Back to Journals » Clinical and Experimental Gastroenterology » Volume 12
Mesenteric panniculitis: comparison of computed tomography findings in patients with and without malignancy
Authors Al-Omari MH , Qararha K, Garaleh M, Smadi MM , Bani Hani M , Elheis M
Received 10 August 2018
Accepted for publication 30 November 2018
Published 27 December 2018 Volume 2019:12 Pages 1—8
DOI https://doi.org/10.2147/CEG.S182513
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Koulaouzidis
Mamoon H Al-Omari,1 Khaleel Qararha,1 Mohammed Garaleh,1 Mahmoud M Smadi,2 Mohammed Bani Hani,3 Mwaffaq Elheis1
1Department of Radiology, Jordan University of Science and Technology, King Abdullah University Hospital, Irbid, Jordan; 2Department of Mathematics and Statistics, Jordan University of Science and Technology, Irbid, Jordan; 3Department of Surgery, Jordan University of Science and Technology, King Abdullah University Hospital, Irbid, Jordan
Purpose: The aim was to compare computed tomography (CT) findings between patients with mesenteric panniculitis (MP) with and without known malignancy.
Patients and methods: We retrospectively analyzed 116 consecutive patients who were diagnosed with MP on the basis of CT findings and categorized them according to the absence (Group 1: 73 patients) or presence (Group 2: 43 patients) of malignancy. Patient age and sex, diameter, size, mass effect, location, and fat density of the MP mass, presence of a pseudocapsule and/or halo sign, and lymph node status were compared between the two groups.
Results: MP tends to be more common in males, and this trend shows statistical significance when combining the findings for both groups (P=0.041). Patients in Group 1 were significantly younger than those in Group 2 (54.29 vs 64.77 years, P=0.001). A well-defined fatty mass at the small bowel root was observed in all patients. The halo sign was present in most cases in both groups. A pseudocapsule was observed in 36 patients (49%) in Group 1 and 29 (67%) in Group 2 (P=0.045). The average craniocaudal diameter of the MP masses on the sagittal view was 11.14 and 12.5 cm in Groups 1 and 2, respectively (P=0.005). The MP fat density was less negative in patients with malignancy (–66 vs –76 HU, P=0.001). Lymph node status was similar in both groups.
Conclusion: Detailed CT features should be evaluated in patients with MP, as some of these features may indicate an associated malignancy, necessitating further investigation and close follow-up.
Keywords: imaging, mesenteric inflammation, paraneoplastic
Introduction
Mesenteric panniculitis (MP) is an idiopathic, localized inflammation involving the adipose tissue of the small bowel mesentery.1,2 Similar mesenteric inflammatory conditions have been described in the literature as retractile mesenteritis, sclerosing mesenteritis, and mesenteric lipodystrophy.3 However, MP has specific radiological features. On computed tomography (CT) scans, MP is characterized by localized mesenteric thickening and stranding covering the blood vessels with a characteristic halo sign, in which the fat around the lymph nodes and blood vessels is spared. MP is also characterized by a pseudocapsule and small lymph nodes engulfed within the inflamed mass.2,4,5
The histopathological findings of different mesenteric inflammatory conditions overlap and are characterized by fibrosis, chronic inflammation, and fat necrosis to variable degrees, depending on the extent and severity of the condition.3,6 The cytomorphological patterns in these cases are characterized by lymphocytic infiltrates, plasma cells, and occasional eosinophils.6 The radiological prevalence of MP varies between 0.16 and 7.8%.4,7–10 Nevertheless, this condition is underdiagnosed and its epidemiology remains uncertain. A few causes have been suggested, including previous abdominal trauma or surgery, autoimmune diseases, ischemia, and infections.5,7,9,10
The association of MP with malignancy has been reported to range from 1 to 75%, with lymphoma being the most common malignancy reported in the literature.5,7,9–11 Clinically, most patients are asymptomatic; however, some patients report nonspecific symptoms, including abdominal pain, weight changes, diarrhea, and occasionally fever.1,7,12 Patients may also show signs and symptoms related to the underlying malignancy (eg, pleural effusion, fever, and jaundice).5,7,8,13
Nevertheless, MP remains a clinical conundrum frequently encountered during routine abdominal CT imaging. It often appears as if further imaging is not required, especially for asymptomatic patients in whom associated factors like previous abdominal surgery may explain the MP finding.10 Furthermore, imaging is not justified for routine follow-up in typical cases when malignancy has been ruled out.14 Otherwise, a high degree of suspicion needs to be maintained for MP patients with a higher risk of malignancy. In this situation, there is a need for guidelines for imaging protocols and follow-up assessments in such patients.13
To the best of our knowledge, no previous report has compared the detailed radiological signs of MP between malignant and nonmalignant conditions. Therefore, the objective of our study was to compare the CT findings of MP between patients with and without known malignancy.
