Back to Journals » Clinical Ophthalmology » Volume 15
Medical History, Clinical Features, Treatment Outcome and Its Predictors Among Infectious Keratitis Patients in Jimma University Medical Center, Southwest Ethiopia: Prospective Observational Study
Authors Dago TR , Woldemichael DK , Daba FB
Received 12 November 2020
Accepted for publication 22 February 2021
Published 22 March 2021 Volume 2021:15 Pages 1223—1237
DOI https://doi.org/10.2147/OPTH.S291880
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Tolcha Regasa Dago,1 Dagmawit Kifle Woldemichael,2 Fekede Bekele Daba3
1School of Pharmacy, College of Medicine and Health Science, Mizan Tepi University, Mizan-Aman, Ethiopia; 2Department of Ophthalmology, Institute of Health, Jimma University, Jimma, Ethiopia; 3School of Pharmacy, Institute of Health, Jimma University, Jimma, Ethiopia
Correspondence: Tolcha Regasa Dago
Mizan-Tepi University, P.O Box 260 Tel +251917233151
Email [email protected]
Background: Infectious keratitis is a major global cause of visual impairment and irreversible blindness among the corneal diseases. Its diagnosis and management remain getting challenge. The clinical and visual outcome remains poor in developing countries. The aim of this study was to determine treatment outcome and its predictors among patients with infectious keratitis.
Methods: Prospective observational study was conducted among adult patients diagnosed with infectious keratitis at the Ophthalmology Department in Jimma University Medical Center from April 1 to September 30, 2019. The primary outcome indicator was response of the ulcer to empirical treatment. Ulcers that did not heal and required surgery had a poor outcome. Variables with a p-value of < 0.25 were entered into a multivariate logistic regression model to determine the independent predictors of poor treatment outcome and variables with a p-value of < 0.05 were considered statistically significant.
Results: The research involved 131 adult patients. Eighty-seven (66.4%) were males. The mean age was 39.38 (± 18.9) years. Eighty-three (63.4%) patients had poor treatment outcome. Mean length of hospital stay was 17.38 (± 12.563) days. Poor visual outcome was observed among 71 (54.2%) participants. Evisceration was done for seven (5.3%) patients. Independent predictors of poor treatment outcome include perforation at admission (AOR=6.1, 95%CI: 1.5– 25.1), presence of comorbidity (AOR=7.7, 95%CI: 2.16– 27.3), poor adherence (AOR=5.3, 95%CI: 1.8– 25.9), traditional medicine use (AOR=6.7, 95%CI: 1.8– 25.4), ulcer depth > 1/3 (AOR=7.6, 95%CI: 2.48– 48.23) and farm workers (AOR=3.59, 95%CI: 1.09– 11.77). Major complications occurred after admissions were perforation (14.5%), followed by endophthalmitis (7.63%) and corneal opacity (6.87%).
Conclusion and Recommendation: Our study found high poor treatment outcomes and high poor visual outcomes. Presence of comorbidity, perforation at admission, traditional medicine use, working on a farm, poor adherence, and ulcer depth were the predictors of poor treatment outcome. This high poor outcome requires a nationwide interventional study and urgent intervention that may reach rural communities.
