has led to the evolution of H. pylori with antibiotic resistance. Correspondingly, the resistance of H. pylori to antibiotics such as clarithromycin, metronidazole, and levofloxacin is annually on the increase, thereby decreasing the eradication rate of H. pylori and endangering public health (Figure 1C). Savoldi et al conducted a global meta-analysis consisting of 178 studies and 66–142 samples (99.5% of endoscopic gastric or duodenal biopsies) from 65 countries, and found that the resistance of H. pylori to clarithromycin, metronidazole, and levofloxacin was 15% or higher in most WHO regions and had increased over time. Among them, China’s metronidazole and levofloxacin resistance rates are among the highest in the world, at 77% and 33%, respectively35 (Figure 1C). To address this challenge, numerous efforts have been made to offer available options to combat H. pylori infection and antibiotic resistance, including the discovery of new drugs [such as potassium-competitive acid blockers (P-CAB); notably, P-CAB are also recommended as an alternative to PPIs; correspondingly, P-CAB-containing regimens have been found to be superior in patients with clarithromycin-resistant strains]20,33,36 or the use of different drug combination approaches (such as sequential, hybrid, and concomitant therapies).37 However, these methods still involve the use of large quantities of antibiotics, which can lead to an increase in H. pylori resistance in the future; consequently, the outcomes will continue to deteriorate. Understanding how current antibiotics fail to achieve their intended effects and how resistance develops is crucial for the development of innovative and potent antibacterial technologies. From a pathogenic perspective, the mechanisms of resistance in H. pylori fall into four main categories: 1) evasion of antibiotic activity via mutations in cellular targets, 2) reduction of intracellular accumulation of antibiotic compounds via alterations in efflux systems or cell membrane permeability, 3) inactivation or impairment of antibiotic activity via enzymes or virulence factors, or 4) prevention of antibiotic clearance via initiation of resistance escape mechanisms1 (Figure 1D). In addition to antibiotic resistance, factors such as the harsh environment of the stomach, which prevents antibiotics from penetrating, as well as the barrier effect of H. pylori biofilm comprise persistent obstacles in the eradication of H. pylori.1,38,39 In addition, the extensive use of antibiotics has had an irreversible effect on the intestinal microecology of patients.37,40 Therefore, to eradicate H. pylori in the future, the reliance on the current antibiotic and the associated treatment protocols present significant risks. Consequently, the search for antibiotic-free strategies with high eradication rates, low adverse effects, and high compliance comprise a promising emerging strategy for addressing the current anti-H. pylori conundrum. In this regard, the development and use of metal-based nanomaterials is a rising star in the fight against H. pylori.
Metal Based Nanoparticles-Based Treatment of H. pylori
Metal-based nanoparticles are substances prepared from metals or their compounds that are less than 100 nm in at least one dimension.41,42 Due to their facile preparation, high reactivity, large specific surface area, and ease of surface modification, they have been widely used in drug delivery, biosensor, pathogen detection and antibacterial applications.27,43 According to the World Health Organization, metal-based nanoparticles have a small size, high penetrability, and selectivity for bacteria in addition to their demonstrated efficacy against specific pathogens.44 In addition, their non-specific bacterial toxicity mechanism not only broadens the spectrum of antibacterial activity, but also makes it difficult for bacteria to develop resistance, attracting increased interest in the field of antimicrobial research, particularly in antibiotic-free antimicrobial strategies.27,45,46
Normally, a majority of metal ions are present in the human organism in the form of complexes, which are involved in tissue composition, growth and development, the metabolism of energy and substances, and the transmission of various signals.47–49 For instance, transition metal ions such as iron (Fe), manganese (Mn), zinc (Zn), copper (Cu), cobalt (Co) and nickel (Ni) act as key components of enzymes and proteins, catalyze various types of reactions, and regulate the transcription, translation and expression of DNA and RNA. When these metal ions are present in excess, however, their effects on cells can be fatal.22,47 Conversely, it is due to this lethality, metal ions are capable of killing pathogens; for instance, recent evidence suggests that macrophages actually poison pathogens by increasing zinc and copper concentrations in phagosomes.50,51 Specific metal compounds will distinguish between bacteria and eukaryotic cells, due to their distinct metal transport systems and metalloproteins, and toxicity to humans can be avoided by altering the route of administration or confining some toxic substances outside the cell membrane.22 This permits metal-based nanomaterials to function as long-lasting antimicrobial agents with little or no effect on the host, thereby resolving the issue of antibiotic resistance.52 In parallel, gold, silver, copper, iron, and other transition metal-based NPs have attracted a great deal of attention in the field of oncology due to their variable size, excellent photoacoustic effects, and ability to precisely target lesions. Accordingly, copper (Cu), silver (Ag), zinc (Zn), titanium (Ti), gold (Au), silicon (Si), bismuth (Bi), iron (Fe), and magnesium (Mg) metal and metal oxide NPs comprise the predominant antibacterial metal nanoparticles that are currently used (Figure 2A). The antibacterial mechanism of these metal-based NPs can be attributed to at least one of the following mechanisms: direct or indirect damage to cell membranes by generating free radicals and ROS, inactivation of enzymes, energy imbalance, photocatalysis.52 However, there is limited research on metal and metal oxide NPs with anti-H. pylori capabilities. In this regard, three primary mechanisms are involved in the killing of H. pylori by metal nanoparticles that have been studied for this purpose: 1) release of metal ions, 2) production of ROS, and 3) light-induced photothermal or photodynamic effects.27,53–55 In the following, a comprehensive summary of the current research progress on metal-based NPs against H. pylori, as well as metal-based NPs that have the potential to be investigated for anti-H. pylori (Table 1).
