Back to Journals » Patient Preference and Adherence » Volume 16
Medication Supports at Transitions Between Hospital and Other Care Settings: A Rapid Scoping Review
Authors Varghese S , Hahn-Goldberg S , Deng Z , Bradley-Ridout G , Guilcher SJT , Jeffs L , Madho C , Okrainec K , Rosenberg-Yunger ZRS , McCarthy LM
Received 6 November 2021
Accepted for publication 24 January 2022
Published 25 February 2022 Volume 2022:16 Pages 515—560
DOI https://doi.org/10.2147/PPA.S348152
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Shawn Varghese,1– 3 Shoshana Hahn-Goldberg,1,4 ZhiDi Deng,1 Glyneva Bradley-Ridout,1,5 Sara JT Guilcher,1,6 Lianne Jeffs,6– 8 Craig Madho,4 Karen Okrainec,9 Zahava RS Rosenberg-Yunger,10 Lisa M McCarthy1,2,11,12
1Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, Ontario, Canada; 2Women’s College Research Institute, Women’s College Hospital, Toronto, Ontario, Canada; 3Michael G.Degroote School Of Medicine, McMaster University, Hamilton, Ontario, Canada; 4OpenLab, University Health Network, Toronto, Ontario, Canada; 5Gerstein Science Information Centre, University of Toronto, Toronto, Ontario, Canada; 6Institute for Health Policy Management and Evaluation, University of Toronto, Toronto, Ontario, Canada; 7Sinai Health, Toronto, Ontario, Canada; 8Lawrence S. Bloomberg Faculty of Nursing, University of Toronto, Toronto, Ontario, Canada; 9Toronto General Research Institute, University Health Network, Toronto, Ontario, Canada; 10Ted Rogers School of Management, School of Health Services Management, Ryerson University, Toronto, Ontario, Canada; 11Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; 12Institute for Better Health, Trillium Health Partners, Mississauga, ON, Canada
Correspondence: Lisa M McCarthy, Clinician Scientist, Institute for Better Health, Trillium Health Partners, Tel +1 416-566-2793, Email [email protected]
Purpose: Transitions in care (TiC) often involves managing medication changes and can be vulnerable moments for patients. Medication support, where medication changes are reviewed with patients and caregivers to increase knowledge and confidence about taking medications, is key to successful transitions. Little is known about the optimal tools and processes for providing medication support. This study aimed to identify describe patient or caregiver-centered medication support processes or tools that have been studied within 3 months following TiC between hospitals and other care settings.
Methods: Rapid scoping review; English-language publications from OVID MEDLINE, OVID EMBASE, Cochrane Library and EBSCO CINAHL (2004-July 2019) that assessed medication support interventions delivered within 3 months following discharge were included. A subset of titles and abstracts were assessed by two reviewers to evaluate agreement and once reasonable agreement was achieved, the remainder were assessed by one reviewer. Eligibility assessment for full-text articles and data charting were completed by an experienced reviewer.
Results: A total of 7671 unique citations were assessed; 60 studies were included. Half of the studies (n = 30/60) were randomized controlled trials. Most studies (n = 45/60) did not discuss intervention development, particularly whether end users were involved in intervention design. Many studies (n = 37/60) assessed multi-component interventions with written/print and verbal education components. Few studies (n = 5/60) included an electronic component. Very few studies (n = 4/60) included study populations at high risk of adverse events at TiC (eg, people with physical or intellectual disabilities, low literacy or language barriers).
Conclusion: The majority of studies were randomized controlled trials involving verbal counselling and/or physical document delivered to the patient before discharge. Few studies involved electronic components or considered patients at high-risk of adverse events. Future studies would benefit from improved reporting on development, consideration for electronic interventions, and improved reporting on patients with higher medication-related needs.
Keywords: continuity of patient care, patient discharge, patient education, rapid scoping review, medication counseling, care transitions
Introduction
The World Health Organization (WHO) has identified transitions in care (TiC) as a key focus area for improving patient safety and minimizing preventable medication-related harm.1 TiC, in which patients move from one care setting to another, often involves changes to medication regimens.2 During transitions, discrepancies and gaps in communication can lead to medication errors,3 which in turn, can cause patient harm and lead to emergency department visits, hospitalization, and even death.4 A study assessing patient’s recall of medication changes one week after discharge found less than half were able to recall whether and which medications changed during their hospital stay, and that nearly 10% experienced changes in their perceived informational needs during their care transition.5 As inadequate knowledge about medication regimens can lead to incorrect use and non-adherence,6 these findings suggest that many patients may be at risk of harm and/or suboptimal therapy following discharge. Furthermore, certain patient groups are at higher risk for adverse events during transitions in case. Patients with language barriers or low literacy levels often have difficulty understanding their discharge medications.7,8 Older people and those with intellectual or physical disabilities experience high rates of multimorbidity and are often prescribed many medications, which need to be reviewed frequently.9–11
Medication support interventions are key to successful transitions.12 We define medication support interventions as those in which patients and caregivers partner with health-care providers to review medication changes with the goal of increasing the patients’ knowledge and confidence about taking their medications. Current knowledge and understanding of the optimal tools for providing medication support during TiC is limited. Further study is required to understand both the design and implementation of successful support tools. As part of this effort, we performed a rapid scoping review to describe medication support tools and processes that have been studied, understand the extent to which elements of user-centered design were included in their development, and to identify opportunities for future research. This rapid scoping review is a component of a multi-method research project to understand the needs and co-develop guidelines and prototypes of potential medication support tool(s) together with patients and providers, with the goals of improving people’s medication experiences and optimizing medication management at TiC.
Objective
This rapid scoping review sought to address the following research question: What patient-centered or caregiver-centered medication support tools or processes have been studied within three months following TiC between hospitals and other care settings?
Methods
We undertook a rapid scoping review with the intent of identifying evidence in a timely fashion to facilitate the integration of our findings into our complementary co-design work to create medication support tools for use during TiC.13 Our methods were guided by the information and recommendations outlined in the Joanna Briggs Institute Manual for Evidence Synthesis on scoping reviews.14 Reporting of our work was guided by the Preferred Reporting Items for Systematic Reviews and Meta-analysis for Scoping Reviews (PRISMA-ScR).15
Eligibility Criteria
Study eligibility criteria were as follows.
P - Population: We included studies that involved patients experiencing TiC, and their caregivers. Caregivers were defined as any person who gives care to people who need help taking care of themselves. Examples included family members, friends, or members of the clergy.16 Only patients experiencing transitions from hospitals to other care settings (home/community, primary care, long-term care, rehabilitation facilities, etc.) were included because this represents a population at high risk for experiencing medication errors and adverse events.17,18
I–Intervention: Studies that involved or assessed a medication support intervention were included. A medication support intervention was defined as any process or tool that aims to increase patients and caregivers’ understanding of their medications, confidence about taking their medication, or aims to increase providers’ understanding of a patient’s preferences and values with respect to their medications. Broader interventions, such as discharge summaries, with medication information were included.