Keywords: microbes, infectious keratitis, treatment outcome, risk factors, Ethiopia
Background
The cornea is a clear, transparent, dome-shaped structure used for visual acuity. It does not contain blood vessels to nourish or defend itself from infection. However, it has a tough collagen structure that protects intraocular content from harmful external agents.1 Infective keratitis is a corneal infection caused by bacteria, fungi, viruses, or protozoa.2,3 Ocular trauma, contact lens use, alcohol use, low socioeconomic status, advanced age, inadequate education, recent ocular surgery, preexisting ocular diseases, dry eyes, lid deformity, reduced corneal sensitivity, chronic use of topical steroids, and prolonged systemic immune suppression are typical predisposing factors for infectious keratitis.4,5 The severity of the corneal infection depends on the degree of pathogenicity of the etiologic organism, the underlying condition of the cornea, and the immunological state of the individual.6–9
The World Health Organization (WHO) estimates that 285 million individuals are visually impaired worldwide and 90% of them live in low-resource countries. Visually impaired individuals, as well as their families, face serious social and economic challenges. For instance, mobility can be limited and active occupational status can be decreased.10,11 These visual impairment and blindness were majorly caused by infectious keratitis. Despite the advanced treatment options, ulcerative infectious keratitis, especially, continues to be a significant global cause of preventable, irreversible, monocular blindness among corneal diseases and is considered an ophthalmic emergency.4,6,12–15 Corneal opacification due to infectious keratitis accounts for 5.1% of worldwide blindness. Nevertheless, in East and Central Africa, it accounts for 12% of blindness.15 The risk of developing blindness in children from infectious keratitis is 20 times more prevalent in poor countries compared with rich countries. Corneal infections are the second most common cause of vision loss next to cataract in poor countries.6–9 Because of the unilateral essence of infectious keratitis in these areas, the incidences of its blinding complications and the burden of its impairment have been underreported.6,12,13
In the US, infectious keratitis required $175 million direct health-care costs, each year. Office and outpatient clinic visits demanded more than 250,000 hours of physician time annually.2 It is the highest problem in developing countries where access to care is limited due to poverty. Even though corneal infections need proper identification of microbe and targeted therapy to get the best outcome, it is unsatisfactory in these countries due to a lack of diagnostic agent’s access.16,17 Many infectious keratitis patients in developed countries do not receive medical services because they undermine their disease. In addition to this the lack of effective drugs, essential checking and operating equipment, well-trained medical care personnel, medical insurance systems, a shortage of cornea grafts, and lack of donor corneas for transplants results in severe outcomes.15,18,19 Infective ulcerative keratitis continues to pose a therapeutic problem, even in some areas where sophisticated testing methods are available and a wide variety of new antimicrobials are available. Visual morbidity continues to be high and clinical visual outcome remains poor in these regions.4,12,20 Antibiotic resistance among ocular pathogens is increasing globally.21,22 In the US, two million people are infected with drug-resistant microbes each year23 and empirical selection of an effective treatment is becoming more problematic because of this. In turn, this exposes patients to corneal melting, scarring, and perforation.12,21,24–27
In Ethiopia, the prevalence of blindness is about 1.6% and 87.4% of the cases are estimated to be caused by untreated bacterial, viral, and fungal infections. Several studies were reported higher prevalence of bacterial keratitis in different regions of the country. These studies also explained the increment of drug-resistant microbes in Ethiopia. Purchase of antibiotics without prescription and irrational use of antibiotics were possible causes of multidrug resistance in Ethiopia.24,28–31 The increased multidrug resistant organism may increase treatment failure and contribute to poor treatment outcome (blindness).32,33 For example, Pseudomonas aeruginosa and Staphylococcus aureus can cause corneal perforation in less than 24 h.33 High prevalence of fungal keratitis was reported in Ethiopia but its diagnosis and treatment also remains a challenge.34 If not diagnosed and treated effectively it will frequently result in corneal melting, visual impairment, and devastating ocular damage.22,35 Recurrence, worsened visual acuity, and corneal ulcer are common and serious ocular diseases caused by viral infection.36 Even though no more study conducted on viral keratitis in Ethiopia previous study conducted in this country showed herpetic keratitis as the common risk factor for bacterial keratitis.37
In general, risk factors, etiologies, diagnosis, and clinical manifestations of infectious keratitis were extensively researched, but previous studies neglected to measure treatment outcome and predictors of poor treatment outcome. Data about treatment outcome and its predictors is very important for identifying the need and timing of penetrating keratoplasty (PKP) to prevent blindness.38–40 The evolving range of microorganisms involved in ocular infections and the development of acquired microbial resistance determine the need for ongoing studies to direct empirical therapy.21,22,37 Despite all these problems, there were no studies conducted on predictors and treatment outcomes of infectious keratitis in Ethiopia. Few studies were undertaken in Africa. Therefore, the aim of this study was to determine medical history, clinical features, treatment outcome, and its predictors among infectious keratitis patients in Southwest Ethiopia.