 |
Table 1 Typical Examples of Metal-Based Nanoparticles as Anti-H. pylori Agents |
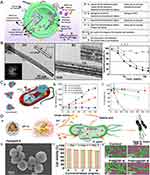 |
Figure 2 Metal-based nanomaterials exert antibacterial action through metal ion release. (A) Schematic diagram of the mechanism of metal ion sterilization released from metal nanocarriers; (B) High resolution TEM images of (BiO)2CO3 nanotube bundles (a and b), and their inhibition of bacteria at different concentrations (c); Application of bismuth nanoparticles for H. pylori eradication; (C) As acarrier, AgNPs can efficiently transport Ag+ into the cytoplasm and membranes of bacteria and their proton motive force will lower the local pH thus enhancing Ag+ release (a); Silver ion release from PEG-AgNPs of different particle sizes (5 nm and 11 nm) under aerobic and anaerobic mitigation (b); Toxicity elimination by AgNP synthesis and exposure under anaerobic conditions, thus preventing oxidative Ag+ release (c); (D) Pd(H)@ZIF-8 release zinc ions to kill H. pylori. The antibacterial mechanism diagram (a); The scanning electron micrograph of Pd(H)@ZIF-8 (b); The cytotoxicity test of Pd(H)@ZIF-8 (c), and the scanning electron micrographs of different materials after co-culture with bacteria (d). (A) Used with permission of Royal Society of Chemistry, from Metal organic framework-based antibacterial agents and their underlying mechanisms. Han D, Liu X, Wu S. 51(16), copyright 2022; permission conveyed through Copyright Clearance Center, Inc.53 (B) Used with permission of Royal Society of Chemistry, from Fabrication of bismuth subcarbonate nanotube arrays from bismuth citrate, Chen R, So MH, Yang J, Deng F, Che C-M, Sun H Chem, (21), copyright 2006; permission conveyed through Copyright Clearance Center, Inc.104, (C) Reprinted with permission from Xiu ZM, Zhang QB, Puppala HL, Colvin VL, Alvarez PJJ. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012;12(8):4271–4275. © 2012 American Chemical Society106, (D) Reproduced with permission from Zhang W, Zhou Y, Fan Y, et al. Metal-organic-framework-based hydrogen-release platform for multieffective Helicobacter pylori targeting therapy and intestinal flora protective capabilities. Adv Mater. 2022;34(2):e2105738. doi:10.1002/adma.202105738. © 2021 Wiley-VCH GmbH.101 |
Metal-Based NPs Based on the Release of Metal Ions
A key bactericidal function of metal-based NPs is their ability to release metal ions. Regarding H. pylori, whose outer membrane is composed of negatively charged lipopolysaccharide,48 positively charged metal ions can adsorb to negatively charged bacterial surfaces, thereby inducing pits and gaps in the bacterial membrane, which leads to cell division.47 On the other hand, a strong coordination bond can be formed between metal ions and the N, O, or S atoms of biomolecules,49 and the binding of metal ions to these biomolecules alters the function of H. pylori, thereby achieving antibacterial effects. In addition, metal ions have been shown to have a variety of other anti-H. pylori mechanisms, such as induction of oxidative stress and inhibition of bacterial surface biofilm production. Moreover, certain metal ions can directly interfere with the nickel in H. pylori urease, thereby inactivating the enzyme, and these mechanisms are typically synergistic.47,49–51
Organic salts of bismuth (ie, bismuth salicylate, bismuth subcitrate, and bismuth subnitrate) have been used to treat various gastrointestinal disorders since the early 19th century.56,57 Bismuth exists in two primary oxidation states, trivalent and pentavalent, with trivalent being the most common and stable form and Bi(III) having a high affinity for sulfur, nitrogen and oxygen ligands.58,59 Li et al demonstrated that colloidal bismuth subcitrate (CBS) inhibits several major antioxidant enzymes and urease produced by H. pylori to varying degrees, validating the key biological pathways (oxidative stress defense system and pH buffering system) of H. pylori that are disrupted by bismuth-based drugs.58 Similarly, this provides a rationale for the release of Bi(III) in anti-H. pylori mechanisms of bismuth nanomaterials. For instance, Chen et al56 synthesized the first bismuth carbonate (BiO)2CO3 nanotubes (Figure 2B), and the group subsequently synthesized well-crystallized bismuth carbonate (BiO)2CO3 NPs with an average particle size of 9.2 nm via the water-in-oil (w/o) microemulsion-assisted hydrothermal method.60 Both (BiO)2CO3 nanomaterials were found to inhibit H. pylori in relation to the bismuth concentration, with the inhibition being greater for nanoparticles (>85%) than for nanotubes (>80%) at 80 μg/mL. Furthermore, the antibacterial activity of these materials was found to be in the following order: (BiO)2CO3 NPs > CBS > bulk (BiO)2CO3 > Bi2O3 NPs. Other than that, Nazari et al produced less than 5 nm Bi NPs and evaluated their antibacterial activity against various clinical isolates and standard H. pylori strains using a serial solid agar dilution method, which demonstrated excellent antibacterial activity against all H. pylori strains tested. The minimum inhibitory concentration (MIC) of bismuth subnitrate against H. pylori (>200 μg/mL) was significantly higher than the MIC of Bi NPs (up to 100 μg/mL).61 Based on the above findings, we can speculate that the antibacterial effect of bismuth in the form of NPs is greater than that of bismuth ions against H. pylori, or that elemental bismuth in the form of NPs enriches the mechanism by which bismuth alone acts on the pathogen, resulting in increased bactericidal efficacy. Although the exact mechanism has not been elucidated, its great potential to combat H. pylori has been demonstrated, and these studies may serve as a foundation for the future development of multiple treatments for H. pylori infection or new nanomedicines.
Due to the large surface area with which silver nanoparticles come into contact with bacteria, these nanoparticles have demonstrated highly effective antibacterial properties. Numerous studies have demonstrated that silver and its oxide nanoparticles release Ag+ in aqueous solution, thereby increasing their bactericidal activity.62,63,106 Xiu et al discovered that Ag NPs could release Ag+ under aerobic conditions, resulting in a significant reduction of bacteria. However, Ag NPs under anaerobic conditions exhibited no bactericidal activity, indicating that antibacterial activity was related to Ag+ release106 (Figure 2C). Based on H. pylori strains, certain of these studies have demonstrated antibacterial activity. For instance, Amin et al successively used methanol extract from Solanum xanthocarpum64 and P. harmala seeds extract65 as a reducing and capping agent to synthesize and reduce silver nanoparticles. It was discovered that Ag NPs had greater antibacterial potency than several antibiotics used to eradicate H. pylori, and it was surprising to find that it also had antibacterial activity against strains exhibiting double and triple resistance. Additionally, Ag NPs with an average size of 20 nm produced by Gurunathan et al,66 and Silver Ultra-NanoClusters (SUNCs) with an average size of less than 5 nm produced by Grande et al,67 demonstrated that silver nanomaterials have anti-biofilm properties and can effectively eradicate mature biofilms produced by H. pylori. It is well known that biofilms can protect H. pylori from the harsh environment in the stomach39,68; therefore, inhibiting the growth of biofilms or eradicating them is advantageous to the eradication of pathogens, which also supports the notion that Ag NPs may function as antibacterial agents for H. pylori. On the other hand, it has been reported that the continued use of Ag NPs as antibacterial agents does not bode well due to genetic alterations in the bacteria;69 however, it will be necessary to conduct additional research in the future to confirm this.
Zinc is an essential trace element whose role in wound healing67 is well-established. Due to this property of zinc and its relatively low toxicity, the FDA (Food and Drug Administration) has approved zinc oxide nanoparticles (ZnO NPs) as a biocompatible antimicrobial agent. Consequently, in the field of anti-H. pylori research, numerous researchers have created new anti-H. pylori complexes by complexing zinc ions with H receptor blockers or antibiotics used to eradicate H. pylori, utilizing the inhibitory effect of zinc on urease. For instance, Amin et al studied a novel zinc(II)-famotidine anti-H. pylori complex, which was found to be nearly as effective against antibiotic-resistant strains of H. pylori as antibiotic-sensitive strains, and its toxicity was significantly lower than that of the parent drug.68 The complex inhibited urease significantly, although the underlying mechanism is unknown. Moreover, the synthesis of ZnO NPs based on Oak galls extract(OGE) by Attia et al,69 as well as the first combination of metal-organic framework and hydrogen therapy for the treatment of H. pylori by Zhang et al, wherein, he designed a metal-organic framework hydrogen production system (Pd(H)@ZIF-8@AP),70 exploited the significant inhibitory effect of zinc ions on urease, thereby resulting in excellent anti-H. pylori activity (Figure 2D). These Zn+-based nanomaterials could, therefore, serve as a safer and more effective alternative drug for treating H. pylori infections in the near future.