C - Comparator: Studies with or without controls were eligible for inclusion.
O - Outcomes: Studies that assessed patient and caregivers’ knowledge/comprehension, patient attitudes (satisfaction, preferences, values) and confidence (self-efficacy), patient behaviour (self-management, adherence) and health system outcomes (health care utilization (eg, physician visits, pharmacy visits, readmission) and mortality) were included. Only studies that measured outcomes within 3 months of discharge were included because this time frame is frequently cited as the period in which the outcome may be associated with the intervention administered at discharge.19
S - Study Designs: Eligible study designs included: experimental studies (randomized controlled trials, quasi-experimental studies with control(s)), observational studies (prospective and historical cohort, case-control, case-crossover, case-cohort, case-time controlled, self-controlled case-series design, cross-sectional studies, time-series, pre-post studies), qualitative studies, and mixed methods studies.20 Descriptive quantitative studies were excluded as these studies do not assess the effectiveness of the intervention. We included published or in-press peer reviewed articles published as full reports. Book chapters, abstracts, conference proceedings were excluded.
Report Characteristics: Given the short time to conduct the review, we confined our search to published literature available in English-language only. We limited eligible studies to those published within the previous 15 years (2004 −2019) due to rapid shifts in clinical practice supporting medication management.
Literature Search Strategy
The search strategy was developed and executed by a university-affiliated librarian co-investigator (Appendix 1). A second librarian with expertise in health literature reviewed the search strategies using the PRESS EBC Checklist.21 The following databases were searched between July 26 and 30, 2019:
- Ovid MEDLINE (1946-present including Epub ahead of print, in-process, and other unindexed citations) Ovid MEDLINE (1946-present including Epub ahead of print, in-process, and other unindexed citations),
- Ovid EMBASE (1947-present),
- Cochrane Library,
- EBSCO CINAHL Plus with Full Text (1981-present).
Search results were exported by the librarian into EndNote and duplicates were removed. After duplicate removal, citations from only the prior 15 years (ie, 2019 to 2004) were identified.
Study Selection
Citation titles and abstracts from the searches were screened to determine study eligibility within Covidence®.22 Interrater agreement was assessed, and any conflicts resolved.
Standardized relevance screening forms with instructions were prepared and piloted to ensure uniform application of eligibility criteria. Titles and abstracts of identified references were reviewed by an experienced member of the study team. A second reviewer verified a subset of the records (5%) examined by the first reviewer and was also consulted for citations about which the first reviewer is uncertain (DG). Agreement on this subset of records was above a minimum kappa value of 0.80 taken to represent substantial agreement.23
Full-text articles were retrieved and reviewed using the process described above for any abstract that required further clarification or was related to the study research question. Disagreements were discussed until consensus was reached or reconciled by a third reviewer.
Team members did not review studies they authored, co-authored or consulted upon.
A review log was created to document all decisions made in relation to selecting studies for inclusion including recommendations of the reviewer(s) and reason(s) for exclusion to facilitate development of a flowchart, recommended by PRISMA.24
Data Charting Process and Data Items
We collected data about study characteristics (eg study design, citation), population characteristics (eg number of patients, age, gender, whether the population included was at high risk for adverse events due to presence of disabilities or language barriers), intervention characteristics (eg physical, verbal, or electronic modes of delivery, components of intervention such as videos, counselling, medication list, follow-up, content of intervention, layout (design), process of the interventions, transition type), study outcomes (eg patient and caregiver knowledge/comprehension, patient and caregiver attitudes and confidence, patient and caregiver behaviour and experience, health system outcomes, other outcomes), and conclusions.
Fields for data abstraction were based on those recommended by the Cochrane Collaboration and the Centre for Reviews and Dissemination.24,25 We were also guided by a systematic review conducted by members of our study team that explored the impact of patient-centered discharge tools.19 Further, the extent of user-centered design approaches used to develop each tool was assessed with the UCD-11 (User-Centred Design for Patient-Centred Tools) instrument.26 The UCD-11 instrument was used to generate a descriptive measure of an intervention’s user-centeredness through assessing the extent of end user (eg patients, caregivers, health professionals) involvement in the design, development, and refinement stages of the intervention.26 If the criteria was not explicitly reported in the article, it was assumed this criteria was not a part of tool development.
A preliminary data abstraction form and accompanying guide were created. Cochrane’s process for developing the data extraction form guided this process: a paper form was designed, two reviewers independently extracted data from 10 studies, and then met to determine the necessary revisions. Feedback was used to modify the form and repeat the pilot before creating the form electronically. Concurrently, a guide with instructions for completing the extraction and decision rules for coding data was created and revised iteratively. Once the fields for extraction and their accompanying coding rules were agreed upon, an electronic form was developed to facilitate data entry and management.
Due to the time constraints in which this rapid scoping review was conducted, one experienced reviewer abstracted data . A second reviewer was consulted in cases of uncertainty . Disagreements that could not be resolved through consensus were reconciled through discussion with the two reviewers in the presence of a third person .
Mechanisms for recording, assessing, and correcting data entry errors were devised.
A descriptive numerical summary and a narrative synthesis were completed to describe study designs, intervention (and comparator intervention, if applicable) characteristics, target population characteristics, and study outcomes, as stated in our study objective. Results were classified based on main conceptual categories (eg timing, mode of delivery, health-care providers involved). Summary tables were created for study characteristics, intervention characteristics, and development of the interventions (eg UCD-11).
Risk of Bias in Individual Evidence Sources
Our primary interest in this body of literature was to create an inventory of design, layout, and process considerations. Consistent with established scoping review methods, we did not appraise the risk of bias for included studies.14,15
Results
Search Results
The search yielded 8231 reports (Figure 1). After removing duplicates, 7671 articles were assessed for eligibility and after title and abstract screening, 113 articles were marked for full-text review. After full-text review, 53 articles were excluded, and 60 articles remained eligible.27–86 For a large number of articles (n = 45/53), the full text could not be found. Other reasons for excluding studies after full-text review included ineligible design (n = 3) or outcome (n = 3) and duplicates (n = 1) that were not identified during the title and abstract screening stage. None of the included studies were co-authored by a team member.