Patients and methods
This retrospective study included 116 patients who underwent abdominal CT scanning at King Abdulla University Hospital between January 2014 and January 2017 and who had been diagnosed with MP on the basis of the CT criteria. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the King Abdulla University Hospital Review Board (approved on April 26, 2018, number: 101-2018). Our institutional review board waived the need to obtain informed consent since this study was a retrospective analysis of patient data and a review of patients’ radiological files. Our hospital information system (HIS) and picture archiving and communicating system (PACS) were used to select patients. Patient data were stored electronically within a secure system, and only authorized health professionals could access the data using their own codes. Patient’s name and identification number were omitted and rendered anonymous before data analysis.
The term “mesenteric panniculitis” was used as a key term to identify the cohort. The CT scans of the selected patients were reviewed by two experienced radiologists to confirm the diagnoses. Patients were excluded from our study if they met any of the following criteria: cases of diagnostic disagreement between the reviewing radiologists; the presence of ascites; the presence of mesenteric congestion due to any cause (eg, liver cirrhosis and mesenteric ischemia); evidence of primary or secondary mesenteric tumors (eg, deposits, mesenteric lymphoma, and desmoid tumor); or positive activity on positron-imaging tomography scans.
CT protocol
All patients underwent abdominal and pelvic multislice CT scans using a 64-slice CT scanner (Brilliance 64; Philips Healthcare, Best, The Netherlands). In 65 cases (56%), the scans were performed following the administration of intravenous (IV) and oral contrast media (iopromide; Ultravist® 370; Schering, Berlin, Germany) according to our institutional protocol. Fifty-one patients (46%) did not receive IV or oral contrast agent because they were scanned to rule out urinary stones, had a renal failure, or a known major allergic reaction to iodinated contrast media. A scout film was obtained for the entire abdomen and pelvis and the CT scan was taken from the base of the lungs down to the level of the symphysis pubis. The collimation was 64 mm ×0.625 mm, the gantry rotation time was 350 ms, the tube current was 200–300 mA, and the voltage was 120–140 kV. CT images were reconstructed using a multiplanar reconstruction algorithm and were reviewed at a window suited to soft tissue analysis. The high rate of nonenhanced scans in our cohort was related to the fact that our hospital is a urology referral center, and many patients with urolithiases undergo scans on a daily basis
CT analysis
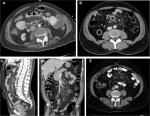
All examinations were reviewed by two radiologists who were blinded to the patients’ diagnoses. Mutual agreement between the two radiologists for the diagnosis and for each study variable was mandatory. Image analysis was performed using a dedicated workstation (PACCS; Fuji, Japan) in the axial, sagittal, and coronal planes to confirm the diagnosis and for assessments of each of the examined criteria. Our CT diagnostic criteria for MP included the presence of at least three of the five known cardinal signs of MP2,7,10: sign 1, a well-defined mesenteric fatty mass; sign 2, a mass with higher density than the adjacent abdominal fat; sign 3, the presence of blood vessels and small lymph nodes inside the mass; sign 4, a “halo sign” or fat ring sign around the lymph nodes and vessels; and sign 5, the presence of a hyperattenuating stripe around the mass, known as a pseudocapsule (Figure 1). The anteroposterior and transverse diameters of the mass were measured from axial views,10,16 while the craniocaudal (CC) diameter was measured from the sagittal views.