Due to H. pylori’s high demand for active urease and the close association of nickel ions with the active site of urease,70,71 nickel accumulation is essential for the organism’s survival in the gastric environment. In addition, the presence of nickel inhibited cobalt (Co) activity in a dose-dependent manner. Sylvaine et al found that cobalt chloride not only had significant anti-H. pylori activity, but also acted specifically against H. pylori.72 In addition, the authors identified a potential mechanism by which cobalt ions inhibit the growth of H. pylori by competing with nickel ions for the active metal binding site of urease or the nickel transport system specific to H. pylori, rather than by mediating toxicity in a urease-dependent manner.72 Although Co NPs have not been investigated in the eradication of H. pylori, their anti-gastric cancer properties have been demonstrated. For instance, Jarestan et al synthesized Co3O4@Glu/TSC NPs via co-condensation reaction and discovered that the complex inhibited the growth of AGS cells and induced apoptosis with its anti-cancer activity.73 Furthermore, a gastric nano-heater iron cobalt alloy shielded with graphitic shells (FeCo@G) was developed to antagonize H. pylori infection using magnetothermal therapy, providing an avenue for the treatment of H. pylori.74 However, the synthesis and mechanism of action of this material are beyond the scope of this review and will not be discussed here.
Metal-Based NPs Based on ROS Generation
In our biological systems, reactive oxygen species (ROS) are produced when electrons leak out of the respiratory chain and consume a small amount of oxygen before being delivered to the terminal oxidase. They are a natural byproduct of the oxygen metabolism process, consisting primarily of superoxide anions (O2•-), hydrogen peroxide (H2O2), singlet oxygen (1O2) and hydroxyl radicals (•OH).75,76 ROS play a crucial role in normal cell function and are involved in a variety of biological processes, including cell signaling and homeostasis76,77 (Figure 3A). In the H. pylori-infected stomach, neutrophils ingest bacteria and transform into phagosomes, where they kill invading pathogens via ROS production catalyzed by NADPH oxidase (NOx) on the phagosome.76,78–82 Similarly, metal nanoparticles use the principle that ROS can cause oxidative stress in cells to damage cell membranes, DNA, and mitochondria in order to kill bacteria83 (Figure 3B). This section will concentrate on metal-based NPs whose primary role is to produce ROS against H. pylori.
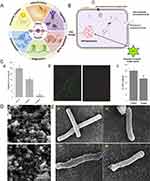 |
Figure 3 Metal-based nanomaterials exert antibacterial action through ROS generation. (A) The mechanism of antibacterial activity of ZnO PEI NP; (B) Function of ROS in normal cells; (C) Growth of H. pylori after treatment with different concentrations of ZnO-PEI NP (a); Staining of living and dead bacteria (b) and quantitative analysis of ATP (c) after incubation of ZnO PEI NP (100mg/mL) with bacteria for 3 hours; (D) Electron microscope scan of zinc-based nanoparticles; (E) Electron microscope scan of zinc-based nanoparticles killing H. pylori through damaging the membrane. (A) Reprinted from from Molecular Cell, 81/18, Lennicke C, Cochemé HM. Redox metabolism: ROS as specific molecular regulators of cell signaling and function, 3691–3707 Copyright 2021, with permission from Elsevier.107 © 2021 Elsevier Inc. (B) and (C) Reproduced from Chakraborti S, Bhattacharya S, Chowdhury R, Chakrabarti P. The molecular basis of inactivation of metronidazole-resistant Helicobacter pylori using polyethyleneimine functionalized zinc oxide nanoparticles. PLoS One. 2013;8(8):e70776. doi:10.1371/journal.pone.0070776105 (D) and (E) Reprinted from Microbial Pathogenesis, 115, Saravanan M, Gopinath V, Chaurasia MK, Syed A, Ameen F, Purushothaman N. Green synthesis of anisotropic zinc oxide nanoparticles with antibacterial and cytofriendly properties. Microb Pathog. 57–63, Copyright 2018, with permission from Elsevier.102 |
The anticancer and antibacterial activity of ZnO nanoparticles is correlated with their capacity to generate ROS and induce apoptosis.84 After treating H. pylori with polyethyleneimine-functionalized zinc oxide nanoparticles (ZnO-PEI NP), Chakraborti et al observed a rapid increase in intracellular ROS production; the amount of ROS generated was proportional to the concentration of ZnO-PEI NP used, and the amount of ROS produced by ZnO NP at similar concentrations was significantly less. Notably, the developed system demonstrated synergistic activity when combined with ampicillin, suggesting that it could be used in conjunction with therapeutic subdoses of antibiotics for the treatment of H. pylori infection85 (Figure 3C). Moreover, Saravanan et al86 and Attia et al69 also demonstrated that the toxicity of biogenic ZnO NPs is primarily a result of oxidative stress. It is well known that the H. pylori-mediated inflammatory response results in excessive ROS production and damage to normal gastric epithelial cells (Figure 3D and E). Therefore, theoretically preventing inflammatory cells from producing excessive ROS can prevent damage to normal epithelial cells, and based on this, Zhang et al developed a novel anti-H. pylori product, Pd(H)@ZIF-8@AP, which the authors assessed to be resistant to oxidative stress using malondialdehyde (MDA) and superoxide dismutase (SOD).70 Whether the objective is to use ROS production to sterilize or to prevent bacteria from producing ROS to damage normal gastric cells, the end goal of both approaches is to protect the gastric mucosa from bacteria. In addition to ZnO NPs, which have been extensively studied for their antimicrobial activity via the release of ROS, Gurunathan et al discovered that biogenic Ag NPs were also effective inducers of ROS and that the production of ROS could ultimately result in cellular DNA breakage.106
In fact, in addition to the above-mentioned metal nanoparticles that have been investigated for anti-H. pylori activity, there are numerous metal nanoparticles, such as Ni, Cu, and Ti NPs, with antibacterial potential against H. pylori primarily via oxidative stress mechanisms. Nickel nanoparticles synthesized with Ocimum sanctum leaf extract (NiGs) were found to be more sensitive to pathogenic Gram-negative bacteria than Gram-positive bacteria, and NiGs-induced formation of ROS disrupted microbial cell membranes and their permeability, resulting in growth inhibition and cell death.84 Additionally, studies have demonstrated that CuO NPs85 and TiO2 NPs86 possess antibacterial properties against both Gram-negative and Gram-positive bacteria, and that these properties are influenced by the particle size, morphology, and chemical composition of the materials. Overall, the production of ROS by metal-based NPs is a crucial mechanism for their antimicrobial activity. ROS-generated metal-based NPs are nevertheless non-selectively toxic, and antibacterial NPs based on this mechanism may also be harmful to mammalian cells. In selecting metal-based nanoparticles with anti-H. pylori potential, therefore, the toxicity of these nanoparticles is unquestionably a factor that cannot be neglected.