Methods
Study Area and Period
The research was performed at the Jimma University Medical Center Ophthalmology Department (JUMCOD) from April 1 to September 30, 2019. JUMC is located 352 km southwest of Addis Ababa, Ethiopia, in Jimma Town. It is the only teaching and referral hospital in the southwestern part of the country with a bed capacity of 800. It provides services for nearly 15,000 inpatients and 160,000 outpatients per annum with a catchment population of about 15 million people. It can serve 11,000 emergency cases and 4500 deliveries per year. It has 1600 staff members and 32 care units. The Ophthalmology department is one of the units. The Ophthalmology department is also the only tertiary and referral eye center that serves for a population of over 10 million living in southwestern Ethiopia. The Ophthalmology Department has also different components such as ward for inpatients, operation room and outpatient departments.
Study Design
A hospital-based prospective observational study design was performed among adult patients with infectious keratitis admitted to Jimma University Medical Center from April 1 to September 30, 2019.
Population
Source Population
All adult patients with infectious keratitis admitted to JUMCOD during the study period.
Study Population
All adult patients with infectious keratitis admitted to JUMCOD who fulfilled the inclusion criteria.
Eligibility Criteria
Inclusion Criteria
All patients older than 15 years of age with the diagnosis of infectious keratitis who attended JUMCOD during the study period.
Exclusion Criteria
Those who were refused to participate.
Patients diagnosed with keratitis who were not taking treatment.
Patients diagnosed with photo keratitis.
Data Collection
Data were collected from the patient interviews and their medical record review while in hospital and during follow-up for discharged patients. Data abstracted included sociodemographics, type of etiologic agents, historical factors, clinical characteristics, diagnostic investigations, and treatment regimens, adherence to treatment, ocular comorbidities, complications and treatment outcome.
Either residents or ophthalmologists examined every patient with the slit-lamp biomicroscope. The size of the ulcer and height of hypopyon was measured with the variable slit-lamp after staining with fluorescein and recorded in millimeters on a standardized form. The size and depth of the stromal infiltrate was also recorded. Visual acuity (VA) was measured under conditions of high contrast, using printed or projected charts with optotypes, typically they are letters or “tumbling Es“. After a detailed ocular examination, the diagnosis was made depending on examination obtained through slit microscopy and clinical manifestation of patients. Then, empirical treatment was prescribed depending on the diagnosis. Corneal scrapings were performed using 20-gauge sterile syringe for severe cases depending on physician decisions and availability of reagent for culture. The scraping was made under aseptic conditions on each corneal ulcer under a microscope by an ophthalmologist. A topical anesthetic (1% tetracaine) was instilled during taking of the corneal specimens. Then the scraping was inserted into a 2-mL brain heart infusion broth (Oxoid, Hampshire, UK) tube. After that, it was immediately inoculated into chocolate and blood agar. The chocolate and a blood agar plate was placed into a candle jar for fastidious bacterial pathogens, which require CO2, while one blood agar plate was placed into a cold chain to maintain the viability of the bacterial pathogens during transport. These three culture plates and one tube were incubated appropriately. After incubation, subcultures were made onto sheep blood agar (5%), chocolate agar, mannitol salt agar and MacConkey agar (Oxoid) using standard methods. The inoculated media plates were incubated at their respective optimal temperatures. The plates were then checked for growth after 48 h. The bacterial isolates from the corneal scrapings wereidentified up to species-level based on different criteria which included morphocultural and biochemical tests. In vitro antibiotic susceptibility testing of the bacterial isolates was performed by Kirby–Bauer disc diffusion method. The interpretation of the results was according to the Clinical and Laboratory Standards Institute methodology as susceptible, intermediate and resistant.
Study Definitions
Good adherence is defined as score of ≥3 out of four questions adherence scale test, which is explained in questionnaire part H supplementary material of this article.41
Indoor occupations: housewives, merchants, civil servants who were working in an office, shopkeepers, and students but outdoor occupations includes laborers and farmers.42
Systemic immune compromising conditions: steroid use, diabetes, cancer, organ failure, HIV, and leprosy.43,44
Comorbidities are the presence of one or more additional conditions often co-occurring with keratitis. Any ophthalmic disease that occurs before the diagnosis of keratitis and persists during the treatment of keratitis can also be considered as comorbidity. For example, cataract, meibomian gland disease and glaucoma.
Empirical treatment is the treatment given based on clinical signs and symptoms without identifying the pathogen (microbe) in microbiology department. It involves the clinical experience of physicians.
Photokeratitis is a painful, temporary eye condition caused by exposure to ultraviolet rays, most commonly from the sun.