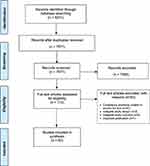 |
Figure 1 PRISMA flow diagram. |
Study Characteristics
Of the 60 studies included, 50.0% (n = 30) were randomized controlled trials,28,29,33–36,39,43,45–47,50,53,54,61–64,68–74,76,80,83,84,86 23.3% (n = 14) were non-randomized trials,30,31,37,40,48,49,51,56–58,60,75,77,78 11.7% (n = 7) were cohort studies,27,42,52,59,66,67,79 8.3% (n = 5) were before-and-after studies,32,41,55,81,82 5.0% (n = 3) were quasi-randomized trials,38,44,85 and 1.7% (n = 1) were case-control studies.65 The majority of studies (53.3%, n = 32/60) were conducted in the United States of America (USA).29,30,32–34,37,39,42,43,45,48,49,51,53,57,59–61,64–67,69–71,75,78,79,81–83,85 Other countries of origin for more than one study included Australia (n = 4),38,40,55,84 Canada (n = 4),44,47,76,80 Brazil (n = 2),36,68 Croatia (n = 2),62,63 France (n = 2),56,72 and Switzerland (n = 2).52,58 The remaining 12 studies originated from other unique countries. Table 1 describes the participants, intervention, comparator and outcome(s) of the included studies. Table 2 provides additional information regarding the interventions of included studies ie, specifies health-care providers delivering the intervention, the timing of the intervention, the various components of multi-pronged interventions, etc.
 |  |  |  |  |  |  |  |  |  |
Table 1 Summary of Studies Included |
 |  |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
Table 2 Key Characteristics of Studies Included |
 |
Table 3 UCD-1126 Criteria of Included Studies |
Many studies selected patients based on age (76.7%, n = 46/60)27–30,33–45,47,49–53,55–57,60–64,66,67,71–76,78,80,81,83–86 and presence of a particular medical condition (50.0%, n = 30/60).29–31,33,34,36,37,40,43–45,47,48,50,53,54,59,60,63,66–69,71,76,78,82–84,86 Fewer studies selected patients based on the medication-class prescribed (30.0%, n = 18/60)29,30,39–41,44,47,48,53,64,68–71,74,78,84,85 or the patients’ number of medications (31.7%, n = 19/60).28,30,33,35,43,46,48,49,52,55,58,61,62,65,70,74,77,84,85 Few studies selected patients based on the presence of an intellectual or physical disability (3.3%, n = 2/60),45,84 low literacy level (n = 1/60),48 or language barrier (n = 1/60).84 There were also only a few studies that included a subgroup analysis involving patients with low literacy (8.3%, n = 5/60),34,38,53,69,71 intellectual or physical disability (5.0%, n = 3/60),34,45,84 and language barriers (n = 1/60).84
UCD-11 Reporting
Only 25% of studies (n = 15/60) explicitly discussed the development of the intervention,29,31,33–36,43,47,51,52,64,76,81,82,86 making it difficult to apply the UCD-11 criteria. Of the 60 articles, none met all 11 criteria and only 1 met 10 of the 11 UCD-11 criteria (Table 3).47 Twenty percent (n = 12/60) of articles met more than 3 of the 11 UCD-11 criteria.29,33–36,43,47,52,64,76,81,86 Of the 15 studies which discussed intervention development, 1 study reported consultation of expert opinion in the development process (n = 1/15),34 and 3 studies reported usage of three or more iterative cycles in which outside opinions were consulted and prototype revisions were made accordingly (n = 3/15).36,47,81 In contrast to these under-reported criteria, end user involvement in evaluation of the intervention, part of our study eligibility criteria, was consistently reported by all 15 studies (n = 15/15) that explicitly discussed intervention development.29,31,33–36,43,47,51,52,64,76,81,82,86
Content
Physical/Electronic Medication List Interventions
For details regarding interventions including physical or electronic medication lists, see “Table 4”. These included any interventions that contained the patient’s medication lists in either a physical (eg paper) or electronic format. While 63.3% (n = 38/60) interventions included a physical or electronic document containing information regarding the patient’s medications (eg customized medication list, medication information pamphlet on a particular medication),28,29,33–43,45–47,50,52,53,55,56,58–68,71,76,77,83,85,86 only 36.7% (n = 22/60) interventions included a list of the patient’s medications.28,29,33–37,43,45,53,55,56,58,60,61,65–67,77,83,85,86 Of the 38 interventions with a physical or electronic document pertaining to the patient’s medications, the content related to medication information included varied. Many included an indication/reason for use (52.6%, n = 20/38),29,33–37,40,43,47,52,53,56,58,61–64,66,76,77 timing of administration (42.1%, n = 16/38),34–37,40,43,52,53,55,60–64,77,86 frequency of administration (42.1%, n = 16/38),34,36,37,40,43,47,52,53,56,58,60,61,64,66,77,86 and side effects (34.2%, n = 13/38).34,36,39,40,50,52,56,58,61–64,66 Ten percent (n = 6/60) of interventions included an electronic component.29,39,44,54,69,81
 |  |  |
Table 4 Characteristics of Written Information in Physical/Electronic Interventions* |
Design
Mode of Delivery
Interventions included in the study were classified based on their mode of delivery into either physical, verbal, electronic or a combination of these modalities. The majority of interventions consisted of multiple components (78.3%, n = 47)27–38,41–43,45–51,53,55–60,63–72,74,75,78,79,82,83,85,86 with 41.7% (n = 25) of interventions including a follow-up component.27,30,33,34,36,37,42,44,45,48,51,53,59,65–67,70,73–75,79,82–85 Sixty-percent (n=37/60) of interventions, used physical and verbal means to deliver various components (eg medication list and patient education).28,29,33–43,45,46,49,50,53,55,56,58–60,62–69,71,74,82,83,85,86 Three of these interventions also included an electronically delivered component.29,39,69 Some interventions had only one mode of delivery: thirty percent (n = 13/60) of interventions were only verbally communicated to the patient,30–32,48,51,57,70,72,73,75,78,79,84 while 10% (n = 6/60) were solely physically provided to the patient47,52,61,76,77,80 and 5% (n = 3/60) were only electronically delivered.44,54,81 A small number of interventions did not involve direct delivery to patients. One of the interventions consisted of a letter mailed to the patient76 and family physician while another intervention involved a referral to outpatient providers if a need was identified.27
Personalization
Interventions were considered “personalized” if they contained any information specific to the patient, rather than a particular medication or medical condition. Most of the interventions (83.3%, n = 50/60) were personalized to the patient.27–30,32–38,42–49,51,53–63,65–70,72–79,82–86 The most common methods of personalization were including the patient’s specific medications list. However, only 13.3% (n = 8/60) involved assessment of the patient’s health priorities (eg if they wanted the medication list in a particular language).33,34,36,43,45,47,49,79 Even fewer (11.7%, n = 7/60) reported integrating the patient’s values and preferences into the care plan.33,34,36,43,45,49,79 Few interventions (20.0%, n = 12/60) addressed the patient’s ability to access medications financially in the community.32–34,36,37,48,51,56,70,73,75,79
Process
Patient Involvement
A large portion (81.7%, n = 49) of interventions included patient counselling.28–46,48–51,53,55–60,62,63,66–75,78,79,82–86 Of these interventions, 53.1% (n = 26/49) directly involved the patient during intervention delivery through strategies such as assessing patient understanding during the delivery or engaging patients in creation of a management plan.33,34,36–38,42,44,45,49,53,55,56,58,60,66,68,70,71,73,74,79,82–86 Twenty-two (44.9%) interventions assessed the patient for understanding,33,34,37,38,42,45,49,56,60,66,68,70,71,73,74,79,82–86 while 22.4% (n = 11/49) of these interventions specifically used teach-back (eg confirm patients’ understanding by asking them to “teach it back” to the health-care provider),34,38,42,45,49,53,71,74,79,82,83 and 12.2% (n = 6/49) assessed the patient’s health priorities (eg values, preferences).33,34,36,45,49,79 Other strategies used included increasing responsibility based on patient’s successful compliance, patient’s own management plan using a template, and positive reinforcement.