Metal-Based NPs Based on Photothermal/Photodynamic Effects
Metal nanoparticle-based phototherapy, including photothermal therapy (PTT) and photodynamic therapy (PDT). PTT refers to the ability of some metallic nanomaterials to kill bacteria by converting light energy into heat and powerfully penetrating the gastric mucosal tissue under the conditions of a near infrared (NIR) laser as a light source. In addition to this, other advantages include locally targeted drug delivery, targeted molecularly modified nanoparticles for drug or nucleic acid delivery, etc. (Figure 4A).55,87 Metal nanoparticle-based PDT is a novel therapeutic approach that employs metal nanoparticles as carriers or photosensitizers (PS). PDT relies on the enhancement of ROS production from metal-based NPs through light irradiation. Light absorption promotes electrons into the high-energy band, where they can readily react with water or oxygen to generate ROS, leading to apoptosis and/or necrosis in bacterial cells.27,75,87 Both therapeutic approaches offer the benefits of minimal invasiveness, low toxicity, good selectivity, and reproducibility, and are promising candidates for the treatment of H. pylori. This section discusses recent advances in anti-H. pylori metal NPs with photothermal/photodynamic effects.
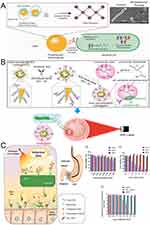 |
Figure 4 Metal-based nanomaterials exert antibacterial action through photothermal/photodynamic effects. (A) Schematic illustration of the photothermal conversion and antibacterial mechanism. Electromagnetic fields cause rapid oscillations of electrons in the conduction band on the surface of metallic nanomaterials, creating a fast-moving electron cloud. This absorbed energy causes vibrations in the metal lattice through electron-phonon coupling, which are subsequently converted into thermal energy, leading to a local temperature increase around the nanomaterial, which leads to cell death through a series of actions. Top right are scanning electron micrographs of E. coli cells before (left) and after (right) treatment with photothermal nanomaterials; (B) The chemical diagram of gold nanostars modified with antibodies and schematic representation of targeted imaging and photothermal treatment of H. pylori in vivo; (C) Schematic illustration of multiple 3SL conjugated PheoA photosensitizers (p3SLP) against H. pylori by an endoscopic laser system. 3SL binds specifically to SabA on the membrane of H. pylori and PheoA produces ROS to inactivate H. pylori using laser irradiation. And the histogram of cytotoxicity and antibacterial activity of different materials at different concentrations (a–c). (A) Reproduced from Cheeseman S, Christofferson AJ, Kariuki R, et al. Antimicrobial metal nanomaterials: from passive to stimuli-activated applications. Adv Sci. 2020;7(10):1902913. doi:10.1002/advs.201902913. © 2020 The Authors. Published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.55 (B) Reproduced from Nenomedicine: Nanotechnology, Biology and Medicine, 20, Zhi X, Liu Y, Lin L, et al. Oral pH sensitive GNS@ab nanoprobes for targeted therapy of Helicobacter pylori without disturbance gut microbiome. Copyright 2019, with permission from Elsevier.91 (C) Reproduced from Biomaterials, 271, Im BN, Shin H, Lim B, et al. Helicobacter pylori-targeting multiligand photosensitizer for effective antibacterial endoscopic photodynamic therapy. Biomaterials. Copyright 2021, with permission from Elsevier.95 |
Due to their exceptional ability to convert light energy into thermal energy, noble metals such as gold and silver are often regarded as promising candidates for antimicrobial nanomaterials. Their exceptional localized surface plasmon resonance (LSPR) properties are the primary reason for the photothermal effect.88,89 In most instances, Au is the material of choice for designing photothermal antimicrobial agents.90 For clinical applications, the physical photothermal effect based on gold nanoparticles (GNPs) has advantages in preventing bacterial drug resistance. Due to their higher absorption to scattering ratio in the near infrared (NIR) spectral region and the presence of more “hot spots” at the branch tips, gold nanostars (GNS) have greater potential for phototherapeutic applications than other GNPs.91–93 It has been reported that a star with eight points can generate 10 times the temperature of a spherical particle.94 Thus, Zhi et al used the photothermal effect of GNS to prepare pH-sensitive gold nanostars@H. pylori-antibodies nanoprobes (GNS@Ab) against H. pylori. They discovered that in gastric tissues containing H. pylori, the oral GNS@Ab nanoprobe captured live H. pylori and retained it for longer, with excess GNS@Ab nanoprobe being quickly removed. In contrast, the oral GNS@Ab nanoprobe was rapidly cleared in gastric tissue devoid of H. pylori. It is reassuring that low power NIR laser irradiation is non-invasive to gastric tissue, that all GNS@Ab nanoprobes can be eliminated from the intestine within 7 days after oral administration, and that the therapeutic dose of GNS@Ab nanoprobes does not disturb the intestinal bacterial balance, making it a safe treatment with great clinical translation potential (Figure 4B).91
The photodynamic effect of metal-based NPs can increase the oxidative stress induced by ROS production, thereby increasing their antibacterial activity, as described in the preceding section and not repeated here. No studies have been published on the use of MNPs photodynamic effects against H. pylori. However, the use of photodynamic effects against H. pylori has been partially demonstrated to be effective, eg, Im et al utilized multiple 3′-sialyllactose (3SL)-conjugated, poly-L-lysine-based photosensitizer (p3SLP) to promote H. pylori-targeting PDT by selectively identifying sialic acid-binding adhesin (SabA) in the H. pylori membrane via 3SL. p3SLP is administered orally to mice infected with H. pylori and irradiated with an endoscopic laser system, resulting in ROS-induced DNA and cell membrane damage. The results display that p3SLP exhibits comparable antimicrobial therapeutic effects to antibiotic-based treatments, with no adverse effects on normal tissues and intestinal flora, and because PDT is unlikely to induce resistance, it is a potential alternative strategy for treating antibiotic-resistant bacteria (Figure 4C).95 In addition, some studies have demonstrated that the combination of PDT and antibiotics has antibacterial synergistic effects without causing significant toxicity96 Although there is a lack of research on the photodynamic effect of MNPs against H. pylori, there have been numerous studies utilizing this effect against other strains of bacteria. Photothermal and photodynamic therapy studies utilizing MNPs for the eradication of H. pylori are expected to increase in the future.
