Back to Journals » Chronic Wound Care Management and Research » Volume 9
Meeting the Challenges in Pediatric Wound Care: Our 15-Year Experience with Dialkylcarbamoyl Chloride-Coated Dressing Technology in Acute and Chronic Wounds
Authors Ciprandi G, Crucianelli S, Grussu F, Spuntarelli G, Marino SFM, Urbani U, Bernaschi P, Sisto A, Rizzo MI, Zama M
Received 27 June 2022
Accepted for publication 19 September 2022
Published 29 November 2022 Volume 2022:9 Pages 23—33
DOI https://doi.org/10.2147/CWCMR.S376889
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Marco Romanelli
Guido Ciprandi,1,2 Serena Crucianelli,2 Francesca Grussu,2 Giorgio Spuntarelli,2 Simone Faustino Maria Marino,2 Urbano Urbani,2 Paola Bernaschi,3 Annamaria Sisto,3 Maria Ida Rizzo,2 Mario Zama4
1Chief Pediatric Wound Care High Specialization, Plastic and Maxillofacial Surgery Unit, Bambino Gesu’ Children’s Hospital, Research Institute, Rome, Italy; 2Consultant, Plastic and Maxillofacial Surgery Unit, Bambino Gesù Children’s Hospital, Research Institute, Rome, Italy; 3Microbiology Unit, Bambino Gesu’ Children’s Hospital, Research Institute, Rome, Italy; 4Head, Plastic and Maxillofacial Surgery Unit, Bambino Gesù Children’s Hospital, Research Institute, Rome, Italy
Correspondence: Guido Ciprandi, via Arturo Colautti, 5, Rome, 00152, Italy, Tel +39.3389400595, Fax +39.06.69592115, Email [email protected]; [email protected]
Abstract: Although similar in structure, pediatric skin is more delicate and vulnerable compared with adult skin and, as a result, is more prone to wounding. The immune response in pediatric skin is underdeveloped because of immature inflammatory cells and lower number of bone marrow progenitor cells. Therefore, pediatric patients, particularly newborns, have weak responses to microorganisms. The use of antimicrobial agents (eg, antibiotics, antimicrobial wound dressings, etc.) to aid in the prevention and/or treatment of wounds prone to or which are infected is one treatment option. Antimicrobial wound dressings using DACC technology physically bind bacteria and reduce the need for chemically active antimicrobial agents. This perspective is intended to highlight the benefit of DACC technology wound dressings for the prevention and treatment of pediatric wounds related to wound infection. We have found that DACC technology dressings are of benefit in the treatment of pediatric wounds and offer a significant resource for the treatment of pediatric wounds.
Keywords: pediatric wounds, infection, DACC technology
Introduction
The skin provides the critical function of assuring a protective barrier between the body’s internal structures and the external environment. Infant skin differs from adult skin at the structural, functional and compositional levels and, together, these differences contribute to the clinically observed differences between infant and adult skin.1 Pediatric skin is different from the skin of an adult,2,3 although it is anatomically mature in terms of the presence of the various skin layers when examined histologically.4 However, pediatric skin is a more delicate and vulnerable structure. For example, the skin of the neonate and infant is thin5,6 and there is reduced cohesion between the epidermis and dermis.7,8 The microvasculature of infant skin is not yet organised,9 and the skin’s subcutaneous is underdeveloped.10 The immune response of pediatric skin is also underdeveloped with white blood cells being immature, low numbers of bone marrow progenitor cells.11 This results in newborns having weak bactericidal functions12–15 which improves as the infant grows and the immune response matures.14
Pediatric Wound Healing
Wound healing progresses along the same physiological processes regardless of age,16 following the three main phases of the inflammatory phase, the proliferative phase, and the maturation phase.17 Clinical experience suggests that pediatric wounds tend to heal quicker than equivalent adult wounds18–20 and it has been proposed that this improved healing is due to the increased cellularity and modified connective tissue of infant skin.10,20 In pediatric patients with wounds, many considerations and risk factors must be considered for any treatment plan that is provided. Additional risk factors for pediatric wounds include a reduced ability for pediatric patients to thermoregulate, increased body surface-to-weight ratio, increased transepidermal water loss, increased susceptibility to skin stripping, and immature biological systems (eg, immune).21 The causes of wounds in pediatric patients differ from those of adults and are more likely to have acute wounds such as from traffic accidents and burns.17 Chronic wounds such as pressure ulcers are largely caused by medical device-related pressure,22 friction and shear.17 Maltreatment of a child can also result in wounds. The failure of a wound to progress towards healing according to the expected course may be considered chronic, and these wounds may be compromised by such occurrences as malnutrition and/or infection.10,23,24 Possessing an immature immune response, pediatric patients are at elevated risk for life-threatening sepsis secondary to bacterial proliferation within the wound bed.25,26
Immobility, neurological impairment, primary system dysfunction, mechanical ventilation in tracheostomy-dependent patients, poor nutritional status and long-lasting surgical procedures are the main risk factors associated with skin breakdown and responsible for limited oxygen supply to the skin and a rapid pressure ulcer development.27–31 Acute and critically ill children are at elevated risk of developing hospital-acquired pressure ulcers.32 The use of medical devices is associated with 50% of pressure ulcers in pediatric inpatients,33,34 although some studies indicate that in pediatric intensive care units the majority were not medical device related.35 The iatrogenic effects of pressure ulcers in children include compromised skin protection, altered thermoregulation, metabolic deficiencies, compromised immunity, and decreased sensation. Newborn and infants exhibit rapidly progressing pressure sores in ICUs, earlier than adults.36 The pre-disposing factors of skin thickness, physiological oedema, and capillary network instability typical in children make for an elevated risk and for the rapid onset and progression from stage I to IV pressure ulcer.37 Finally, the skin immunity is clearly responsible for a healing process and for a stable reconstruction.38