Timing
The majority of interventions were delivered to the patient before leaving the hospital (51.7%, n = 31).28,29,31,32,35,38–41,43,46,47,49,50,52,55–58,61–64,68,71,72,75,77,80,81,86 Thirty percent (n = 18) of interventions had elements delivered before and after the patients left the hospital,30,34,36,37,42,45,48,51,66,67,70,74,78,79,82–85 16.7% (n = 10) of interventions were delivered exclusively after the patients left the hospital,27,33,44,53,54,59,60,65,73,76 and for one of the studies, it was not clear when the intervention was delivered to the patient based on the manuscript.69
Healthcare Providers Involved and Number of Providers
Many interventions involved delivery from pharmacists (61.7%, n = 37),27–32,34–38,41,42,46,48,51–53,55–57,59,65–68,70,72–75,78,79,82,84–86 physicians (25.0%, n = 15),27,31,40,47,58–60,62–64,66,67,69,77,82 and nurses (25.0%, n = 15).30,32,33,39,43,44,49,50,53,55,59,61,66,67,83 Thirteen interventions (21.7%) involved delivery from multiple health-care providers.27,30–32,53,55,59,65–67,79,82,83 Only 26.7% (n = 16/60) studies reported training the staff administering the intervention.33,34,36,38,40,43,45,48,49,53,60,64,67,68,71,83 Twenty percent (n = 12) of interventions were shared with other health-care practitioners outside the intervention setting.35,47,48,53,57,60,72,73,76,83–85
Reported Outcomes
“Table 5: Outcome Measure of Studies” presents details regarding outcome measures ie, which of the included studies assessed a particular outcome category, and those that demonstrated a clinically significant impact in the intervention group. Overall, 63% (38/60)27–38,42,45–49,51,54,56,57,60,62,65–68,70,71,73–75,78,79,81,83,85 of included studies reported health system outcomes (eg readmission rate, mortality rate) and 52% (31/60)28,30,33,36,37,40,43–47,49,50,53–56,58,59,62–64,68,69,71,74,75,80,84,86 explored patient and caregiver behavior and experience (eg behaviours related to adherence, life-style management). Thirty-percent (18/60)28,31,33,35,39,41,43,45,50,52,53,58,61,64,70,77,81,84 examined patient and caregiver knowledge/comprehension (eg, understanding of condition and medication), 28% (17/60)33,38,42,46,49,52–54,58,60,61,69,73–75,80,81, reported patient and caregiver attitudes and confidence (eg, satisfaction, preferences, values and self-efficacy; and 27% (16/60)30,31,35,40,43,50,51,54,63,65,70,75,77,79,82,85 explored other outcome measures (eg, health-care provider insights, economic impact).
 |  |  |
Table 5 Outcome Measures of Studies* |
With respect to included studies reporting significant findings, 68% (21 of 31 studies)28,30,33,36,40,46,47,50,55,56,59,62,64,68,69,71,74–76,84,86 of studies reported significant impact on patient and caregiver behavior and experience. Sixty-seven percent (12 of 18 studies)31,35,39,41,45,50,52,58,61,64,77,84 assessing patient and caregiver knowledge, 55% (21 of 38 studies)27–33,35,36,47–49,60,62,66,70,71,75,78,79,85 assessing health system outcomes, 44% (7 of 16 studies)30,31,50,51,54,77,85 assessing other outcome measures (eg health-care provider insights, economic impact) and 41% (7 of 17 studies)33,38,49,69,74,75,81 assessing patient and caregiver attitudes and confidence demonstrated a clinically significant impact in the intervention group.
Discussion
Our rapid scoping review of medication support-related TiC interventions identified 60 relevant studies. The majority of studies were randomized controlled trials involving verbal counselling and/or physical document delivered to the patient before discharge. Most studies assessed health system outcomes and patient/caregiver behavior and experience, with the majority of studies showing a significant improvement in these outcomes. We identified four key findings, which differ from previous reviews on the topic of medication support and care transitions:87,88 1) the need for improved reporting of intervention development and characteristics; 2) the need to include high-risk populations when designing studies; 3) the potential for future research to apply user-centred design approaches to intervention development, 4) the opportunity for future studies to examine electronic interventions.
Our first key finding is that included studies lacked reporting on the development and characteristics of interventions. The majority of included studies failed to mention the development of their tool entirely, making it difficult to understand the rationale and elements of the interventions, and to assess whether the interventions addressed patient needs. Further, studies were lacking in their description of interventions for both physical and counselling interventions. For example, Lam 2011 mentioned a “medication list with detailed instructions” but did not specify these details.55 Also, Chakravarthy 2018 suggested that “discharge instructions” were provided by nursing staff but did not describe the content or components of the instructions.39
Importantly, these gaps in reporting make it difficult to discern what was tested, thereby diminishing the replicability of particular studies in situations where beneficial impact in the intervention group was demonstrated. We recommend increased use of the TIDieR reporting guideline to ensure that authors provide sufficient details when describing their intervention.89 Further, similarly detailed reporting of the elements of usual care comparators will help readers better understand the “baseline” and assess applicability to their own health systems. This review identified that few staff administering interventions were trained prior to the study. It was unclear whether training was not reported or if it was not provided. A possible explanation may be that interventions were delivered by health-care providers performing their regular scope of practice and did not require additional training. Regardless, additional reporting in publications regarding training, or the lack thereof, is encouraged.
Second, our scoping review identified an important gap with respect to patient populations that have been studied. Very few studies included or considered at-risk populations beyond older adults, such as patients with low literacy, language barriers, or disabilities, in their patient selection or sub-group analysis. These groups are at increased risk of adverse events and more likely to experience systemic barriers to care.8,10,90 Based on the theory of design by exception, considering these “extreme” users when designing an intervention, instead of designing for the average, will result in more equitable interventions that are compatible with the large majority of users and does so more efficiently than other mainstream design approaches.13,91
Third, we found that very few studies met the UCD-11 criteria, which is a way to describe an intervention’s user centredness ie, explores end user involvement in design, development and refinement stages of an intervention.26 This may be partially but not fully explained by poor reporting of how interventions were developed. The strengths of participatory action and user-centered design approaches when developing complex interventions are increasingly recognized, as evidenced, for example, by their inclusion in recent UK Medical Research Council guidance.92 Members of our research team have successfully used these approaches to engage patients, caregivers, and health-care providers in co-developing patient-oriented discharge instructions, which has subsequently undergone wide-scale implementation and evaluation at the provincial level with positive results.90,93 The application of these approaches when developing medication-focused interventions at care transitions is an important opportunity for researchers to explore.