Summary and Perspectives
According to the 15th edition of the carcinogen report in the United States,97,98 H. pylori infection is a global health concern and a known carcinogen. In addition, according to the H. pylori Taipei Global Consensus, the majority of gastric cancers can be avoided if H. pylori infection is eradicated from the population.99 Therefore, the global eradication of H. pylori is of great clinical importance, particularly in regions with a high incidence of gastric cancer. Currently, the anti-H. pylori agents rely primarily on the combination of two antibiotics, which is reported to be highly effective with >90% eradication rates.33 However, the prolonged and excessive use of antibiotics has facilitated the emergence of H. pylori with antibiotic resistance. In addition, the potential impact of widespread antibiotic use on gut microecology raises additional concerns regarding the eradication of H. pylori. Therefore, it is becoming increasingly risky to rely solely on antibiotics and related methods in the future to eradicate H. pylori. Accordingly, an urgent demand exists for new antibiotic-independent strategies that can maintain the ecological homeostasis of the gut ecosystem. In this review, we the current therapeutic strategies for H. pylori and the metal-based nanocarriers designed for the treatment of H. pylori infection, as well as a forecast of the potential future metal-based nanocarriers for the treatment of H. pylori. Summarizing, as a new strategy to eradicate H. pylori, the eradication mechanism of metal-based nanoparticles for H. pylori mainly includes the following aspects (Figure 5): (1) Metal ion release; (2) ROS production; (3) Photothermal and photodynamic effects. In addition, metal-based NPs can be prepared as a multifunctional platform with two or more H. pylori-killing capabilities due to their high surface-to-volume ratio, inherent positive charge, and amenability to surface modification. Moreover, the different chemical composition, size, and morphology of metal-based NPs can affect their antimicrobial efficacy and spectrum of action; these findings will likely contribute to the development of effective antimicrobial agents that do not develop resistance, thereby replacing current multidrug-resistant antibiotic therapies. Nevertheless, despite the proposition that metal-based NPs can be used for the detection and treatment of bacteria is undeniable, there are still hurdles to cross before these NPs can be applied as clinical anti-H. pylori agents: (1) Limited stability. Although it has been demonstrated that metal-based NPs exhibit potent antibacterial activity, their stability in the ambient environment, particularly in the harsh acidic environment of the stomach, may pose challenges for their future applications. Therefore, the greatest challenge for the future application of metal-based nanoparticles for the eradication of H. pylori is to determine how to improve their stability in the stomach; (2) Toxicity to cells. Cytotoxicity is an unavoidable aspect of nanoparticle design involving metals. Metal-based nanoparticles can be biologically toxic while killing H. pylori, so their second challenge is to reduce their toxic effects on cells. Copper ions, for instance, have a potent bactericidal effect; however, excessive copper can result in copper poisoning, which can cause spasms, neurodegenerative diseases, and even death. However, the risk of copper toxicity can be mitigated by the slow release of copper ions. Therefore, slowing the release of metal particles is an essential strategy for overcoming this challenge; (3) In vivo retention effect. The use of metal-based NPs for the eradication of H. pylori usually requires increasing the retention time of the drug at the site of H. pylori colonisation to achieve better bactericidal effects, but the long-term retention of nanomaterials in the human body would significantly compromise their biosafety. Thereby, a metal with enhanced biocompatibility, such as zinc, could be chosen as the core component of anti-H. pylori materials. In addition, some studies have combined metal nanoparticles and physiochemical therapies to produce synergistic effects and thus enhance the killing of H. pylori; (4) High cost. Gold and silver have high antimicrobial activity and a long history as antimicrobial materials, making them the material of choice for anti-H. pylori nanoparticle design. However, the use of gold and silver in clinical translation for mass production is frequently unrealistic. In designing H. pylori-resistant metal-based nanoparticles, we can therefore use inexpensive metals or investigate simple and inexpensive synthesis techniques.
The ultimate goal of scientific research is the successful clinical translation of study designs from the laboratory to the patient’s bedside. Moreover, in order to achieve clinical translation, the design of metal-based nanocarriers requires considerable attention. Firstly, the designed metal-based NPs must be highly effective against H. pylori and stable in the harsh acidic environment of the stomach. Secondly, to maintain the stability of the gastrointestinal microecology after H. pylori eradication, the formulation design should incorporate the ability of pathogenic microorganism selectivity or localizability to target infected areas. Last but not least, cost-effectiveness and mass production are the most important factors in achieving eventual clinical translation; additionally, a simple and relevant method of administration in clinical settings must be considered.
By meeting these criteria and through continuous innovation, metal nanomaterial-based strategies demonstrate great potential as antimicrobial technologies of the next generation for H. pylori treatment. Thus, we are confident that metal-based nanoparticles will help us achieve our ultimate goal of eradicating H. pylori in the near future.
Abbreviations
H. pylori, Helicobacter pylori; NPs, Nanoparticles; SPR, Surface plasmon resonance; ROS, Reactive oxygen species; BQT, Bismuth quadruple therapy; PPIs, Proton pump inhibitors; P-CAB, Potassium-competitive acid blockers; Fe, Iron; Mn, Manganese; Zn, Zinc; Cu, Copper; Co, Cobalt; Ni, Nickel; Ag, Silver; Ti, Titanium; Au, Gold; Si, Silicon; Bi, Bismuth; Mg, Magnesium; CBS, Colloidal bismuth subcitrate; MIC, Minimum inhibitory concentration; SUNCs, Silver Ultra-NanoClusters; ZnO NPs, Zinc oxide nanoparticles; OGE, Oak galls extract; O2•-, Superoxide anions; H2O2, Hydrogen peroxide; 1O2, Singlet oxygen; •OH, Hydroxyl radicals; MDA, Malondialdehyde; SOD, Superoxide dismutase; NIR, Near infrared; PS, Photosensitizers; PTT, Photothermal therapy; PDT, Photodynamic therapy; GNPs, Gold nanoparticles; GNS, Gold nanostars; SabA, Sialic acid-binding adhesin.
Acknowledgments
We acknowledge funding by National Natural Science Foundation of China (Nos. 82072051 and 81771964) and the Strategic Consulting Research Project of Chinese Academy of Engineering (2021-ZX-12), Project of Shanghai Municipal Health Commission (2019SY001), and Research project of National Digestive Disease Clinical Medical Center. Besides, we also thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Disclosure
The authors declare that there are no competing interests.
References
1. Tshibangu-Kabamba E, Yamaoka Y. Helicobacter pylori infection and antibiotic resistance - from biology to clinical implications. Nat Rev Gastroenterol Hepatol. 2021;18(9):613–629. doi:10.1038/s41575-021-00449-x
2. D’Elios MM, Appelmelk BJ, Amedei A, Bergman MP, Del Prete G. Gastric autoimmunity: the role of Helicobacter pylori and molecular mimicry. Trends Mol Med. 2004;10(7):316–323. doi:10.1016/j.molmed.2004.06.001
3. Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345(2):196–202. doi:10.1016/j.canlet.2013.08.016
4. Crowe SE. Helicobacter infection, chronic inflammation, and the development of malignancy. Curr Opin Gastroenterol. 2005;21(1):32–38.
5. van der Voort PH, van der Hulst RW, Zandstra DF, Geraedts AA, van der Ende A, Tytgat GN. Prevalence of Helicobacter pylori infection in stress-induced gastric mucosal injury. Intensive Care Med. 2001;27(1):68–73. doi:10.1007/s001340000773
6. Franceschi F, Covino M, Roubaud Baudron C. Review: Helicobacter pylori and extragastric diseases. Helicobacter. 2019;24(Suppl 1):e12636. doi:10.1111/hel.12636