Other CT findings, including the number of lymph nodes and the short axis of the largest lymph node, were recorded.4 In addition, the densities of the mesenteric and retroperitoneal fat, presented in Hounsfield units (HU), were measured by choosing a circular region of interest approximately 1 cm in diameter away from the vessels and lymph nodes within the mass of the MP.10
Patient selection
The patients were divided into the following two groups: Group 1 included 73 patients with MP and no evidence of known primary malignancy and Group 2 included 43 patients with MP and known malignancy. All patients in both groups were selected consecutively, regardless of age and sex. The imaging studies and medical records were retrieved from the PACS and HIS to confirm the presence or absence of malignancy.
Statistical analyses
All analyses were performed using the SPSS (SPSS Inc., Chicago, IL, USA) version 12.1. Both descriptive and inferential statistics were used in the statistical analysis. Among descriptive statistics, we determined the mean, SD, and range for the continuous variables and percentages, OR, and RR for the categorical variables. Inferential statistics included the results of Chi-squared tests for independence between pairs of categorical variables; for comparisons of two groups, proportions were tested for categorical variables, while the means of continuous variables were compared using two independent-sample t tests. Univariate analysis was used to determine the proposed risk factors of associated malignancy in MP patients. P values <0.05 were considered statistically significant.
Results
Patient demographics and clinical presentations
In the study population of 116 patients, MP was more common in males (P=0.041; Table 1). Of the 73 patients in Group 1, 45 (62%) patients were males and 28 (38%) patients were females; the mean age of the men was 53.96 years (range, 23–83 years), and the mean age of the females was 54.82 years (range, 23–70 years); the male-to-female ratio was 1.6:1. Of the 43 patients in Group 2, 24 (56%) patients were males and 19 (44%) patients were females; the mean age of the men was 65.67 years (range, 50–94 years) and the mean age of the females was 63.63 years (range, 46–103 years); the male-to-female ratio was 1.3:1.
Patients in Group 2 were significantly older (P<0.001), with a mean age of 64.77 years, while the mean age of patients in Group 1 was 54.29 years (Table 1). The peak incidence of MP in Group 1 was noted in individuals between 50 and 59 years of age, while in Group 2, the peak was noted in individuals aged between 60 and 69 years (Figure 2). The calculated risk of malignancy in patients with MP above the age of 60 years was statistically significant (OR: 3.668, RR: 2.228).
  | Figure 2 Percentage of mesenteric panniculitis cases in both groups according to age group. |
The main indications for CT among patients in Group 1 were as follows: renal colic (33 cases; 45%); abdominal pain (16 cases; 22%); trauma (six cases; 8%); hematuria, leg swelling, renal cysts, and examination to rule out masses (three cases each; 4% for each condition); fever and jaundice (two cases each; 2.7% for each condition); and anemia and pancreatitis (one case each; 1.4% for each condition). CT scanning in Group 2 was performed for cancer staging in 32 (74.4%) patients and to rule out malignancy in 11 (25.6%) patients. None of our patients were diagnosed with malignancy after the diagnosis of MP. The associated malignancies in the Group 2 patients are listed in Table 2.
  | Table 2 Associated malignancies of the patients in Group 2 Abbreviation: CML, chronic myeloid leukemia. |
CT features
The cardinal signs of MP were evaluated in both groups (Table 3). The following criteria were universally present in both groups: a well-defined mesenteric fatty mass originating from the small bowel mesentery, a higher density in the mass than in the adjacent abdominal fat, and the presence of blood vessels and small lymph nodes inside the mass. A pseudocapsule was present in 36 (49%) patients in Group 1 and 29 (67%) patients in Group 2 (P=0.05). The halo sign was present in 63 (86%) patients in Group 1 and 37 (86%) patients in Group 2 (P=0.967).
  | Table 3 CT characteristics of mesenteric panniculitis in both groups Abbreviation: CT, computed tomography. |
The total number of positive criteria (PC) was calculated in both groups (Table 4). Since the diagnosis requires the presence of at least three of the five CT signs of MP, the minimum and maximum numbers of total PC were 3 and 5, respectively. The majority of patients in Group 2 (63%) met five PC, while only 48% of the patients in Group 1 met five PC; however, there was no significant difference between the two groups (P=0.115).