Finally, we note that most interventions included in this review involved providing written information and verbal instructions or education to patients and caregivers. Electronic interventions were the least frequently used. The paucity of electronic interventions was somewhat unexpected considering the shift toward technology in health care. Considering that the technology is available for various tools, such as medication time reminder, missed medication alerts, applications across multiple devices and much more, future assessment and use of these tools is another opportunity for future studies.
Strengths and Limitations
Limitations of this study are consistent with those known for rapid reviews.94 After piloting the process for title and abstract screening to attain agreement on 5% of the studies, subsequent studies were assessed for eligibility by one reviewer. Our review was limited to articles published between January 2004 and July 2019 in English, articles indexed in well-known databases, and those known to context experts. Grey literature sources were not searched and as an optional component of scoping reviews, risk of bias for included studies was not assessed.
Despite these limitations, our study has several strengths building on previous research conducted examining medication interventions during care transitions. For example, Tomlinson et al87 conducted a systematic review and meta-analysis of randomized controlled trials evaluating interventions that support medication continuity in older adults at or started within one month of discharge from hospital. Consistent with scoping review methods, we used a broader search strategy (ie, included more constructs), and included study designs beyond randomized controlled trials, more eligible populations, and studies with a range of outcomes. Another systematic review and meta-analysis by Daliri et al88 examining the impact of prospective studies of medication-related interventions at care transitions on outcomes, is complementary to our review. They used meta-analyses to estimate the impact of interventions on outcomes while we focused on describing patient and caregiver-centered medication support interventions and how user-centered design elements were considered in the development of these interventions. Our study builds on their review by expanding the breadth of study designs and outcomes eligible for inclusion.
Conclusion
There is global interest in understanding the components and impact of medication-related interventions on patient outcomes during care transitions, as evidenced by recent systematic reviews exploring this topic. In this rapid scoping review, focused on describing patient and caregiver-centered medication support interventions and the role of user-centred design, the majority of studies included were randomized controlled trials involving verbal counselling and/or physical document delivered to the patient before discharge. Most studies selected patients based on age or medical condition, and few studies considered patients at high-risk of adverse events. Few studies reported on the development of the intervention. The majority of studies included multiple components, most commonly involving verbal or physical modalities, rather than electronic interventions. With respect to outcomes, most studies assessed health system outcomes and patient and caregiver behavior and experiences. Less commonly explored were patient and caregiver knowledge, attitudes or confidence. Studies assessing medication support interventions at TiC would benefit from improved reporting on development, characteristics of the intervention and usual care comparators, and training administered. As well, active involvement of people with higher medication-related needs, such as those with low literacy, language barriers, and disabilities, is highly encouraged.
Data Sharing Statement
All search strategies are included in Appendix 1 and included studies are cited herein. Citations for excluded studies are available on reasonable request from the corresponding author.
Acknowledgments
The authors wish to thank Allie Dai, Dana Green-Campbell, Sanket Patel, Miles Luke, Clara Korenvain for their contributions to data collection, David Lightfoot for his assistance with reviewing the search strategy, and Melanie Dissanayake for reviewing and editing the manuscript. We also wish to thank our larger investigator team including Audrey Chaput, Michelle Ransom, Angela Gonzales, Tai Huynh, Aisha Lofters, Yona Lunsky, Carol Mulder for their contributions to conceptualization of the study.
Author Contributions
Conceptualization: LMM, SHG, GBR, SJTG, LJ, KO, ZRY, SV
Formal analysis: SV, ZD, LMM
Investigation: SV, ZD, LMM
Methodology: SV, SHG, ZD, GBR, SJTG, LJ, CM, KO, ZRY, LMM
Project administration: SV, LMM
Supervision: LMM
Visualization: SV, SHG, ZD, GBR, SJTG, LJ, CM, KO, ZRY, LMM
Writing – original draft: SV, ZD, LMM
Writing – reviewing and editing: SV, SHG, ZD, GBR, SJTG, LJ, CM, KO, ZRY, LMM
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Canadian Institutes of Health Research (CIHR) FRN: 163065. Stipends for ZhiDi Deng and Shawn Varghese were partially supported by the Leslie Dan Faculty of Pharmacy, University of Toronto.
Disclosure
Dr Lisa M McCarthy reports grants from Canadian Institutes for Health Research during the conduct of the study. The authors have declared no potential conflicts of interest.
References
1. WHO. WHO Global Patient Safety Challenge: Medication Without Harm. WHO. Available from: http://www.who.int/patientsafety/medication-safety/medication-without-harm-brochure/en/.
2. Kee KW, Char CWT, Yip AYF. A review on interventions to reduce medication discrepancies or errors in primary or ambulatory care setting during care transition from hospital to primary care. J Fam Med Prim Care. 2018;7(3):501–506. doi:10.4103/jfmpc.jfmpc_196_17
3. Wheeler AJ, Scahill S, Hopcroft D, Stapleton H. Reducing medication errors at transitions of care is everyone’s business. Aust Prescr. 2018;41(3):73–77. doi:10.18773/austprescr.2018.021
4. Sultana J, Cutroneo P, Trifirò G. Clinical and economic burden of adverse drug reactions. J Pharmacol Pharmacother. 2013;4(Suppl1):S73–S77. doi:10.4103/0976-500X.120957
5. Eibergen L, Janssen MJ, Blom L, et al. Informational needs and recall of in-hospital medication changes of recently discharged patients. Res Soc Admin Pharm. 2018;14(2):146–152.
6. Saqib A, Atif M, Ikram R, Riaz F, Abubakar M, Scahill S. Factors affecting patients’ knowledge about dispensed medicines: a Qualitative study of healthcare professionals and patients in Pakistan. PLoS One. 2018;13(6):e0197482. doi:10.1371/journal.pone.0197482
7. Karliner LS, Auerbach A, Nápoles A, Schillinger D, Nickleach D, Pérez-Stable EJ. Language barriers and understanding of hospital discharge instructions. Med Care. 2012;50(4):283–289. doi:10.1097/MLR.0b013e318249c949
8. de Moissac D, Bowen S. Impact of language barriers on quality of care and patient safety for official language minority francophones in Canada. J Patient Exp. 2019;6(1):24–32. doi:10.1177/2374373518769008
9. Coleman EA, Smith JD, Raha D, Min S. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med. 2005;165(16):1842–1847. doi:10.1001/archinte.165.16.1842
10. Guilcher SJT, Hogan M-E, Calzavara A, et al. Prescription drug claims following a traumatic spinal cord injury for older adults: a retrospective population-based study in Ontario, Canada. Spinal Cord. 2018;56(11):1059–1068. doi:10.1038/s41393-018-0174-z
11. Sullivan WF, Diepstra H, Heng J, et al. Primary care of adults with intellectual and developmental disabilities: 2018 Canadian consensus guidelines. Can Fam Physician Med Fam Can. 2018;64(4):254–279.