Although the sacrum, buttock, and heels are the most prevalent locations for pressure injuries in adults,39 the most common locations of pressure ulcers in pediatric patients in a tertiary care hospital were the ears, occiput, and heels.40–42 The pressure ulcer incident rate among pediatric patients has been reported to range between 0.29 and 7.3%.33,43 Studies specifically examining incidence rates in select clinical settings have found incidence rates in neonatal intensive care units of 3.9–16%,44,45 and in pediatric critical care units incidence rates have been cited as ranging from 0.8 to 27%.32,35,46 A descriptive analysis evaluating 39,984 patients identified a 1.1% (range, 0.57–4.6%) rate of hospital-acquired pressure ulcers (HAPUs) among pediatric patients (aged 1 day to 18 years).32 It is noteworthy that a retrospective study of 5346 pediatric intensive care patients demonstrated damage to the epidermis/dermis from a pressure ulcer injury increased the risk for infection, other care complications, and later psychosocial effects related to tissue damage and scarring.46 Nie highlights the unique aspects of pediatric physiology that can result in an increased risk for pressure injury and the subsequent complications both physical and psychological.47
Maintenance of skin integrity and prompt diagnosis of pressure ulcers in the pediatric population is nowadays a high priority, especially when caring for the critically ill child. Pediatric skin is functionally still developing, and the impaired barrier function makes it more susceptible to local or systemic infections compared with adults.48 In pediatric patients, the epidermal skin is loosely bound to the dermis, making them more susceptible to epidermal tears and blisters.49 The decreased epidermal-dermal cohesion, immature epidermal barrier, and immune system10 can leave the pediatric patient more prone to infections.17 Prevention or treatment of infection in pediatric wounds is a significant challenge for the clinician; for example, it has recently been reported that 67% of stage 3 pressure ulcers in children less than 10 years of age are critically contaminated and/or infected and from a microbiological point of view a polymicrobial profile affected the lesions in 92% of these infected cases.50 The reasons for this increased liability of infection are multifactorial, for example, in the infant skin barrier which differ from that of adults in the host immunological defense ability relating to the high percent of immature neutrophils, which demonstrated compromised adhesion and phagocytosis and are therefore more prone to develop bacterial infections.51
Overall, there are many causes that determine a greater propensity to infections of pediatric complex skin lesions and therefore we can assert that the final genesis is in any case multifactorial and multicausal. The causes are also wide and varied and include skin thickness, the poor dermal capillary bundles, a true physiological oedema capable of interfering with the transport of oxygen on the skin surface, a huge skin critical colonization, the systemic immunity depletion, salivary, fecal, urine incontinence, the small size of the various parts of the body that make self-on-self pressure ulcers easier as well as the skin tears, patients with the highest number of devices per body surface available when admitted to critical intensive areas.
Pediatric Wounds and Wound Dressings
In both pediatric patients and adults, wound healing generally proceeds through the same four phases: coagulation, inflammation, proliferation, and maturation.52 However, there are physiological differences in pediatric skin that affect wound healing and require consideration during treatment.53 Most wound dressings are developed from adult research studies, and in many cases pediatric wound care specialists must adapt these products for use in children.49 Wound dressings do not come in small enough sizes for easy use on pediatric patients.54 One of the key goals of holistic wound management in children is to minimise pain and lessen emotional distress.17 However, as the skin of pediatric patients is more susceptible to epidermal tears and blistering,49 tissue damage on the removal of adhesive wound dressings is likely to be elevated due to higher risk of epidermal stripping. The advent of non-adhesive wound dressings and the associated atraumatic dressing change will reduce levels of pain experienced by pediatric patients and the associated anxiety of patients and carers.
Regarding wound infection and dressings in pediatric wounds, DACC-coated wound dressings are a group of wound dressing that utilises the hydrophobic interactions between the fatty acid DACC and bacteria.55 These bacteria become physically bound to the fibres of the DACC-coated dressing and are removed when the dressing is removed.56 The antimicrobial effect of this dressing is beneficial as it reduces the need to use chemically active antimicrobial agents thereby reducing the risk of the development of bacterial resistance.56 The evidence for the use of DACC-coated wound dressings in the treatment of pediatric wounds is limited (Table 1). The efficacy of Cutimed Sorbact was recently demonstrated in donor site wounds after a split-thickness graft in children aged <16 years.57 This prospective RCT that included three wound dressings showed that DACC wound dressing was just as effective as the other dressings in time to re-epithelialisation, pain, and itch scores. A small observational case series in 10 children (mean age = 2.49 years, range 11 months – 8 years) with superficial-partial burns (TBSA range 4–14%, mean = 8%) showed that DACC wound dressing was effective at promoting wound progression in these burn wounds, with 50% of wounds healing within seven days and 100% of wounds healing within 21 days.58 Few clinical studies have examined the effect of DACC wound dressings on the incidence of wound infection. Meberg and Schøyen59 reported on umbilical cord care and prevention of infection in a large prospective randomised trial of 2441 infants who received either DACC wound dressing or daily cleansing of the wound with 0.5% chlorhexidine/70% ethanol. The results showed that there was no difference between total infection rates: 16.3% and 14.6% in the DACC wound dressing group and chlorhexidine group, respectively (p > 0.05).