7. HHS. 15th report on carcinogens. 2021.
8. Xie C, Li N, Wang H, et al. Inhibition of autophagy aggravates DNA damage response and gastric tumorigenesis via Rad51 ubiquitination in response to H. pylori infection. Gut Microbes. 2020;11(6):1567–1589. doi:10.1080/19490976.2020.1774311
9. Xie C, Yi J, Lu J, et al. N-acetylcysteine reduces ROS-mediated oxidative DNA damage and PI3K/Akt pathway activation induced by Helicobacter pylori infection. Oxid Med Cell Longev. 2018;2018:1874985. doi:10.1155/2018/1874985
10. Li N, Feng Y, Hu Y, et al. Helicobacter pylori CagA promotes epithelial mesenchymal transition in gastric carcinogenesis via triggering oncogenic YAP pathway. J Exp Clin Cancer Res. 2018;37(1):280. doi:10.1186/s13046-018-0962-5
11. Li WQ, Zhang JY, Ma JL, et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a randomized intervention trial. BMJ. 2019;366:l5016. doi:10.1136/bmj.l5016
12. Chiang TH, Chang WJ, Chen SL, et al. Mass eradication of Helicobacter pylori to reduce gastric cancer incidence and mortality: a long-term cohort study on Matsu Islands. Gut. 2021;70(2):243–250. doi:10.1136/gutjnl-2020-322200
13. Yan L, Chen Y, Chen F, et al. Effect of Helicobacter pylori eradication on gastric cancer prevention: updated report from a randomized controlled trial with 26.5 years of follow-up. Gastroenterology. 2022;163:154–162.e3. doi:10.1053/j.gastro.2022.03.039
14. Ren S, Cai P, Liu Y, et al. Prevalence of Helicobacter pylori infection in China: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2022;37(3):464–470. doi:10.1111/jgh.15751
15. Mégraud F, Graham DY, Howden CW, et al. Rates of antimicrobial resistance in Helicobacter pylori isolates from clinical trial patients across the US and Europe. Am J Gastroenterol. 2022;118:269–275. doi:10.14309/ajg.0000000000002045
16. Chen J, Li P, Huang Y, Guo Y, Ding Z, Lu H. Primary antibiotic resistance of in different regions of China: a systematic review and meta-analysis. Pathogens. 2022;11(7):786. doi:10.3390/pathogens11070786
17. Mutalik C, Krisnawati DI, Patil SB, et al. Phase-dependent MoS2 nanoflowers for light-driven antibacterial application. ACS Sustain Chem Eng. 2021;9(23):7904–7912. doi:10.1021/acssuschemeng.1c01868
18. Yougbaré S, Chou H-L, Yang C-H, et al. Facet-dependent gold nanocrystals for effective photothermal killing of bacteria. J Hazard Mater. 2021;407:124617. doi:10.1016/j.jhazmat.2020.124617
19. Mutalik C, Lin IH, Krisnawati DI, et al. Antibacterial pathways in transition metal-based nanocomposites: a mechanistic overview. Int J Nanomedicine. 2022;17:6821–6842. doi:10.2147/IJN.S392081
20. Mutalik C, Okoro G, Krisnawati DI, et al. Copper sulfide with morphology-dependent photodynamic and photothermal antibacterial activities. J Colloid Interface Sci. 2022;607(Pt 2):1825–1835. doi:10.1016/j.jcis.2021.10.019
21. Sahli C, Moya SE, Lomas JS, Gravier-Pelletier C, Briandet R, Hémadi M. Recent advances in nanotechnology for eradicating bacterial biofilm. Theranostics. 2022;12(5):2383–2405. doi:10.7150/thno.67296
22. Lemire J, Harrison J, Turner R. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol. 2013;11(6):371–384. doi:10.1038/nrmicro3028
23. Stoimenov PK, Zaikovski V, Klabunde KJ. Novel halogen and interhalogen adducts of nanoscale magnesium oxide. J Am Chem Soc. 2003;125(42):12907–12913. doi:10.1021/ja030195l
24. Sharma H, Mishra PK, Talegaonkar S, Vaidya B. Metal nanoparticles: a theranostic nanotool against cancer. Drug Discov Today. 2015;20(9):1143–1151. doi:10.1016/j.drudis.2015.05.009
25. Kotrange H, Najda A, Bains A, Gruszecki R, Chawla P, Tosif MM. Metal and metal oxide nanoparticle as a novel antibiotic carrier for the direct delivery of antibiotics. Int J Mol Sci. 2021;22(17):9596. doi:10.3390/ijms22179596
26. Wang S, McGuirk CM, d’Aquino A, Mason JA, Mirkin CA. Metal-organic framework nanoparticles. Adv Mater. 2018;30(37):e1800202. doi:10.1002/adma.201800202
27. Yuan P, Ding X, Yang YY, Xu Q-H. Metal nanoparticles for diagnosis and therapy of bacterial infection. Adv Healthc Mater. 2018;7(13):e1701392. doi:10.1002/adhm.201701392
28. Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C Mater Biol Appl. 2014;44:278–284. doi:10.1016/j.msec.2014.08.031
29. Ge X, Cao Z, Chu L. The antioxidant effect of the metal and metal-oxide nanoparticles. Antioxidants. 2022;11(4):25.
30. Toller IM, Neelsen KJ, Steger M, et al. Carcinogenic bacterial pathogen Helicobacter pylori triggers DNA double-strand breaks and a DNA damage response in its host cells. Proc Natl Acad Sci U S A. 2011;108(36):14944–14949. doi:10.1073/pnas.1100959108
31. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52(24):6735–6740.
32. Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353–1367. doi:10.1136/gutjnl-2015-309252
33. Malfertheiner P, Megraud F, Rokkas T, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022;71:1724–1762.
34. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut. 2017;66(1):6–30. doi:10.1136/gutjnl-2016-312288
35. Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterology. 2018;155(5):1372–1382.e17. doi:10.1053/j.gastro.2018.07.007
36. Shinozaki S, Kobayashi Y, Osawa H, et al. Effectiveness and safety of vonoprazan versus proton pump inhibitors for second-line Helicobacter pylori eradication therapy: systematic review and meta-analysis. Digestion. 2021;102(3):319–325. doi:10.1159/000504939
37. Lai Y, Wei W, Du Y, Gao J, Li Z. Biomaterials for Helicobacter pylori therapy: therapeutic potential and future perspectives. Gut Microbes. 2022;14(1):2120747. doi:10.1080/19490976.2022.2120747
38. Gerrits MM, van Vliet AHM, Kuipers EJ, Kusters JG. Helicobacter pylori and antimicrobial resistance: molecular mechanisms and clinical implications. Lancet Infect Dis. 2006;6(11):699–709. doi:10.1016/S1473-3099(06)70627-2
39. Hathroubi S, Servetas SL, Windham I, Merrell DS, Ottemann KM. Helicobacter pylori biofilm formation and its potential role in pathogenesis. Microbiol Mol Biol Rev. 2018;82(2). doi:10.1128/MMBR.00001-18
40. Lange K, Buerger M, Stallmach A, Bruns T. Effects of antibiotics on gut microbiota. Digest Dis. 2016;34(3):260–268. doi:10.1159/000443360
41. Stewart S, Wei Q, Sun Y. Surface chemistry of quantum-sized metal nanoparticles under light illumination. Chem Sci. 2020;12(4):1227–1239. doi:10.1039/D0SC04651E
42. Wu Y, Rong X, Zhang C, et al. Response of the intertidal microbial community structure and metabolic profiles to zinc oxide nanoparticle exposure. Int J Environ Res Public Health. 2020;17(7):35.
43. Sweet MJ, Chesser A, Singleton I. Review: metal-based nanoparticles; size, function, and areas for advancement in applied microbiology. Adv Appl Microbiol. 2012;80:113–142.