Statistical Analysis
Data was entered using EpiData version 4.4.1.0 and exported to SPSS version 23.0 software to perform statistical tests. To examine predictors of treatment outcome, first a bivariate logistic regression between independent variables and treatment outcome was done. Then, variables with a p-value of <0.25 were entered into a multivariate logistic regression model to determine the independent predictors of poor treatment outcome and variables with a p-value of <0.05 were considered statistically significant.
Outcome Measures and Validating Method
Empirical treatment was given for all patients depending on their clinical manifestation and diagnosis. Patients were followed daily during their stay in wards and after discharge, they were followed-up weekly up to 21 days. After 21 days, discharged patients were followed-up weekly, every two weeks, or monthly depending on physician's decision up to three months postenrolment. At each follow-up ulcer, visual acuity, infiltrates, hypopyon, comorbidities, and complications that occurred were recorded.
Primary Outcome
The main outcome measure was response of ulcer to treatment within 21 days.
Treatment outcome was defined as “poor“ if the ulcer had not healed within 21 days despite medical treatment or required any surgical treatment (evisceration, Gunderson flap or other conjunctival flap and graft etc, as required) or major complication occurred like descemetocele, endophthalmitis, anterior staphyloma, perforation after treatment.43,45–47
Treatment outcome was classified as “good“ if the ulcer had completely healed within 21 days (epithelial defect <0.5 mm, evidence of scar tissue through slit lamp biomicroscopy, reduced infiltrate size by more than 20%, decreased eyelid edema or decreased chemosis.43,45–47
Visual outcome is classified as a good outcome if final visual acuity at three months postenrollment was better than or the same as VA at admission but classified as a poor outcome if it was worse than VA at admission.14,43,48,49
According to WHO severity of keratitis classification visual acuity of <3/60 were considered as blind.Ulcer size >5mm,hypopyon >3mm,VA,3/60,ulcer depth that leads to full cornea and ulcer area >14mm2 are considered as very severe cases(grade IV) (Table 1).
 |
Table 1 Grade of Severity for Infectious Keratitis |
Results
Participant Enrolment
One hundred and forty-six adult patients with infectious keratitis were admitted to JUMCOD for medical management from April 1 to September 30, 2019. One hundred and thirty-one patients were included in the study, but 15 participants were excluded from the study due to the reasons listed in (Figure 1).
 |
Figure 1 Participants enrollment at Jimma University Medical Center Ophthalmology Department from April 1 to September 30, 2019. |
Sociodemographic Characteristics
Among 131 patients included for analysis, 66.4% were males. The mean age of participants was 39.38 (±18.901) years. The majority of them (69.5%) were married and more than 51% had indoor occupations. About 68% of patients were residing in the rural area and 84% were from distances greater than five kilometers; about 40% of participants had primary education (Table 2).
 |
Table 2 Sociodemographic Characteristics of Study Participants Among at JUMCOD from April to September 2019 (n=131) |
Medical History
Most of the study participants (90.8%) had a history of other health-care visits before admission. Mean time of presentation was 27.5 (±21.94) days. Seventy-nine (60.3%) patients had trauma prior to admission. Of these wooden stick trauma accounted for 25 (31.6%) of the participants and vegetative trauma accounted for 24 (30.3%) participants. Systemic immune compromising condition occurred among 96 (73.3%) of the study participants. Steroid use (dexamethasone eye drops) was observed among 88 (91.67%) of participants. Forty-five (34.4%) patients used traditional herbal medicine. One hundred and twenty participants (91.6%) were using topical eye drops before admission (Table 3).
 |
Table 3 Historical Characteristics of Study Participants at JUMCOD During April to September 2019 |
Clinical Characteristics
All studied participants had ulcers along the major axis and minor axis of the affected eye at admission. The mean ulcer sizes of the eyes along the larger axis, minor axis, and ulcer area at admission were 5.2 (±2.7) mm, 3.85 (±2.50) mm and 25.7 (±31.0) mm2, respectively. Hypopyon occurred only among 40 patients at admission. The mean of hypopyon was 2 (±1.0) mm. Ninty-four patients had ulcer depth more than one third (Table 4).
 |
Table 4 Characteristics of Ulcer at Admission and Other Objectively Measured Clinical Feature During April to September 2019 (n=131) |
Clinical manifestation like redness, pain, discharge, photophobia and reduction of vision was recorded. Mucoid discharge was common among bacterial keratitis, watery discharge and absence of discharge was seen among viral keratitis. Twenty-three patients were diagnosed clinically as sole fungal infection and co-infection based on clinical manifestation like satellite lesions, whitish pigmentation, feathery margins, and granular infiltrate. Fever, recurrence, foreign body sensation and upper respiratory tract infection were the distinctive clinical features of viral keratitis, in addition to dendrite lesion observed through microscopic finding (Table S1).