12. Cawthon C, Walia S, Osborn CY, Niesner KJ, Schnipper JL, Kripalani S. Improving care transitions: the patient perspective. J Health Commun. 2012;17(Suppl 3):312–324. doi:10.1080/10810730.2012.712619
13. Hahn-Goldberg S, Chaput A, Rosenberg-Yunger Z, et al. Tool development to improve medication information transfer to patients during transitions of care: a participatory action research and design thinking methodology approach. Res Soc Adm Pharm. 2021. doi:10.1016/j.sapharm.2021.04.002
14. Peters M, Godfrey C, McInerney P, Munn Z, Trico A, Khalil H. Chapter 11: scoping Reviews. In: Aromataris E, Munn Z editors. JBI Manual for Evidence Synthesis. JBI;2020. doi:10.46658/JBIMES-20-12
15. Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi:10.7326/M18-0850
16. Definition of caregiver - NCI Dictionary of Cancer Terms - National Cancer Institute; 2011. Available from: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/caregiver.
17. Vira T, Colquhoun M, Etchells E. Reconcilable differences: correcting medication errors at hospital admission and discharge. Qual Saf Health Care. 2006;15(2):122–126. doi:10.1136/qshc.2005.015347
18. Forster AJ, Clark HD, Menard A, et al. Adverse events among medical patients after discharge from hospital. CMAJ Can Med Assoc J J Assoc Medicale Can. 2004;170(3):345–349.
19. Okrainec K, Lau D, Abrams HB, et al. Impact of patient-centered discharge tools: a systematic review. J Hosp Med. 2017;12(2):110–117. doi:10.12788/jhm.2692
20. Deeks JJ, Dinnes J, D’Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess Winch Engl. 2003;7(27):
21. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi:10.1016/j.jclinepi.2016.01.021
22. Covidence - Better systematic review management. Available from: https://www.covidence.org/.
23. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174.
24. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Med. 2009;6(7):e1000100. doi:10.1371/journal.pmed.1000100
25. Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLOS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
26. Witteman HO, Vaisson G, Provencher T, et al. An 11-item measure of user- and human-centered design for personal health tools (UCD-11): development and validation. J Med Internet Res. 2021;23(3):e15032. doi:10.2196/15032
27. Acomb C, Laverty U, Smith H, Fox G, Petty D. Medicines optimisation on discharge. The Integrated Medicines oPtimisAtion on Care Transfer (IMPACT) project: 0171. Int J Pharm Pract. 2013;21:123–124.
28. Al-Hashar A, Al-Zakwani I, Eriksson T, et al. Impact of medication reconciliation and review and counselling, on adverse drug events and healthcare resource use. Int J Clin Pharm. 2018;40(5):1154–1164. doi:10.1007/s11096-018-0650-8
29. Anderegg SV, Demik DE, Carter BL, et al. Acceptance of recommendations by inpatient pharmacy case managers: unintended consequences of hospitalist and specialist care. Pharmacotherapy. 2013;33(1):11–21. doi:10.1002/phar.1164
30. Bailey JE, Surbhi S, Wan JY, et al. Effect of intensive interdisciplinary transitional care for high-need, high-cost patients on quality, outcomes, and costs: a Quasi-experimental study. J Gen Intern Med. 2019;34(9):1815–1824. doi:10.1007/s11606-019-05082-8
31. Baky V, Moran D, Warwick T, et al. Obtaining a follow-up appointment before discharge protects against readmission for patients with acute coronary syndrome and heart failure: a quality improvement project. Int J Cardiol. 2018;257:12–15. doi:10.1016/j.ijcard.2017.10.036
32. Balling L, Erstad BL, Weibel K. Impact of a transition-of-care pharmacist during hospital discharge. J Am Pharm Assoc JAPhA. 2015;55(4):443–448. doi:10.1331/JAPhA.2015.14087
33. Barnason S, Zimmerman L, Hertzog M, Schulz P. Pilot testing of a medication self-management transition intervention for heart failure patients. West J Nurs Res. 2010;32(7):849–870. doi:10.1177/0193945910371216
34. Bell SP, Schnipper JL, Goggins K, et al. Effect of pharmacist counseling intervention on health care utilization following hospital discharge: a randomized control trial. J Gen Intern Med. 2016;31(5):470–477. doi:10.1007/s11606-016-3596-3
35. Bolas H, Brookes K, Scott M, McElnay J. Evaluation of a hospital-based community liaison pharmacy service in Northern Ireland. Pharm World Sci PWS. 2004;26(2):114–120. doi:10.1023/b:phar.0000018601.11248.89
36. Bonetti AF, Bagatim BQ, Mendes AM, et al. Impact of discharge medication counseling in the cardiology unit of a tertiary hospital in Brazil: a randomized controlled trial. Clin Sao Paulo Braz. 2018;73:e325. doi:10.6061/clinics/2018/e325
37. Budiman T, Snodgrass K, Komatsu Chang A. Evaluation of pharmacist medication education and post-discharge follow-up in reducing readmissions in patients with ST-Segment Elevation Myocardial Infarction (STEMI). Ann Pharmacother. 2016;50(2):118–124. doi:10.1177/1060028015620425
38. Cabilan CJ, Boyde M, Currey E. The effectiveness of pharmacist- led discharge medication counselling in the emergency department (ExPLAIN): a pilot quasi-experimental study. Patient Educ Couns. 2019;102(6):1157–1163. doi:10.1016/j.pec.2019.01.020
39. Chakravarthy B, Somasundaram S, Mogi J, et al. Randomized pilot trial measuring knowledge acquisition of opioid education in emergency department patients using a novel media platform. Subst Abuse. 2018;39(1):27–31. doi:10.1080/08897077.2017.1375061
40. Chan BS, Mills N, Chiew A. Does physician education and factsheet impact on safe opioid use in emergency patients? Can J Addict. 2018;9(2):26–33. doi:10.1097/CXA.0000000000000019
41. Chedepudi PR, Mounika O, Chandrika G, et al. Impact of pharmacist led anticoagulation monitoring and patient education on oral anticoagulation therapy with acenocoumarol. Asian J Pharm Clin Res. 2017:314–317. doi:10.22159/ajpcr.2017.v10i10.16459.