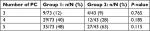  | Table 4 Total number of PC Notes: n; number of patients with PC, N; total number of patients. Abbreviation: PC, positive criteria. |
MP origin in relation to the root of the small bowel mesentery was identified in both groups. Group 1 patients showed a fixed origin from the mesentery of the jejunum located in the left upper outer quadrant of the abdominal cavity. In the second group, there was some variation: in 40 (93%) patients, the MP originated in the left upper quadrant; in two (4.6%) patients, MP was related to the root of the jejunum, in the right upper quadrant; and in one (2.3%) patient, it was related to the ilium, in the left lower quadrant. There was a trend toward some variation in Group 2.
The lymph node status showed no significant variation in the either group. The average number of lymph nodes in Groups 1 and 2 was 20.2 (range, 6–35) and 21.08 (range, 5–40), respectively (P=0.525). The mean size of the largest lymph node in Group 1 was 6.91 mm, while in Group 2, it was 7.1 mm (P=0.701).
The volume was estimated using the basic ellipsoid equation commonly used in radiology for volume measurement (p/6× L × W × D), which is equivalent to height × transverse × anteroposterior ×0.52.15 Group 2 patients tended to have a larger-sized masses than Group 1 patients. The average size was 301.8 cm3 in Group 1 and 390.61 cm3 in Group 2. A mass effect on the adjacent bowel loops was present in 78% of the cases in Group 1 and 84% of those in Group 2 (P=0.448). The density of the mass lesion was measured in HU. The average HU in Group 1 was –76 and that in Group 2 was –66 (P=0.001). A similar finding was observed regarding the density of the peritoneal fat in both groups (P=0.001).
A summary of all variables is presented in Table 5, and the proposed high-risk factors for associated malignancy are summarized in Table 6.
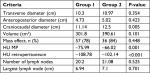  | Table 5 Measurement of the dimensions, volume, mass effect, fat density, and lymph nodes of the MP masses in both groups Abbreviations: HU, Hounsfield unit; MP, mesenteric panniculitis. |
  | Table 6 Risk factors for associated malignancy in patients with MP: RR with 95% CIs and ORs Abbreviation: HU, Hounsfield unit; MP, mesenteric panniculitis. |
Discussion
We found some morphological differences between MP associated with malignant and nonmalignant conditions. In paraneoplastic conditions, MP was associated with more hyperdense fat (misty mesentery), a higher frequency of pseudocapsule formation, and a longer mean CC diameter than MP in nonmalignant conditions. Our CT findings for some of the cardinal signs of MP correspond with those of previous research reports.2,4,7 The detailed description of these signs presented in our study is novel; furthermore, the criteria for distinguishing between MP in non-malignant and malignant conditions have not been reported to date.
In the present study, the first three cardinal CT signs of MP were present in all patients in both groups, indicating that these signs are essential for the diagnosis of MP. This is similar to previously published findings. Badet et al11 investigated 158 patients with MP and found that hyperattenuation of a fatty mass containing small lymph nodes with evidence of a mass effect on adjacent bowel loops was present in all cases; similar results were also reported by Coulier.4
The halo sign or fat ring sign is characterized by clear fat around the lymph nodes, with blood vessels inside, and is considered to be one of the specific signs of MP.2,4 Our study showed that this sign was observed almost equally in both groups. This indicates that MP spares the fat around the vessels and lymph nodes regardless of the clinical status of the patient. Some previous studies reported that the halo sign was present in 50–60% of cases,10,11,13 while in our study, this sign was seen in 86% of the cases overall. The higher incidence of this sign in our study is mostly related to the fact that, in our study, two radiologists were looking for each sign simultaneously, making it unlikely that the signs would be missed. Furthermore, images were interpreted on axial, sagittal, and coronal views, which increased the likelihood of detecting this sign. We noticed that, in a significant number of patients, the halo sign was seen only on the coronal and/or sagittal views, but not on axial views; most of the previous studies utilized axial images to detect this sign.