Key Laboratory and Instruments Used for Diagnosis
Corneal scrape was performed in 51 (38.9%) cases. Of these, 27 samples were sent for culture and 24 samples were sent for gram stain and KOH mount. Culture was done only for 27 patients because it was not always feasible. Out of 27 samples sent to microbiology, six samples (four pure bacterial, one mixed bacterial and fungal, and one pure fungal growth) showed growth, but from 24 gram stain and KOH mount 10 samples (five fungal hyphae, two mixed fungal hyphae and bacterial, and three gram-positive cocci) were isolated. Ophthalmic ultrasound and ophthalmic CT scan was done for 20 patients. It was used to differentiate keratitis from endophthalmitis and other ocular diseases as well as to identify whether perforation occurred or not, and existence of the eye globe. Drug susceptibility test for four gram-negative species of bacteria showed that all isolated organisms were multidrug resistant. Nevertheless, all isolated organisms from culture were rare etiology in causing infectious keratitis (Tables 5 and 6)
 |
Table 5 Key Laboratory and Instrumental Diagnostic Used Among Study Participants at JUMCOD During April to September 2019 |
 |
Table 6 Drug Susceptibility Test for Isolated Organism Among Study Participants at JUMCOD from April to September 2019 |
Types of Infectious Keratitis Diagnosed During the Study Period
Based on clinical manifestation and slit lamp examination Ophthalmologist’s diagnosed 52 bacterial, 47-mixed bacterial and viral, 15 mixed bacterial and fungal, nine viral, six fungal, one mixed viral, fungal, and bacterial, one mixed fungal and viral keratitis. Seventy-eight (59.78%) of the diagnoses occurred in the right eye.
Types of Comorbidities Occurred
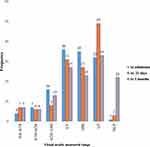
Fifty comorbidities were observed among study participants. The most common were meibomian gland (22%), cataract (20%), glaucoma (18%), blepharitis (14%), entropion (8%), conjunctivitis (6%), blephroconjuctivities (4%), pterygium (4%), and bullouskeratophathy (4%)(Figure 2).
Medical Treatment
A combination of fortified vancomycin eye drops 50 mg/mL (5%) and fortified gentamicin eye drops (14 mg/mL) (1.4%) were prescribed for 110 (83.97%) participants. Vancomycin fortified eye drops 50 mg/mL (5%) and fortified ceftazidime eye drop 50 mg/mL were prescribed for 21 (16.03%) patients. Patients diagnosed with fungal keratitis were 23 patients as co-infection and as pure fungal infection, all these patients had used fluconazole 0.3% eye drops in addition to fortified eye drops. Systemic antifungal, fluconazole 200 mg per oral was added for severe cases and during discharge. Acyclovir 3% eye ointment was prescribed for 59 viral (mixed infection and viral) keratitis. Acyclovir 400 mg oral was combined with acyclovir eye ointment and in some patients; it was prescribed solely during follow-up for improved patients. Topical ciprofloxacin 0.3% eye drops (zoxan D-eye drops), chloramphenicol eye drops and gentamicin 0.3% eye drops were used by some patients before admission and after discharge but in a few patients they were also used in wards. Tetracycline eye ointment was used among all patients before admission; either after discharge or in ward. Tropicamide 0.3% eye drops were prescribed for 123 patients whereas acetazolamide 250 mg were prescribed for 47 patients (Table 7).