42. Christy S, Sin B, Gim S. Impact of an integrated pharmacy transitions of care pilot program in an urban hospital. J Pharm Pract. 2016;29(5):490–494. doi:10.1177/0897190014568674
43. Cordasco KM, Asch SM, Bell DS, et al. A low-literacy medication education tool for safety-net hospital patients. Am J Prev Med. 2009;37(6 Suppl 1):S209–216. doi:10.1016/j.amepre.2009.08.018
44. Côté J, Godin G, Ramirez-Garcia P, et al. Virtual intervention to support self-management of antiretroviral therapy among people living with HIV. J Med Internet Res. 2015;17(1):e6. doi:10.2196/jmir.3264
45. Davis KK, Mintzer M, Dennison Himmelfarb CR, Hayat MJ, Rotman S, Allen J. Targeted intervention improves knowledge but not self-care or readmissions in heart failure patients with mild cognitive impairment. Eur J Heart Fail. 2012;14(9):1041–1049. doi:10.1093/eurjhf/hfs096
46. Sáez de la Fuente J, Granja Berná V, Lechuga Vázquez P, et al. [Efficiency of the information given at discharge and adherence of polymedicated patients]. Farm Hosp Organo of Expresion Cient Soc Espanola Farm Hosp. 2011;35(3):128–134. Spanish. doi:10.1016/j.farma.2010.06.005
47. Ducharme FM, Zemek RL, Chalut D, et al. Written action plan in pediatric emergency room improves asthma prescribing, adherence, and control. Am J Respir Crit Care Med. 2011;183(2):195–203. doi:10.1164/rccm.201001-0115OC
48. Feldmann JD, Otting RI, Otting CM, Witry MJ. A community pharmacist-led service to facilitate care transitions and reduce hospital readmissions. J Am Pharm Assoc JAPhA. 2018;58(1):36–43. doi:10.1016/j.japh.2017.09.004
49. Hyrkas K, Wiggins M. A comparison of usual care, a patient-centred education intervention and motivational interviewing to improve medication adherence and readmissions of adults in an acute-care setting. J Nurs Manag. 2014;22(3):350–361. doi:10.1111/jonm.12221
50. Jiang R, Chen M, Li Y. Effectiveness of picture description education on compliance behaviors of diabetics in western Sichuan district. Int J Nurs Sci. 2016;3(3):229–234. doi:10.1016/j.ijnss.2016.08.008
51. Jones CD, Anthony A, Klein MD, et al. The effect of a pharmacist-led multidisciplinary transitions-of-care pilot for patients at high risk of readmission. J Am Pharm Assoc JAPhA. 2018;58(5):554–560. doi:10.1016/j.japh.2018.05.008
52. Kaestli LZ, Noble S, Combescure C, et al. Drug information leaflets improve parental knowledge of their child’s treatment at paediatric emergency department discharge. Eur J Hosp Pharm. 2016;23(3):151–155. doi:10.1136/ejhpharm-2015-000776
53. Kapoor A, Landyn V, Wagner J, et al. Supplying pharmacist home visit and anticoagulation professional consultation during transition of care for patients with venous thromboembolism. J Patient Saf. 2020;16(4):e367–e375. doi:10.1097/PTS.0000000000000571
54. Khonsari S, Subramanian P, Chinna K, Latif LA, Ling LW, Gholami O. Effect of a reminder system using an automated short message service on medication adherence following acute coronary syndrome. Eur J Cardiovasc Nurs J Work Group Cardiovasc Nurs Eur Soc Cardiol. 2015;14(2):170–179. doi:10.1177/1474515114521910
55. Lam P, Elliott RA, George J. Impact of a self-administration of medications programme on elderly inpatients’ competence to manage medications: a pilot study. J Clin Pharm Ther. 2011;36(1):80–86. doi:10.1111/j.1365-2710.2009.01157.x
56. Leguelinel-Blache G, Dubois F, Bouvet S, et al. Improving Patient’s Primary Medication Adherence: the Value of Pharmaceutical Counseling. Medicine (Baltimore). 2015;94(41):e1805. doi:10.1097/MD.0000000000001805
57. Li H, Guffey W, Honeycutt L, Pasquale T, Rozario NL, Veverka A. Incorporating a pharmacist into the discharge process: a unit-based transitions of care pilot. Hosp Pharm. 2016;51(9):744–751. doi:10.1310/hpj5109-744
58. Louis-Simonet M, Kossovsky MP, Sarasin FP, et al. Effects of a structured patient-centered discharge interview on patients’ knowledge about their medications. Am J Med. 2004;117(8):563–568. doi:10.1016/j.amjmed.2004.03.036
59. Lingyun L, Cynthia J, de Leon N, Alberta W, Donald C, Freny M. Impact of a multi-disciplinary heart failure post-discharge management clinic on medication adherence. J Am Coll Cardiol. 2014;63(12_Supplement):A539–A539. doi:10.1016/S0735-1097(14)60539-8
60. Luder HR, Frede SM, Kirby JA, et al. TransitionRx: impact of community pharmacy postdischarge medication therapy management on hospital readmission rate. J Am Pharm Assoc JAPhA. 2015;55(3):246–254. doi:10.1331/JAPhA.2015.14060
61. Manning DM, O’Meara JG, Williams AR, et al. 3D: a tool for medication discharge education. Qual Saf Health Care. 2007;16(1):71–76. doi:10.1136/qshc.2006.018564
62. Marusic S, Gojo-Tomic N, Erdeljic V, et al. The effect of pharmacotherapeutic counseling on readmissions and emergency department visits. Int J Clin Pharm. 2013;35(1):37–44. doi:10.1007/s11096-012-9700-9
63. Marušić S, Meliš P, Lucijanić M, et al. Impact of pharmacotherapeutic education on medication adherence and adverse outcomes in patients with type 2 diabetes mellitus: a prospective, randomized study. Croat Med J. 2018;59(6):290–297.
64. McCarthy DM, Wolf MS, McConnell R, et al. Improving patient knowledge and safe use of opioids: a randomized controlled trial. Acad Emerg Med off J Soc Acad Emerg Med. 2015;22(3):331–339. doi:10.1111/acem.12600
65. Miller DE, Roane TE, McLin KD. Reduction of 30-day hospital readmissions after patient-centric telephonic medication therapy management services. Hosp Pharm. 2016;51(11):907–914. doi:10.1310/hpj5111-907
66. Moye PM, Chu PS, Pounds T, Thurston MM. Impact of a pharmacy team-led intervention program on the readmission rate of elderly patients with heart failure. Am J Health-Syst Pharm. 2018;75(4):183–190. doi:10.2146/ajhp170256
67. Murphy JA, Schroeder MN, Rarus RE, Yakubu I, McKee SOP, Martin SJ. Implementation of a cardiac transitions of care pilot program: a prospective study of inpatient and outpatient clinical pharmacy services for patients with heart failure exacerbation or acute myocardial infarction. J Pharm Pract. 2019;32(1):68–76. doi:10.1177/0897190017743129
68. Oliveira-Filho AD, Morisky DE, Costa FA, Pacheco ST, Neves SF, Lyra-Jr DP. Improving post-discharge medication adherence in patients with CVD: a pilot randomized trial. Arq Bras Cardiol. 2014;103(6):502–512. doi:10.5935/abc.20140151
69. Olives TD, Patel RG, Thompson HM, Joing S, Miner JR. Seventy-two-hour antibiotic retrieval from the ED: a randomized controlled trial of discharge instructional modality. Am J Emerg Med. 2016;34(6):999–1005. doi:10.1016/j.ajem.2016.02.046
70. Phatak A, Prusi R, Ward B, et al. Impact of pharmacist involvement in the transitional care of high-risk patients through medication reconciliation, medication education, and postdischarge call-backs (IPITCH Study). J Hosp Med. 2016;11(1):39–44. doi:10.1002/jhm.2493
71. Press VG, Arora VM, Trela KC, et al. Effectiveness of interventions to teach metered-dose and diskus inhaler techniques. A randomized trial. Ann Am Thorac Soc. 2016;13(6):816–824. doi:10.1513/AnnalsATS.201509-603OC
72. Renaudin P, Baumstarck K, Daumas A, et al. Impact of a pharmacist-led medication review on hospital readmission in a pediatric and elderly population: study protocol for a randomized open-label controlled trial. Trials. 2017:18. doi:10.1186/s13063-017-1798-6.