 |
Table 1 Clinical Evidence for Use of DACC Wound Dressings on Paediatric Wounds |
Considering this and the evidence that has been presented in the previous sections of this article that the skin of pediatric patients demonstrates greater fragility and increased risk of damage and infection, the authors propose a ten-point criteria for an ideal wound dressing for pediatric patients (Table 2).
 |
Table 2 Areas of Concern for Wound Care of Paediatric Patients |
This paper describes our fifteen-year experience in wound care and clinical experience with DACC technology in pediatric patients and highlights the challenges of pediatric wound care.
The Pediatric Patients and Their Treatments
The patients were treated at the Bambino Gesu’ Children’s Hospital, Rome, Italy, between July 2007 and January 2021. A total of 4223 patients (aged 0–16 years) with a variety of wounds were admitted and 1232 children affected by complex wounds underwent treatment with DACC wound dressings (Cutimed Sorbact or Cutimed Sorbact gel). Exclusion criteria included ulcers of vascular or diabetic in origin or if the ulcers were drug-resistant in nature. Patients with a known sensitivity to the dressing or its components were also excluded. The following data were collected: demographics, signs and symptoms of wound infection (using the parameters defined in the International Wound Infection Institute’s Principles of Best Practice),60 wound healing progression (as measured by a change in surface wound area and volume reduction as measured with digital planimetry), assessment of pain experienced upon dressing removal (as measured by the FLACC Behavioural Pain Assessment Scale and VAS),61,62 ease of use and dressing acceptability in relation to the physical and performance characteristics of the dressing (eg, conformability, ease of handling, dislocation of dressing), the psychological impact of the wound care (eg, child’s anxiety), and peri-wound skin damage. A multidisciplinary team (including pediatric clinical nurse specialists, plastic surgeon, microbiologist, dermatologist, oncohematologist, psychologist, occupational therapist, immunologist) participated in various aspects of studies and treatment.
Regarding treatment with the DACC wound dressing, use of the dressing was stopped if wound bioburden did not decrease within 10 days (4–5 dressing changes) or if the patients began to exhibit signs of fever. Together, a specialised team from a number of disciplines (including pediatric nurses, surgeons, microbiologists, psychologists, pharmacologists and other allied professionals) was consulted as needed and is necessary for a holistic child and wound care view especially in immobile pediatric patients with a permanent high skin breakdown risk and a severe to moderate cerebral disability together with a primary multisystem dysfunction.63–65
Our Clinical Experience
Of the 1232 pediatric patients affected by complex wounds who underwent treatment with DACC wound dressing 56% presented with an infected wound. Of these, 66% were under 5 years of age. In approximately 33% of the cases the lesions were in the cephalic extremity, in the occipital area in case of pressure injuries, in the vertex site or to cover a craniotomy for cranioplasty to prevent SSI. Nearly 50% of infected lesions were due to ulcers or MDRI. Our experience with using DACC wound dressing in these patients is very positive. Almost 92% of patients treated with the antimicrobial wound dressing showed complete healing of their wounds, 1.5% of wounds deteriorated and 5% did not show stable progression. The remainder of patients were lost to follow-up. Patients with pressure ulcers showed no recurrence when seen after a mean follow-up period of 90 days. The microbiological evaluations highlight the polymicrobial nature of the wounds we have treated. MRSA, Pseudomonas aeruginosa, Acinetobacter baumannii, Staphylococcus epidermidis, Serratia marcescens and Enterococcus faecalis are common species isolated from these pediatric wounds.
Significant positive results relating to the treatment of the patients with DACC wound dressing included a reduction in hyperemia of the peri-wound tissues, a reduction in wound malodour, exudate, and the alleviation of pain at dressing removal after the first 2–5 changes. No adverse wound or peri-lesional effects were recorded, and the dressing did not cause itching or interfere with play activities. No other treatment modalities (eg, other dressings or topical antibiotics) were used in the treatment of these wounds, and this resulted in a reduction in treatment costs (including a reduction in the nursing time costs). The reduction in the nursing time and time taken to change dressings was particularly important for neonate and infant patients as this helped reduce the level of anxiety experienced.