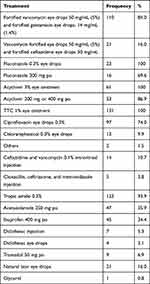 |
Table 7 Treatment for Infectious Keratitis Patients at JUMCOD from April to September 2019 |
Treatment Outcome
After being treated for 21 days 83 (63.36%) participant's eye ulcers had not healed (poor outcome). Out of this poor treatment outcome, surgery was performed for 36 patients. Evisceration was done for seven patients. The mean for healing time for patients whose ulcers had healed was 33.93 (±16.704) days. Poor visual outcome at three months was seen among 71 (54.2%) participants. The total person days for acute complications was 1915 days whereas the incidence rate for occurrence of acute complication was 0.0433420 and 95%CI: 0.034925–0.0537453. It can be converted to years by dividing it by 365 then it will give us 5.24=six years. And the incidence rate can also be calculated by dividing 0.0433420 by six which will give 0.0072 per year (Table 8)
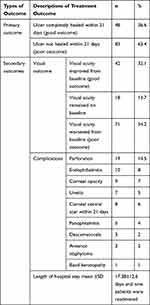 |
Table 8 Treatment Outcome of Patients Among Study Participants from April to September 2019 at JUMCOD (n=131) |
The mean and standard deviation of length of hospital stay was 17.38±12.563. Nine patients were readmitted. Surgery was performed for 36 patients. Of these, the highest number were conjunctival flap followed by evisceration. At the end of three months 22 patients cannot see light (NLP=blind) (Figures 3 and 4).
 |
Figure 3 Surgical treatments for infectious keratitis attending JUMCOD from April to September 2019. |
Predictors of Poor Treatment Outcome
Outdoor occupation, ulcer depth, traditional medicine use, poor adherence to medications, ocular comorbid conditions and perforation or thinning at admission were found to be significant independent predictors of poor treatment outcome (Table 9).
 |
Table 9 Predictors of Poor Treatment Outcome from April to September 2019 |
Discussion
The present study reveals that 63.36% of study participants had poor treatment outcome (ulcer not healed within 21 days postenrollment). This is higher than the prospective study conducted at India (51.5%),46 Nepal (24.2%)47 and other study conducted at India where poor treatment outcome rate was 54.8% (23.5% slow healing, and 31.3% of patient’s refractory to treatment).50 The reason for the discrepancy may be lack of definitive therapy in our case, worsened cases that had failed treatment at local health institutions referred to this institution, inappropriate treatment at a lower health institution before referral, like steroid prescription for fungal and bacterial keratitis, and traditional medicine use before admission. The finding of this study was also very high compared to a retrospective study conducted in Tanzania (18%).14 The reason may be a difference in study design, time of healing not settled, and a difference in the aim of assessing outcome in Tanzania.
In our study, 36 patients (27.48%) required surgical therapy after failed medical therapy. This is lower than a study conducted in Thailand (41.3%).51 The difference between the findings might be due to different treatment protocol, study design, and availability of surgical treatment. Evisceration was done for seven (5.34%) patients in our study. It is higher than studies conducted in Nepal 2.4%,52 Israel (1%),51 and another study done in Nepal (1.1%),47 but it is lower than studies from Tanzania (8%)14 KCMC (25%),53 and Thailand (25%).51 This difference could be due to difference in treatment protocol. In our study the conjunctival flap was done for 20 (15.27%) patients. It was lower than study from Thailand (39.8%).51
The finding of this study also identified more than half (54.2%) of the patients had a poor visual outcome at the end of three months. This finding is higher than the prospective studies conducted in Pakistan (52. 21%)48 and China (9.3%).43 The reason for the discrepancy might be the difference in treatment modalities, as in the Chinese studyin which they used definitive therapy, penetrating keratoplasty, and different diagnostic techniques. However, ours is lower than a retrospective study conducted in Tanzania (66%)14 and the reason might be due to lack of pertinent information in the case of the retrospective study.
One of the acute complications of keratitis occurred in our study was perforation or thinning of cornea at admission (34.35%). It was higher than in the studies from Tanzania (30%)14 and Pakistan (15.8%).48 Posttreatment perforation rate in our study was 14.5%, which was lower than two studies from India (16%) and (19%),54,55 but slightly higher than a study from Tanzania (14%).14 The discrepancy from India might be due to different etiologic agents that caused the disease. Our study also found that 10 patients (7.6%) developed endophthalmitis. It was higher than studies conducted in Thailand (4.2%),51 Germany (0.29% incidence rate),56 and China (1.54%).43 This difference might be due to lack of definitive therapy and lack of knowledge on severity of the case by patients.