73. Salmany SS, Ratrout L, Amireh A, et al. The impact of pharmacist telephone calls after discharge on satisfaction of oncology patients: a randomized controlled study. J Oncol Pharm Pract off Publ Int Soc Oncol Pharm Pract. 2018;24(5):359–364. doi:10.1177/1078155217709616
74. Sanii Y, Torkamandi H, Gholami K, Hadavand N, Javadi M. Role of pharmacist counseling in pharmacotherapy quality improvement. J Res Pharm Pract. 2016;5(2):132–137. doi:10.4103/2279-042X.179580
75. Sarangarm P, London MS, Snowden SS, et al. Impact of pharmacist discharge medication therapy counseling and disease state education: pharmacist Assisting at Routine Medical Discharge (project PhARMD). Am J Med Qual off J Am Coll Med Qual. 2013;28(4):292–300. doi:10.1177/1062860612461169
76. Schwalm J-D, Ivers NM, Natarajan MK, et al. Cluster randomized controlled trial of Delayed Educational Reminders for Long-term Medication Adherence in ST-Elevation Myocardial Infarction (DERLA-STEMI). Am Heart J. 2015;170(5):903–913. doi:10.1016/j.ahj.2015.08.014
77. Send AFJ, Schwab M, Gauss A, Rudofsky G, Haefeli WE, Seidling HM. Pilot study to assess the influence of an enhanced medication plan on patient knowledge at hospital discharge. Eur J Clin Pharmacol. 2014;70(10):1243–1250. doi:10.1007/s00228-014-1723-9
78. Shaver A, Morano M, Pogodzinski J, Fredrick S, Essi D, Slazak E. Impact of a community pharmacy transitions-of-care program on 30-day readmission. J Am Pharm Assoc JAPhA. 2019;59(2):202–209. doi:10.1016/j.japh.2018.10.011
79. Shull MT, Braitman LE, Stites SD, DeLuca A, Hauser D. Effects of a pharmacist-driven intervention program on hospital readmissions. Am J Health-Syst Pharm. 2018;75(9):e221–e230. doi:10.2146/ajhp170287
80. Singh S, Clarke C, Lawendy AR, et al. First Place: a prospective, randomized controlled trial of the impact of written discharge instructions for postoperative opioids on patient pain satisfaction and on minimizing opioid risk exposure in orthopaedic surgery. Curr Orthop Pract. 2018;29(4):292–296.
81. Sinha S, Dillon J, Dargar SK, et al. What to expect that you’re not expecting: a pilot video education intervention to improve patient self-efficacy surrounding discharge medication barriers. Health Informatics J. 2019;25(4):1595–1605. doi:10.1177/1460458218796644
82. Smith L Reducing Acute Myocardial Infarction Readmissions [dissertation]. USA: Grand Canyon University, 2017.
83. Tuttle KR, Alicic RZ, Short RA, et al. Medication therapy management after hospitalization in CKD: a randomized clinical trial. Clin J Am Soc Nephrol CJASN. 2018;13(2):231–241. doi:10.2215/CJN.06790617
84. Vuong T, Marriott JL, Kong DCM, Siderov J. Implementation of a community liaison pharmacy service: a randomised controlled trial. Int J Pharm Pract. 2008;16(3):127–135. doi:10.1211/ijpp.16.3.0002
85. Walker PC, Bernstein SJ, Jones JNT, et al. Impact of a pharmacist-facilitated hospital discharge program: a quasi-experimental study. Arch Intern Med. 2009;169(21):2003–2010. doi:10.1001/archinternmed.2009.398
86. Zerafa N, Zarb Adami M, Galea J. Impact of drugs counselling by an undergraduate pharmacist on cardiac surgical patient’s compliance to medicines. Pharm Pract. 2011;9(3):156–161.
87. Tomlinson J, Cheong VL, Fylan B, et al. Successful care transitions for older people: a systematic review and meta-analysis of the effects of interventions that support medication continuity. Age Ageing. 2020;49(4):558–569.
88. Daliri S, Boujarfi S, El Mokaddam A, et al. Medication-related interventions delivered both in hospital and following discharge: a systematic review and meta-analysis. BMJ Qual Saf. 2021;30(2):146–156.
89. Hoffmann T, Glasziou P, Boutron I, et al. Die TIDieR Checkliste und Anleitung – ein Instrument für eine verbesserte Interventionsbeschreibung und Replikation [Better Reporting of Interventions: template for Intervention Description and Replication (TIDieR) Checklist and Guide]. Gesundheitswesen. 2016;78(03):175–188. doi:10.1055/s-0041-111066
90. Hahn-Goldberg S, Okrainec K, Huynh T, Zahr N, Abrams H. Co-creating patient-oriented discharge instructions with patients, caregivers, and healthcare providers. J Hosp Med. 2015;10(12):804–807. doi:10.1002/jhm.2444
91. Huynh TM Design by exception: outliers, misfits and the design of extraordinary healthcare [dissertation]. Canada: OCAD University; 2015: 70.
92. O’Cathain A, Croot L, Duncan E, et al. Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open. 2019;9:e029954. doi:10.1136/bmjopen-2019-029954
93. Hahn-Goldberg S, Huynh T, Chaput A, et al. Implementation, spread and impact of the Patient Oriented Discharge Summary (PODS) across Ontario hospitals: a mixed methods evaluation. BMC Health Serv Res. 2021;21(1):361. doi:10.1186/s12913-021-06374-8
94. WHO. Rapid Reviews to Strengthen Health Policy and Systems: A Practical Guide. WHO. Available from: http://www.who.int/alliance-hpsr/resources/publications/rapid-review-guide/en/.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