The hallmark of this study is evidenced by the presence of sign 5. Patients with MP in Group 2 were significantly more likely to have a pseudocapsule than patients in Group 1; this finding has not been reported previously. This may indicate that the paraneoplastic MP is more defined and organized, probably due to its slow-growing characteristic. Therefore, radiologists should pay marked attention to this sign, because when it was present, the likelihood of malignancy increases significantly (OR: 2.129, RR: 1.625, P=0.045). This capsule is actually fibrous tissue surrounding the mass, which might be secondary to chronic inflammation. We noticed that in some cases, the capsule was continuous, while in others, it was interrupted; these findings require further investigation.
In the current study, MP was more frequent in individuals aged 60–70 years, which is similar to the findings of previous reports.4,10,11,15,16 Our study found that patients in Group 2 were significantly older than those in Group 1 (the mean age in Groups 1 and 2 was 54.29 and 64.77 years, respectively); this is consistent with the natural history of malignancy, where increased frequency is observed with age.17 The incidental finding of MP on routine abdominal CT in patients aged above 60 years should be cautiously considered, and hidden malignancy should be ruled out (OR: 3.7, RR: 2.2).
Most previous studies have reported that the disease is more common in men, with a male-to-female ratio of 2–3:1.4,10,14 In both groups in the present study, MP was more common in males (P=0.041); the male-to-female ratios in Group 1 and Group 2 were 1.6:1 and 1.3:1, respectively, with an overall male-to-female ratio of 1.5:1. In our study, the male predominance appeared less evident than in previous reports; this may be related to the ethnicity of our patients (Arab), as several reports have indicated that MP is more common in Caucasian males.16
Previous studies have reported the presence of an increased density of fat within MP tissue as compared to normal fat in the mesentery or the subcutaneous tissue; however, we found only two studies that had measured the actual density of such fat, although no correlation with malignancy was found.10,18 In our study, there was a significant difference in terms of the HU between both groups. MP in patients with malignancy had more dirty fat (HU, –66 to –76); the likelihood of associated malignancy in patients with MP with a fat density above –70 was 2.43-fold higher than in patients with an MP fat density lower than –70 HU (OR: 2.429, RR: 1.714, P=0.02). A similar finding was observed in terms of the density of the normal-looking peritoneal fat in both groups (P=0.001). This finding is clinically significant and novel; it might be partially explained by the fact that patients with malignancy usually had weight loss that would be directly reflected in the fat density as measured by CT scan.
In our study, there was a significant association between the CC diameter of the MP mass as measured on sagittal views and malignancy. Patients with an MP mass of more than 11.5 cm in CC diameter had more than a twofold greater risk of malignancy as compared to those with an MP mass shorter than 11.5 cm (OR: 2.129, RR: 1.625, P=0.004). This finding has not been reported previously and needs to be confirmed by larger studies.
The current literature on this subject is conflicting and describes vague relationships between MP and different types of cancer. In our study, we were able to identify some imaging signs of MP that were associated with a higher risk of associated malignancy. Our data suggest that the mere diagnosis of MP on CT scans might be inadequate, and a detailed description of the imaging signs would be more appropriate. Guidelines regarding further investigation and imaging follow-up for subgroups of patients with MP are lacking and need to be established by the scientific community.
This retrospective study had some limitations. First, the CT database was searched using the terms “mesenteric panniculitis” to define the cohort; some patients could have been overlooked as a result. Second, MP was diagnosed radiologically and none of the patients were diagnosed on the basis of histopathological findings. Third, the CT scan protocol was not standardized; some patients received oral and IV contrast, while others did not. Finally, there were no long-term follow-up assessments for the control group to detect possible future malignancy and no age- and sex-matched groups to compare confounding factors.
Conclusion
Detailed CT features of MP should be evaluated in all patients and should not be overlooked by radiologists. Some features might be relevant in terms of clinical prediction of an associated neoplasm. Patients with high-risk criteria for associated malignancy should be thoroughly investigated and might require regular follow-up. Larger prospective, blinded, and matched randomized studies are necessary to confirm our findings.