44. Sánchez-López E, Gomes D, Esteruelas G, et al. Metal-based nanoparticles as antimicrobial agents: an overview. Nanomaterials. 2020;10(2):292. doi:10.3390/nano10020292
45. Ren R, Lim C, Li S, et al. Recent advances in the development of lipid-, metal-, carbon-, and polymer-based nanomaterials for antibacterial applications. Nanomaterials. 2022;12(21). doi:10.3390/nano12213855
46. Wang Y, Yang Y, Shi Y, Song H, Yu C. Antibiotic-free antibacterial strategies enabled by nanomaterials: progress and perspectives. Adv Mater. 2020;32(18):e1904106. doi:10.1002/adma.201904106
47. Chandrangsu P, Rensing C, Helmann JD. Metal homeostasis and resistance in bacteria. Nat Rev Microbiol. 2017;15(6):338–350. doi:10.1038/nrmicro.2017.15
48. Begg SL. The role of metal ions in the virulence and viability of bacterial pathogens. Biochem Soc Trans. 2019;47(1):77–87. doi:10.1042/BST20180275
49. Botella H, Stadthagen G, Lugo-Villarino G, de Chastellier C, Neyrolles O. Metallobiology of host-pathogen interactions: an intoxicating new insight. Trends Microbiol. 2012;20(3):106–112. doi:10.1016/j.tim.2012.01.005
50. Stafford SL, Bokil NJ, Achard MES, et al. Metal ions in macrophage antimicrobial pathways: emerging roles for zinc and copper. Biosci Rep. 2013;33(4). doi:10.1042/BSR20130014
51. White C, Lee J, Kambe T, Fritsche K, Petris MJ. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem. 2009;284(49):33949–33956. doi:10.1074/jbc.M109.070201
52. Godoy-Gallardo M, Eckhard U, Delgado LM, et al. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: from mechanisms to applications. Bioact Mater. 2021;6(12):4470–4490. doi:10.1016/j.bioactmat.2021.04.033
53. Han D, Liu X, Wu S. Metal organic framework-based antibacterial agents and their underlying mechanisms. Chem Soc Rev. 2022;51(16):7138–7169. doi:10.1039/D2CS00460G
54. de Souza MPC, de Camargo BAF, Sposito L, et al. Highlighting the use of micro and nanoparticles based-drug delivery systems for the treatment of Helicobacter pylori infections. Crit Rev Microbiol. 2021;47(4):435–460. doi:10.1080/1040841X.2021.1895721
55. Cheeseman S, Christofferson AJ, Kariuki R, et al. Antimicrobial metal nanomaterials: from passive to stimuli-activated applications. Adv Sci. 2020;7(10):1902913. doi:10.1002/advs.201902913
56. Briand GG, Burford N. Bismuth compounds and preparations with biological or medicinal relevance. Chem Rev. 1999;99(9):2601–2658. doi:10.1021/cr980425s
57. Lambert JR, Midolo P. The actions of bismuth in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 1997;11(Suppl 1):27–33. doi:10.1046/j.1365-2036.11.s1.13.x
58. Li H, Wang R, Sun H. Systems approaches for unveiling the mechanism of action of bismuth drugs: new medicinal applications beyond Helicobacter pylori infection. Acc Chem Res. 2019;52(1):216–227. doi:10.1021/acs.accounts.8b00439
59. Ge R, Chen Z, Zhou Q. The actions of bismuth in the treatment of Helicobacter pylori infections: an update. Metallomics. 2012;4(3):239–243. doi:10.1039/c2mt00180b
60. Chen R, Cheng G, So MH, et al. Bismuth subcarbonate nanoparticles fabricated by water-in-oil microemulsion-assisted hydrothermal process exhibit anti-Helicobacter pylori properties. Mater Res Bull. 2010;45(5):654–658. doi:10.1016/j.materresbull.2009.12.035
61. Nazari P, Dowlatabadi-Bazaz R, Mofid MR, et al. The antimicrobial effects and metabolomic footprinting of carboxyl-capped bismuth nanoparticles against Helicobacter pylori. Appl Biochem Biotechnol. 2014;172(2):570–579. doi:10.1007/s12010-013-0571-x
62. Matsumura Y, Yoshikata K, Kunisaki S-I, Tsuchido T. Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl Environ Microbiol. 2003;69(7):4278–4281. doi:10.1128/AEM.69.7.4278-4281.2003
63. Morones JR, Elechiguerra JL, Camacho A, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16(10):2346–2353. doi:10.1088/0957-4484/16/10/059
64. Amin M, Anwar F, Janjua MR, Iqbal MA, Rashid U. Green synthesis of silver nanoparticles through reduction with Solanum xanthocarpum L. berry extract: characterization, antimicrobial and urease inhibitory activities against Helicobacter pylori. Int J Mol Sci. 2012;13(8):9923–9941. doi:10.3390/ijms13089923
65. Amin M, Hameed S, Ali A, et al. Green synthesis of silver nanoparticles: structural features and in vivo and in vitro therapeutic effects against Helicobacter pylori induced gastritis. Bioinorg Chem Appl. 2014;2014:135824. doi:10.1155/2014/135824
66. Gurunathan S, Jeong J-K, Han JW, Zhang X-F, Park JH, Kim J-H. Multidimensional effects of biologically synthesized silver nanoparticles in Helicobacter pylori, Helicobacter felis, and human lung (L132) and lung carcinoma A549 cells. Nanoscale Res Lett. 2015;10:35. doi:10.1186/s11671-015-0747-0
67. Grande R, Sisto F, Puca V, et al. Antimicrobial and antibiofilm activities of new synthesized silver ultra-nanoclusters (SUNCs) against Helicobacter pylori. Front Microbiol. 2020;11:1705. doi:10.3389/fmicb.2020.01705
68. Carron MA, Tran VR, Sugawa C, Coticchia JM. Identification of Helicobacter pylori biofilms in human gastric mucosa. J Gastrointest Surg. 2006;10(5):712–717. doi:10.1016/j.gassur.2005.10.019
69. Graves JL, Tajkarimi M, Cunningham Q, et al. Rapid evolution of silver nanoparticle resistance in Escherichia coli. Front Genet. 2015;6:42. doi:10.3389/fgene.2015.00042
70. Mobley HL, Island MD, Hausinger RP. Molecular biology of microbial ureases. Microbiol Rev. 1995;59(3):451–480. doi:10.1128/mr.59.3.451-480.1995
71. Carter EL, Flugga N, Boer JL, Mulrooney SB, Hausinger RP. Interplay of metal ions and urease. Metallomics. 2009;1(3):207–221. doi:10.1039/b903311d
72. Bruggraber SFA, French G, Thompson RPH, Powell JJ. Selective and effective bactericidal activity of the cobalt (II) cation against Helicobacter pylori. Helicobacter. 2004;9(5):422–428. doi:10.1111/j.1083-4389.2004.00264.x
73. Jarestan M, Khalatbari K, Pouraei A, et al. Preparation, characterization, and anticancer efficacy of novel cobalt oxide nanoparticles conjugated with thiosemicarbazide. 3 Biotech. 2020;10(5):230. doi:10.1007/s13205-020-02230-4
74. Xia X, Yin Z, Yang Y, et al. Upregulating heat shock protein 70 via gastric nano-heaters for the interference of infection. ACS Nano. 2022;16(9):14043–14054. doi:10.1021/acsnano.2c03911
75. Yang B, Chen Y, Shi J. Reactive oxygen species (ROS)-based nanomedicine. Chem Rev. 2019;119(8):4881–4985. doi:10.1021/acs.chemrev.8b00626