A significant number of patients with infected pressure ulcers have been treated within the clinical centre in the most recent years of this 15-year reflection (September 2015 to September 2020). During this period, 402 infants with infected stage I or stage II pressure ulcers66 were treated with DACC wound dressing. Patients treated with DACC wound dressing (gel) received no other topical therapy nor was there any systemic antibiotic treatment. As a response measure of the DACC wound dressing, the parameter of WSR was measured. Three-hundred and sixty-three (90.7%) patients demonstrated a mean 75% WSR at the end of the evaluation period, 25 (6.3%) patients showed incomplete or partial WSR (10–23%), and the remaining 12 (3.0%) patients had wounds that showed a deterioration in wound condition in a mean time of 10 days. In most patients (92.6%) with a mean 75% WSR there was a reduction in exudate levels that corresponded with the WSR data. There was also a 10-fold decrease in bacterial counts after the first two dressing changes. However, in seven patients of this group, exudate levels only started decreasing after seven dressing changes. These patients showed a polymicrobial profile including species such as Pseudomonas aeruginosa, Enterococcus faecalis and Klebsiella pneumoniae, and the clinical decision was taken to subsequently treat these patients with systemic antibiotics. In patients with incomplete or partial WSR, wounds showed polymicrobial cultures of 3–7 pathogens including MRSA. Patients exhibiting a deterioration in wound condition showed signs of localised and systemic infection. In these cases, DACC wound dressing was stopped, and alternative therapies (including systemic antibiotics) were started.
The use of DACC wound dressings for the prevention of SSI is an application used at our clinical setting. The protocol for the prevention of SSI in craniotomy suture lines the Bambino Gesu’ Children’s Hospital begins 48 hours prior to surgery and continues during and after the surgical procedure, and then into the immediate, subsequent hospitalisation period both in the ICU and on the return of the patient to the ward. The effectiveness of DACC wound dressing (gel) on the prevention of SSI was assessed in 45 pediatric patients affected by mono- and polysutural craniosynostosis and therefore midfacial malformations such as Apert and Crouzon Syndrome.67 Children were treated with a medicated shampoo containing 4% chlorhexidine digluconate during the day of operation. Prior to surgery, the skin was treated with PHMB followed by Chloraprep (2% chlorhexidine digluconate/isopropyl alcohol). After the surgical procedure was complete, sutures had been applied the DACC wound dressing. Superficial SSI was managed with the DACC wound dressing, and the infection was resolved within 2 weeks resulting in an absence of clinical signs of SSI. No additional antimicrobial therapies were required, and the duration of any antibiotic administration was reduced. The removal of the DACC wound dressing was assessed as being painless (VAS assessment). Crying and agitation were reported in 7/40 (17.5%) of cases, and always at the beginning of the dressing change procedure. This was only seen at the first dressing change and was therefore done in the presence of drainage and with the central venous catheters in place and was felt was not due to the dressing.
The Benefits of DACC Technology Dressings
In our experience, DACC wound dressings have been used successfully in a variety of different skin conditions and wounds including to protect the wound and prevent ingress of contaminants and microorganisms in cutaneous mucositis (a reaction after chemotherapy), in palliative care (both oncohematological and terminal congenital pathologies), to protect partial or complete open abdomens, congenital conditions such as omphalocele or acquired conditions (post-liver transplant),68 and in dehiscence of the anterior abdominal wall in inflammatory chronic intestinal disease. DACC wound dressing has also been used in the treatment of first, second and third degree burns in patients of pediatric age, reducing re-epithelialisation times with minimal psychological distress and no side effects in a group of ten patients.58 In our experience, DACC wound dressing is extremely effective in the treatment of burns of the extremities, where it prevents the scar fusion of the digits, controls pain and reduces the appearance of interphalangeal keloids.
The peri-wound skin plays a critical role in the wound healing process. It not only provides a structural stability that allows wound healing to progress but it also provides the cellular (ie, keratinocytes) and biochemical nutrients and signals (eg, growth factors) that are required for stimulating the new tissue’s integral components.69 Recent studies have demonstrated the importance of peri-wound skin in promoting healing (in patients with venous leg ulcers) or importantly in that peri-wound skin subject to various insults will delay healing.70 It is essential therefore that the skin is recognised as a vital part of healing a wound by healthcare workers treating a wound and this recognition is especially so in pediatric wounds. Experience has shown that, in a variety of indications during fifteen consecutive years, DACC wound dressing (Sorbact) has been shown to protect the peri-wound skin from the progression of the inflammation and possible infection that enables superficial tissue erosion, hyperemia, edema, dullness and pain. Particularly useful in pediatrics is the application of DACC wound dressing gel beyond the limits of the lesion in the perigenital sites; areas sometimes made moist by a physiological urinary incontinence. In these cases, the gel is also capable of effectively counteracting the salinity and acidity of the urine.68
There is no contraindication for DACC wound dressing in neonates and infants except for the width and shape of the dressing, even the smallest sizes of dressing.50 When lesions are circumferential – as is the case in amniotic bridle or extravasation lesions – a wrap-around dressing can easily be applied to include the peri-wound skin. The physical and non-medicated nature of DACC wound dressing’s antimicrobial action makes it particularly useful in neonatal age and premature babies with congenital wounds such as aplasia cutis. Infant skin is still developing, and the impaired barrier function of newborn skin means that any wound care provider must give critical consideration to the use of wound care products and the ingredients in these products and topicals to identify known irritants or if some components are the mechanism of absorption and clearance of dressing components (eg, silver).3,49,71 The use of a dressing that acts via physical means and binds microorganisms rather than relying on the use of active agents such as topical antibiotics will minimise deleterious effects on the patient. The ability of DACC wound dressings (eg, gel) to reduce pain in a short time until it is abolished makes it a key element in the therapy and prevention of complex pediatric lesions.72
Scalp defects, including occipital (posterior skull region) wounds, can be a particularly difficult problem,73 particularly in terms of the congenital malformations that are due to structural anomalies of the craniofacial complex. As with adults, pediatric patients are at risk for pressure ulcers74 with the occipital region at increased risk in infants and children compared to adults.75 As with wounds in anatomical areas difficult to dress, the curvature of the head may make the application of wound dressings difficult and the retention of these dressings may be problematic due to the mobility of the pediatric patient particularly if dressings are painful or uncomfortable. Pediatric patients may attempt to remove the dressings.71
Pediatric patients with craniomaxillofacial defects require early and careful intervention because of the effects they might have on growth and development and for improving quality of life, brain, and cognitive functions, and stomatognathic aspects during growth.76 The approach with craniotomies and subsequent cranioplasty must observe a precise protocol for the prevention of post-surgical infections and the consequent complications (eg, wound dehiscence, infection, etc.). The literature reports an incidence of SSI in pediatric patients undergoing craniotomies ranging from 6.9% to 28%.77 Our experience has shown that the use of DACC wound dressing (gel) in the treatment of occipital surgical wounds for the prevention of SSI has been highly successful.