The mean and standard deviation of length of hospital stay in our study was 17.38±12.563; it was very high compared to a study from Israel 5.69±5.508,49 but lower than a study from Thailand 20.3±19.1.51 The reason that our finding is higher than the study from Israel might be use of empirical therapy rather than definitive and lack of ophthalmologists at lower health institutions.
This study found that presence of comorbidity, perforation at admission, traditional medicine use, working in farm, poor adherence and ulcer depth, were predictors of a poor treatment outcome.
Traditional herbal medicine use was one of the independent predictors of a poor outcome. Traditional medicine users were five times more likely to develop poor ulcer healing compared to nonusers (p=0.005). It is very similar to a study conducted in Uganda where traditional medicine users developed a poor outcome more often than nonusers (p=0.005).57 Traditional medicine use causes burning to the cornea and widens the ulcer and these required more time to heal. As a result, most of the traditional medicine users faced poor vision. We observed significant association between traditional medicine use and larger ulcer area at admission >14 mm2 (p=0.01). Some patients undermined their condition and believed it would heal with locally available herbal remedies and did not visit the health institution until perforation occurred.
Patients having outdoor occupations in our study were 2.381 more likely to develop poor treatment outcome compared to indoor occupations (p=0.035). It is similar to recent review, which described patients with agriculture-based activities were at 1.33 times greater risk of developing microbial keratitis poor outcome (p<0.001).42 It is also in line with the finding from China where farm-working trauma was an independent predictor of poor outcome.43 Powder or ash around roads in rural areas, repetitive trauma from leaves, mud, and sticks while working on a farm or living in a rural area might hinder ulcer healing.
Poor adherence to treatment was an independent predictor of poor ulcer healing. Most of the fortified eye drops prescribed for patients were every 15 min, for the first one hour then every one hour, two hours and the like during the start of medication. Patients were forgetting to add their medication during nighttime. Some of the patients also did not believe the eye drops could cure their disease and they always require either injection or surgical treatment. Precipitation of medications especially ciprofloxacin eye drops in some patients were seen as cause of nonadherence. Ciprofloxacin precipitation was formed among patients with mixed viral and bacterial keratitis. The precipitation formed was very painful and because of this patients would not volunteer to take any eye drops; they wanted only an injection. This finding of precipitation of ciprofloxacin eye drops is similar to the recent report that ciprofloxacin 0.3% precipitation on the ocular surface delayed recovery from viral ocular surface infection and resulted in poor outcome.58 The irregular surface formed by precipitates of ciprofloxacin causes discomfort, reduces visual acuity, and delays recovery. Older patients treated with topical ciprofloxacin for bacterial keratitis have a higher risk of corneal precipitation and delay epithelial healing of ulcerative keratitis.59
We observed that patients with ocular comorbidities to infectious keratitis was an independent predictor of poor outcome. This is similar to a study that found ocular comorbidities such as glaucoma, cataracts, uveitis, age-related macular degeneration, and dry eyes, had the greatest input for poor treatment outcome.60
A nationwide interventional study is required to get more evidence about infectious keratitis. Creating awareness about infectious keratitis can help to prevent the delay of patients visiting the ophthalmic clinic. This in turn can decrease perforation and other complications. The created awareness can hinder preventable blindness. Especially rural communities who were far from internet access will benefit from the media of the country like radio because they do not have awareness about infectious keratitis.
Strengths and Limitations of the Study
This is the first study conducted on infectious keratitis treatment outcome in Ethiopia so this will create the highest awareness regarding the infectious keratitis treatment outcome and initiating other health-care professionals located at a lower level of health institution in regard to immediate infectious keratitis treatment and referral. The data from this finding may contribute to determine time required for penetrating keratoplasty. This will prevent avoidable blindness.
However, our study had several limitations First, our study suffers the same limitations as all observational studies that means limited sample size although we prolonged the study period, still the final sample size was limited, and our study lacks external validity. Second, most of the diagnoses were clinical because culture was not accessible most of the time. Third, there was lack of local data on treatment outcome to compare our results with other findings.