Acknowledgments
We would like to thank the Deanship of Research at the Jordan University of Science and Technology for the approval and continuous support of this study. We would also like to thank Editage (www.editage.com) for English language editing. No financial support was received for this study.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Issa I, Baydoun H. Mesenteric panniculitis: various presentations and treatment regimens. World J Gastroenterol. 2009;15(30):3827–3830. | ||
Coulier B. Mesenteric panniculitis part 1: mdct – pictorial review. J Belg Soc Radiol. 2011;94(5):229–240. | ||
Emory TS, Monihan JM, Carr NJ, Sobin LH. Sclerosing mesenteritis, mesenteric panniculitis and mesenteric lipodystrophy: a single entity? Am J Surg Pathol. 1997;21(4):392–398. | ||
Coulier B. Mesenteric panniculitis. Part 2: prevalence and natural course: MDCT prospective study. J Belg Soc Radiol. 2011;94(5):241–246. | ||
Zissin R, Metser U, Hain D, Even-Sapir E. Mesenteric panniculitis in oncologic patients: PET-CT findings. Br J Radiol. 2006;79(937):37–43. | ||
Levy AD, Rimola J, Mehrotra AK, Sobin LH. From the archives of the AFIP: benign fibrous tumors and tumorlike lesions of the mesentery: radiologic-pathologic correlation. Radiographics. 2006;26(1):245–264. | ||
Daskalogiannaki M, Voloudaki A, Prassopoulos P, et al. CT evaluation of mesenteric panniculitis: prevalence and associated diseases. AJR Am J Roentgenol. 2000;174(2):427–431. | ||
Wilkes A, Griffin N, Dixon L, Dobbs B, Frizelle FA. Mesenteric panniculitis: a paraneoplastic phenomenon? Dis Colon Rectum. 2012;55(7):806–809. | ||
Smith ZL, Sifuentes H, Deepak P, Ecanow DB, Ehrenpreis ED. Relationship between mesenteric abnormalities on computed tomography and malignancy: clinical findings and outcomes of 359 patients. J Clin Gastroenterol. 2013;47(5):409–414. | ||
Mahafza WS, Manzalawi KA, Gharaibeh AA, Khayat OW, Shahait A, Juweid ME. Diagnosis of mesenteric panniculitis in the multi-detector computed tomography era. Association with malignancy and surgical history. Saudi Med J. 2017;38(10):1013–1018. | ||
Badet N, Sailley N, Briquez C, Paquette B, Vuitton L, Delabrousse É. Mesenteric panniculitis: still an ambiguous condition. Diagn Interv Imaging. 2015;96(3):251–257. | ||
Hirano H, Yoshida A, Sasae Y, Sakuta T, Morita Y. Mesenteric panniculitis: a rare cause of fever. Int J Rheum Dis. 2012;15(2):e40–e42. | ||
Scheer F, Spunar P, Wiggermann P, Wissgott C, Andresen R. Mesenteric panniculitis (MP) in CT – a predictor of malignancy? Rofo. 2016;188(10):926–932. | ||
Nyberg L, Björk J, Björkdahl P, Ekberg O, Sjöberg K, Vigren L. Sclerosing mesenteritis and mesenteric panniculitis – clinical experience and radiological features. BMC Gastroenterol. 2017;17(1):75. | ||
Dachman AH, MacEneaney PM, Adedipe A, Carlin M, Schumm LP. Tumor size on computed tomography scans: is one measurement enough? Cancer. 2001;91(3):555–560. | ||
van Putte-Katier N, van Bommel EF, Elgersma OE, Hendriksz TR. Mesenteric panniculitis: prevalence, clinicoradiological presentation and 5-year follow-up. Br J Radiol. 2014;87(1044):20140451. | ||
Bao Q, Pan J, Qi H, et al. Aging and age-related diseases--from endocrine therapy to target therapy. Mol Cell Endocrinol. 2014;394(1–2):115–118. | ||
Küpeli A, Cansu A, Oğuz Ş, et al. Evaluation of mesenteric panniculitis with computed tomography: benign condition or paraneoplastic syndrome? Turk J Med Sci. 2018;14:569–575. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.