The success of DACC wound dressing when applied to pediatric patients regarding the reduction of wound bioburden is due to the ability of the DACC component of the dressing to irreversibly bind pathogenic bacteria.55,78,79 The pathogens bind to the wound dressing when they encounter the hydrophobic surface under the moist conditions of infected wounds. Pathogens bind to the DACC permanently and are then removed when the dressing is changed. The hydrophobic interaction technology does not induce bacterial resistance, has no contraindications, and since it does not release any material or pharmacological component into the tissues it is unable to induce skin intolerances or allergies nor systemic effects.80,81
Pediatric patients, especially neonates and infants, are vulnerable to pressure ulcer formation, and it is becoming increasingly clear to clinicians that pediatric patients with pressure ulcers require special considerations, protocols, guidelines and standardised approaches to pressure ulcer prevention and treatment.82 Pediatric pressure injury prevalence has been estimated to be between 0.47 and 35% with the estimates rising to between 7.1 and 44% in pediatric intensive care units.82 Successful pediatric pressure injury prevention and treatment can be achieved through the standardized and concentrated efforts of interprofessional teams and the use of specialized wound dressings that address infection, enable wound healing, reduce pain, and hence provide psychological support for the pediatric patient. This clinical experience has shown the effectiveness of DACC wound dressings in this respect.
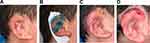
Figures 1–3 show clinical case summaries that exemplify treatment of complex pediatric wounds with wound dressings with DACC technology.
Conclusion
This article has presented a historical perspective (over a period of 15 years) from 1232 pediatric patients relating to the use of DACC wound dressing in the treatment of a variety of chronic and complex pediatric wounds. The evidence gathered over this time has shown that DACC wound dressing has been a successful dressing in that it enabled prevention/treatment of bacterial wound infections, supported healing progression and reduced pain thus having a positive psychological impact upon both the patient and their family.
Abbreviations
DACC, dialkyl carbamoyl chloride; FLACC, Faces, Legs, Activity, Cry and Consolability; ICUs, intensive care units; MDRI, medical device-related pressure injury; MRSA, methicillin-resistant Staphylococcus aureus; PHMB, polyhexamethylene biguanide; RCT, randomised controlled trial; SSI, surgical site infection; TBSA, total body surface area; VAS, Visual Analogue Scale; WSR, wound size reduction.
Ethics Approval and Consent to Participate
Formalized studies conducted during the 15-year period followed relevant Italian Law, and European Medical Device Guidelines. The studies were approved by the Institutional Review Board of the Bambino Gesu’ Children’s Hospital. Informed consent was provided by parent/foster parent/caregiver prior to enrollment into any study. Patients/parents/foster parents/social caregivers gave their consent for data relating to treatment being analysed and used to support publications available in the public domain. All aspects of these studies were in accordance with the principles set forth by the Declaration of Helsinki.
Acknowledgments
We acknowledge the contributions of the staff and residents of our department in conducting this study. The license for this publication was paid for by Essity AB, but they have had no involvement with the content of the article.
Disclosure
The authors report no conflicts of interest, financial or otherwise, in relation to this work.
References
1. Walters RM, Khanna P, Chu M, Mack MC. Developmental changes in skin barrier and structure during the first 5 years of life. Skin Pharmacol Physiol. 2016;29(3):111–118. doi:10.1159/000444805
2. Telofski LS, Morello AP, Mack Correa MC, Stamatas GN. The infant skin barrier: can we preserve, protect, and enhance the barrier? Dermatol Res Pract. 2012;2012:198789. doi:10.1155/2012/198789
3. Oranges T, Dini V, Romanelli M. Skin physiology of the neonate and infant: clinical implications. Adv Wound Care. 2015;4(10):587–595. doi:10.1089/wound.2015.0642
4. King A, Balaji S, Keswani SG. Biology and function of fetal and pediatric skin. Facial Plast Surg Clin North Am. 2013;21(1):1–6. doi:10.1016/j.fsc.2012.10.001
5. Vitral GLN, Aguiar RAPL, de Souza IMF, et al. Skin thickness as a potential marker of gestational age at birth despite different fetal growth profiles: a feasibility study. PLoS One. 2018;13(4):e0196542. doi:10.1371/journal.pone.0196542
6. Stamatas GN, Nikolovski J, Luedtke MA, et al. Infant skin microstructure assessed in vivo differs from adult skin in organization and at the cellular level. Pediatr Dermatol. 2010;27(2):125–131. doi:10.1111/j.1525-1470.2009.00973.x
7. Evans NJ, Rutter N. Development of the epidermis in the newborn. Biol Neonate. 1986;49(2):74–80. doi:10.1159/000242513
8. Lund C, Kuller J, Lane A, et al. Neonatal skin care: the scientific basis for practice. Neonatal Netw. 1999;18(4):15–27. doi:10.1891/0730-0832.18.4.15
9. Perera P, Kurban AK, Ryan TJ. The development of the cutaneous microvascular system in the newborn. Br J Dermatol. 1970;82(s5):86–91.