76. Jain U, Saxena K, Chauhan N. Helicobacter pylori induced reactive oxygen Species: a new and developing platform for detection. Helicobacter. 2021;26(3):e12796. doi:10.1111/hel.12796
77. D’Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8(10):813–824. doi:10.1038/nrm2256
78. Davies GR, Simmonds NJ, Stevens TR, et al. Helicobacter pylori stimulates antral mucosal reactive oxygen metabolite production in vivo. Gut. 1994;35(2):179–185. doi:10.1136/gut.35.2.179
79. Naito Y, Yoshikawa T. Molecular and cellular mechanisms involved in Helicobacter pylori-induced inflammation and oxidative stress. Free Radic Biol Med. 2002;33(3):323–336. doi:10.1016/S0891-5849(02)00868-7
80. Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4(3):181–189. doi:10.1038/nri1312
81. Handa O, Naito Y, Yoshikawa T. Helicobacter pylori: a ROS-inducing bacterial species in the stomach. Inflamm Res. 2010;59(12):997–1003. doi:10.1007/s00011-010-0245-x
82. Ding S-Z, Minohara Y, Fan XJ, et al. Helicobacter pylori infection induces oxidative stress and programmed cell death in human gastric epithelial cells. Infect Immun. 2007;75(8):4030–4039. doi:10.1128/IAI.00172-07
83. Li Y, Zhang W, Niu J, Chen Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano. 2012;6(6):5164–5173. doi:10.1021/nn300934k
84. Jeyaraj Pandian C, Palanivel R, Dhanasekaran S. Screening antimicrobial activity of nickel nanoparticles synthesized using Ocimum sanctum leaf extract. J Nanoparticles. 2016;2016:1–13. doi:10.1155/2016/4694367
85. Yoon K-Y, Hoon Byeon J, Park J-H, Hwang J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci Total Environ. 2007;373(2–3):572–575. doi:10.1016/j.scitotenv.2006.11.007
86. Simon-Deckers A, Loo S, Mayne-L’hermite M, et al. Size-, composition- and shape-dependent toxicological impact of metal oxide nanoparticles and carbon nanotubes toward bacteria. Environ Sci Technol. 2009;43(21):8423–8429. doi:10.1021/es9016975
87. Hou X, Tao Y, Pang Y, Li X, Jiang G, Liu Y. Nanoparticle-based photothermal and photodynamic immunotherapy for tumor treatment. Int J Cancer. 2018;143(12):3050–3060. doi:10.1002/ijc.31717
88. Gopinath V, Priyadarshini S, MubarakAli D, et al. Anti-Helicobacter pylori, cytotoxicity and catalytic activity of biosynthesized gold nanoparticles: multifaceted application. Arab J Chem. 2019;12(1):33–40. doi:10.1016/j.arabjc.2016.02.005
89. Jain PK, Huang X, El-Sayed IH, El-Sayed MA. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc Chem Res. 2008;41(12):1578–1586. doi:10.1021/ar7002804
90. Xu J-W, Yao K, Xu Z-K. Nanomaterials with a photothermal effect for antibacterial activities: an overview. Nanoscale. 2019;11(18):8680–8691. doi:10.1039/C9NR01833F
91. Zhi X, Liu Y, Lin L, et al. Oral pH sensitive GNS@ab nanoprobes for targeted therapy of Helicobacter pylori without disturbance gut microbiome. Nanomedicine. 2019;20:102019. doi:10.1016/j.nano.2019.102019
92. Liang S, Li C, Zhang C, et al. CD44v6 monoclonal antibody-conjugated gold nanostars for targeted photoacoustic imaging and plasmonic photothermal therapy of gastric cancer stem-like cells. Theranostics. 2015;5(9):970–984. doi:10.7150/thno.11632
93. Xia F, Hou W, Zhang C, et al. pH-responsive gold nanoclusters-based nanoprobes for lung cancer targeted near-infrared fluorescence imaging and chemo-photodynamic therapy. Acta Biomaterialia. 2018;68:308–319. doi:10.1016/j.actbio.2017.12.034
94. Rodríguez-Oliveros R, Sánchez-Gil JA. Gold nanostars as thermoplasmonic nanoparticles for optical heating. Opt Express. 2012;20(1):621–626. doi:10.1364/OE.20.000621
95. Im BN, Shin H, Lim B, et al. Helicobacter pylori-targeting multiligand photosensitizer for effective antibacterial endoscopic photodynamic therapy. Biomaterials. 2021;271:120745. doi:10.1016/j.biomaterials.2021.120745
96. Baccani I, Faraoni P, Marini M, et al. Synergistic effect of photodynamic therapy at 400 nm and doxycycline against. Future Microbiol. 2019;14:1199–1205. doi:10.2217/fmb-2019-0129
97. Chen X, Zou Y, Zhang S, et al. Multi-functional vesicles improve Helicobacter pylori eradication by a comprehensive strategy based on complex pathological microenvironment. Acta Pharma Sinica B. 2022;12(9):3498–3512. doi:10.1016/j.apsb.2022.05.014
98. Lunn RM, Mehta SS, Jahnke GD, Wang A, Wolfe MS, Berridge BR. Cancer hazard evaluations for contemporary needs: highlights from new national toxicology program evaluations and methodological advancements. J Natl Cancer Inst. 2022;114(11):1441–1448. doi:10.1093/jnci/djac164
99. Liou JM, Malfertheiner P, Lee YC, et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut. 2020;69(12):2093–2112. doi:10.1136/gutjnl-2020-322368
100. Attia HG, Albarqi HA, Said IG, Alqahtani O, Raey MAE. Synergistic effect between amoxicillin and zinc oxide nanoparticles reduced by oak gall extract against. Molecules. 2022;27(14):4559. doi:10.3390/molecules27144559
101. Zhang W, Zhou Y, Fan Y, et al. Metal-organic-framework-based hydrogen-release platform for multieffective Helicobacter pylori targeting therapy and intestinal flora protective capabilities. Adv Mater. 2022;34(2):e2105738. doi:10.1002/adma.202105738
102. Saravanan M, Gopinath V, Chaurasia MK, Syed A, Ameen F, Purushothaman N. Green synthesis of anisotropic zinc oxide nanoparticles with antibacterial and cytofriendly properties. Microb Pathog. 2018;115:57–63. doi:10.1016/j.micpath.2017.12.039
103. Thung I, Aramin H, Vavinskaya V, et al. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43(4):514–533. doi:10.1111/apt.13497
104. Chen R, So MH, Yang J, Deng F, Che C-M, Sun H. Fabrication of bismuth subcarbonate nanotube arrays from bismuth citrate. Chem Commun. 2006;(21):2265–2267. doi:10.1039/b601764a
105. Chakraborti S, Bhattacharya S, Chowdhury R, Chakrabarti P. The molecular basis of inactivation of metronidazole-resistant Helicobacter pylori using polyethyleneimine functionalized zinc oxide nanoparticles. PLoS One. 2013;8(8):e70776. doi:10.1371/journal.pone.0070776
106. Xiu ZM, Zhang QB, Puppala HL, Colvin VL, Alvarez PJJ. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012;12(8):4271–4275. doi:10.1021/nl301934w
107. Lennicke C, Cochemé HM. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol Cell. 2021;81(18):3691–3707. doi:10.1016/j.molcel.2021.08.018
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.