10. Baharestani MM. An overview of neonatal and pediatric wound care knowledge and considerations. Ostomy Wound Manage. 2007;53(6):34–36, 38, 40.
11. Cairo MS. Neonatal neutrophil host defense. Prospects for immunologic enhancement during neonatal sepsis. Am J Dis Child. 1989;143(1):40–46. doi:10.1001/archpedi.1989.02150130050014
12. Nussbaum C, Gloning A, Pruenster M, et al. Neutrophil and endothelial adhesive function during human fetal ontogeny. J Leukoc Biol. 2013;93(2):175–184. doi:10.1189/jlb.0912468
13. Filias A, Theodorou GL, Mouzopoulou S, et al. Phagocytic ability of neutrophils and monocytes in neonates. BMC Pediatr. 2011;11:29. doi:10.1186/1471-2431-11-29
14. Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282(1821):20143085. doi:10.1098/rspb.2014.3085
15. Melvan JN, Bagby GJ, Welsh DA, et al. Neonatal sepsis and neutrophil insufficiencies. Int Rev Immunol. 2010;29(3):315–348. doi:10.3109/08830181003792803
16. Rodgers A. Wound care. In: Trigg E, Mohammaed TA, editors. Practices in Children’s Nursing: Guidelines for Hospital and Community.
17. Wounds UK. Best Practice Statement. Principles of Wound Management in Paediatric Patients. London: Wounds UK; 2017.
18. Bale S, Jones V. Caring for children with wounds. J Wound Care. 1996;5(4):177–180. doi:10.12968/jowc.1996.5.4.177
19. Garvin G. Wound healing in pediatrics. Nurs Clin North Am. 1990;25(1):181–192.
20. Wysocki AB. Anatomy and physiology of skin and soft tissue. In: Bryant RA, editor. Acute and Chronic Wounds: Nursing Management.
21. Patel S, Tomic-Canic M. Neonatal debridement: tricks or treats. J Wound Technol. 2014;1(23):12–13.
22. Gefen A, Alves P, Ciprandi G, et al. Device-related pressure ulcers: SECURE prevention. J Wound Care. 2020;29(Sup2a):S1–S52. doi:10.12968/jowc.2020.29.Sup2a.S1
23. Carletti M, Ciprandi G, Di Felice G, Rivosecchi M. Infected pressure ulcers in disabled children: the microbiological assessment. Int J Infect Control. 2011;7(1):42.
24. Leonard M, Dain E, Pelc K, et al. Nutritional status of neurologically impaired children: impact on comorbidity. Arch Pediatr. 2020;27(2):95–103. doi:10.1016/j.arcped.2019.11.003
25. Keener KE. The surgical neonate. In: Wise BV, McKenna C, Garvin G, Harmon BJ, editors. Nursing Care of the General Pediatric Surgical Patient. Gaithersburg, Md: Aspen Publishers, Inc; 2000:167–180.
26. Rustogi R, Mill J, Fraser JF, Kimble RM. The use of Acticoat in neonatal burns. Burns. 2005;31(7):878–882. doi:10.1016/j.burns.2005.04.030
27. Suddaby EC, Barnett S, Facteau L. Skin breakdown in acute care pediatrics. Pediatr Nurs. 2005;31(2):132–38, 148.
28. Noonan C, Quigley S, Curley MA. Skin integrity in hospitalized infants and children: a prevalence survey. J Pediatr Nurs. 2006;21(6):445–453. doi:10.1016/j.pedn.2006.07.002
29. McCaskey MS, Kirk L, Gerdes C. Preventing skin breakdown in the immobile child in the home care setting. Home Healthc Nurse. 2011;29(4):248–255. doi:10.1097/NHH.0b013e3182119794
30. Freundlich K. Pressure injuries in medically complex children: a review. Children. 2017;4(4):25. doi:10.3390/children4040025
31. Gefen A, Ciprandi G. Biometry impairments: the specific challenges in preventing pressure ulcers in patients with chronic spasticity. J Wound Care. 2019;28(11):699–700. doi:10.12968/jowc.2019.28.11.699
32. Razmus I, Bergquist-Beringer S. Pressure ulcer risk and prevention practices in pediatric patients: a secondary analysis of data from the National Database of Nursing Quality Indicators®. Ostomy Wound Manage. 2017;63(2):28–32.
33. Willock J, Harris C, Harrison J, Poole C. Identifying the characteristics of children with pressure ulcers. Nurs Times. 2005;101(11):40–43.
34. Visscher M, King A, Nie AM, et al. A quality-improvement collaborative project to reduce pressure ulcers in PICUs. Pediatrics. 2013;131(6):e1950–e1960. doi:10.1542/peds.2012-1626
35. Curley MA, Quigley SM, Lin M. Pressure ulcers in pediatric intensive care: incidence and associated factors. Pediatr Crit Care Med. 2003;4(3):284–290. doi:10.1097/01.PCC.0000075559.55920.36
36. Neuhaus K, Meuli M, Koenigs I, Schiestl C. Management of”difficult” wounds. Eur J Pediatr Surg. 2013;23(5):365–374. doi:10.1055/s-0033-1354588
37. Ciprandi G, Oranges T, Schluer AB. Pressure ulcers in paediatric patients. In: Romanelli M, Clark M, Gefen A, Ciprandi G, editors. Science and Practice of Pressure Ulcer Management.
38. Nguyen AV, Soulika AM. The dynamics of the skin’s immune system. Int J Mol Sci. 2019;20(8):1811. doi:10.3390/ijms20081811
39. Vangilder C, Macfarlane GD, Meyer S. Results of nine international pressure ulcer prevalence surveys: 1989 to 2005. Ostomy Wound Manage. 2008;54(2):40–54.
40. Groeneveld A, Anderson M, Allen S, et al. The prevalence of pressure ulcers in a tertiary care pediatric and adult hospital. J Wound Ostomy Continence Nurs. 2004;31(3):108–120. doi:10.1097/00152192-200405000-00004
41. Cummins KA, Watters R, Leming-Lee TS. Reducing pressure injuries in the pediatric intensive care unit. Nurs Clin North Am. 2019;54(1):127–140. doi:10.1016/j.cnur.2018.10.005
42. Rivolo M, Dionisi S, Olivari D, et al. Heel pressure injuries: consensus-based recommendations for assessment and management. Adv Wound Care. 2020;9(6):332–347. doi:10.1089/wound.2019.1042
43. Baldwin KM. Incidence and prevalence of pressure ulcers in children. Adv Skin Wound Care. 2002;15(3):121–124. doi:10.1097/00129334-200205000-00007
44. Fujii K, Sugama J, Okuwa M, et al. Incidence and risk factors of pressure ulcers in seven neonatal intensive care units in Japan: a multisite prospective cohort study. Int Wound J. 2010;7(5):323–328. doi:10.1111/j.1742-481X.2010.00688.x
45. Visscher M, Taylor T. Pressure ulcers in the hospitalized neonate: rates and risk factors. Sci Rep. 2014;4:7429. doi:10.1038/srep07429
46. Schindler CA, Mikhailov TA, Kuhn EM, et al. Protecting fragile skin: nursing interventions to decrease development of pressure ulcers in pediatric intensive care. Am J Crit Care. 2011;20(1):26–34. doi:10.4037/ajcc2011754
47. Nie AM. Pressure injury prevention and treatment in critically ill children. Crit Care Nurs Clin North Am. 2020;32(4):521–531. doi:10.1016/j.cnc.2020.08.003
48. Eichenfield LF, Hardaway CA. Neonatal dermatology. Curr Opin Pediatr. 1999;11(5):471–474. doi:10.1097/00008480-199910000-00017
49. McCord SS, Levy ML. Practical guide to pediatric wound care. Semin Plast Surg. 2006;20(3):192–199.
50. Ciprandi G, Crucianelli S, Pomponi M, et al. Physical approach to infected pressure ulcers in a pediatric population: impact of a DACC non-medicated technology in bioburden management.
51. Visscher MO, Utturkar R, Pickens WL, et al. Neonatal skin maturation – vernix caseosa and free amino acids. Pediatr Dermatol. 2011;28(2):122–132. doi:10.1111/j.1525-1470.2011.01309.x
52. Buganza Tepole A, Kuhl E. Systems-based approaches toward wound healing. Pediatr Res. 2013;73(4 Pt 2):553–563. doi:10.1038/pr.2013.3
53. McNamara SA, Hirt PA, Weigelt MA, et al. Traditional and advanced therapeutic modalities for wounds in the paediatric population: an evidence-based review. J Wound Care. 2020;29(6):321–334. doi:10.12968/jowc.2020.29.6.321
54. White R, Rodgers A, O’Connor L, Anthony D. Paediatric wound care: neonates and infants. Wounds UK. 2016;12(3):8–11.
55. Ljungh A, Yanagisawa N, Wadström T. Using the principle of hydrophobic interaction to bind and remove wound bacteria. J Wound Care. 2006;15(4):175–180. doi:10.12968/jowc.2006
56. Butcher M. Introducing a new paradigm for bioburden management. J Wound Care. 2011;20(Supplt3):4–19.
57. McBride CA, Kimble RM, Stockton KA. Prospective randomised controlled trial of Algisite™ M, Cuticerin™, and Sorbact® as donor site dressings in paediatric split-thickness skin grafts. Burns Trauma. 2018;6:33. doi:10.1186/s41038-018-0135-y
58. Kusu-Orkar TE, Islam U, Hall B, et al. The use of a non-medicated dressing for superficial-partial thickness burns in children: a case series and review. Scars Burn Heal. 2019;5:2059513119896954. doi:10.1177/2059513119896954
59. Meberg A, Schøyen R. Hydrophobic material in routine umbilical cord care and prevention of infections in newborn infants. Scand J Infect Dis. 1990;22(6):729–733. doi:10.3109/00365549009027128
60. International Wound Infection Institute (IWII). Wound Infection in Clinical Practice. Principles of Best Practice. Wounds International; 2016.
61. Willis MH, Merkel SI, Voepel-Lewis T, Malviya S. FLACC behavioral pain assessment scale: a comparison with the child’s self-report. Pediatr Nurs. 2003;29(3):195–198.
62. Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. doi:10.1016/0304-3959(83)90126-4
63. Ousey K, McIntosh C. Topical antimicrobial agents for the treatment of chronic wounds. Br J Community Nurs. 2009;14(9):S6, S8, S10. doi:10.12968/bjcn.2009.14.Sup4.43909
64. Stojadinovic A, Carlson JW, Schultz GS, et al. Topical advances in wound care. Gynecol Oncol. 2008;111(2 Suppl):S70–S80. doi:10.1016/j.ygyno.2008.07.042
65. Ciprandi G. Pressure ulcers. Prevention and skin rehabilitation. First step of a complex project.
66. Beeckman D, Schoonhoven L, Fletcher J, et al. EPUAP classification system for pressure ulcers: European reliability study. J Adv Nurs. 2007;60(6):682–691. doi:10.1111/j.1365-2648.2007.04474.x
67. Carinci F, Pezzetti F, Locci P, et al. Apert and Crouzon syndromes: clinical findings, genes and extracellular matrix. J Craniofac Surg. 2005;16(3):361–368. doi:10.1097/01.scs.0000157078.53871.11
68. Gjiergji M, Ciaralli I, Ciliento G, et al. Improving prevention and treatment of incontinence associated dermatitis (IAD) in onco-haematological children.
69. Schultz GS, Sibbald RG, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen. 2003;11(Suppl 1):S1–S28. doi:10.1046/j.1524-475x.11.s2.1.x
70. Dini V, Janowska A, Oranges T, et al. Surrounding skin management in venous leg ulcers: a systematic review. J Tissue Viability. 2020;29(3):169–175. doi:10.1016/j.jtv.2020.02.004
71. King A, Stellar JJ, Blevins A, Shah KN. Dressings and products in pediatric wound care. Adv Wound Care. 2014;3(4):324–334. doi:10.1089/wound.2013.0477
72. Seckam AM, Twardowska-Saucha K, Heggemann J, et al. Clinical performance and quality of life impact of an absorbent bacteria-binding foam dressing. Br J Nurs. 2021;30(5):S21–S30. doi:10.12968/bjon.2021.30.5.S21
73. Orseck MJ, Trujillo MG, Ritter EF. Screw fixation of dermal regeneration template for scalp reconstruction. Ann Plast Surg. 2012;68(5):457–460. doi:10.1097/SAP.0b013e318243390b
74. Curley MA, Razmus IS, Roberts KE, Wypij D. Predicting pressure ulcer risk in pediatric patients: the Braden Q Scale. Nurs Res. 2003;52(1):22–33. doi:10.1097/00006199-200301000-00004
75. Hamill PV, Drizd TA, Johnson CL, et al. Physical growth: National Center for Health Statistics Percentiles. Am J Clin Nutr. 1979;32(3):607–629. doi:10.1093/ajcn/32.3.607
76. Dufresne CR, Manson PN. Pediatric craniofacial trauma: challenging pediatric cases craniofacial trauma. Craniomaxillofac Trauma Reconstr. 2011;4(2):73–84. doi:10.1055/s-0031-1275387
77. Thenier-Villa JL, Sanromán-álvarez P, Miranda-Lloret P, Plaza Ramírez ME. Incomplete reossification after craniosynostosis surgery-incidence and analysis of risk factors: a clinical radiological assessment study. J Neurosurg Pediatr. 2018;22(2):120–127. doi:10.3171/2018.2.PEDS17717
78. Geroult S, Phillips RO, Demangel C. Adhesion of the ulcerative pathogen Mycobacterium ulcerans to DACC-coated dressings. J Wound Care. 2014;23(8):417–418, 422–424. doi:10.12968/jowc.2014.23.8.417
79. Ronner AC, Curtin J, Karami N, Ronner U. Adhesion of meticillin-resistant Staphylococcus aureus to DACC-coated dressings. J Wound Care. 2014;23(10):484, 486–488. doi:10.12968/jowc.2014.23.10.484
80. Cutting K, McGuire J. In vitro and clinical experience of Cutimed Sorbact: the evidence base. J Wound Care. 2015;24(5 Suppl):S6–S30. doi:10.12968/jowc.2015.24.Sup5a.S6
81. Cutting K, McGuire J. Safe, long-term management of bioburden that helps promote healing Evidence review of DACC technology. J Wound Care. 2015;24(5 Suppl):S3–S5. doi:10.12968/jowc.2015.24.Sup5a.S3
82. Delmore B, Deppisch M, Sylvia C, et al. Pressure injuries in the pediatric population: a National Pressure Ulcer Advisory Panel White Paper. Adv Skin Wound Care. 2019;32(9):394–408. doi:10.1097/01.ASW.0000577124.58253.66
83. Boyar V. Efficacy of dialkylcarbamoylchloride-coated dressing in management of colonized or infected neonatal and pediatric wounds. J Wound Ostomy Continence Nurs. 2016;43(5):547–550. doi:10.1097/WON.0000000000000266
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.