Conclusions
Our study found high poor-treatment outcomes and high poor-visual outcomes. Presence of comorbidity, perforation at admission, traditional medicine use, working on farm, poor adherence and ulcer depth, were the predictors of poor treatment outcome. Many vision-threatening complications and longer hospital stays were the major findings of this study. This high poor-outcome requires a nationwide interventional study and urgent intervention that may reach rural communities.
Abbreviations
JUMCOD, Jimma University Medical Center Ophthalmology Department; PKP, penetrating keratoplasty; VA, visual acuity.
Data Sharing Statement
This article contains all the data produced or analyzed during this research. During the current analysis, the datasets used and/or analyzed are available on request from the corresponding author.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki. Ethical clearance and approval was obtained from Institutional Review Board of Jimma University with reference number of JHRPGD/548/2019, which was granted on April 15, 2019. For patients 15–18 years voluntarily written assents were first obtained from the patients whether they involve their parents or not then, their parents voluntarily signed informed consent forms before enrollment. However, all subjects older than 18 years voluntarily participated in our study and signed informed consent forms before enrollment.
Acknowledgments
Authors wish to thank data collectors, Morisky–Green–Levine four-item medical adherence scale creators, study participants, and Jimma University and JUMCOD staffs.
Author Contributions
All authors made a significant contribution to the reported work, whether in the conception, design of the study, execution, data acquisition, analysis and interpretation, or in all of these areas; participated in the drafting, revision or critical review of the article; gave final approval of the version to be published; agreed on the journal to which the article was submitted; and agreed to accept the drafting of the article.
Funding
This study was supported by (data collection fee) Jimma University. The funders played no role in the study.
Disclosure
The authors report no conflicts of interests in this work.
References
1. Khurana A. Community Ophthalmology in Comprehensive Ophthalmology, Chapter 20. New Delhi: New Age International Limited Publisher; 2007:443–457.
2. Collier SA, Gronostaj MP, MacGurn AK, et al. Estimated burden of keratitis—United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(45):1027.
3. Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27(1):22–27. doi:10.1097/ICO.0b013e318156caf2
4. Srigyan D, Gupta M, Behera HS. Keratitis: an inflammation of cornea. EC Ophthalmol. 2017;6(6):7.
5. Song X, Xie L, Tan X, et al. A multi-center, cross-sectional study on the burden of infectious keratitis in China. PLoS One. 2014;9(12):e113843. doi:10.1371/journal.pone.0113843
6. Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–221.
7. Robles-Contreras A, Perez-Cano HJ, Babayan-Sosa A, Baca-Lozada O. Bacterial Keratitis infection: a battle between virulence factors and the immune response. Common Eye Infect Intech Open. 2013.
8. Foster C. Fungal keratitis. Infect Dis Clin North Am. 1992;6(4):851–857. doi:10.1016/S0891-5520(20)30486-4
9. Norina T, Raihan S, Bakiah S, Ezanee M, Liza-Sharmini A, Wan Hazzabah W. Microbial keratitis: aetiological diagnosis and clinical features in patients admitted to Hospital Universiti Sains Malaysia. Singapore Med J. 2008;49(1):67–71.
10. Mariotti SP, Pascolini D, Rose-Nussbaumer J. Trachoma: global magnitude of a preventable cause of blindness. Br J Ophthalmol. 2009;93(5):563–568. doi:10.1136/bjo.2008.148494
11. Evans T. Socioeconomic consequences of blinding onchocerciasis in west Africa. Bull World Health Organ. 1995;73(4):495.
12. Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology. 2017;124(11):1678–1689. doi:10.1016/j.ophtha.2017.05.012
13. Chirinos-Saldaña P, de Lucio VMB, Hernandez-Camarena JC, et al. Clinical and microbiological profile of infectious keratitis in children. BMC Ophthalmol. 2013;13(1):54. doi:10.1186/1471-2415-13-54
14. Burton MJ, Pithuwa J, Okello E, et al. Microbial keratitis in East Africa: why are the outcomes so poor? Ophthalmic Epidemiol. 2011;18(4):158–163. doi:10.3109/09286586.2011.595041
15. Whitcher JP, Srinivasan M. Corneal ulceration in the developing world—a silent epidemic. Br J Ophthalmol. 1997;81(8):622–623. doi:10.1136/bjo.81.8.622
16. Ansari Z, Miller D, Galor A. Current thoughts in fungal keratitis: diagnosis and treatment. Curr Fungal Infect Rep. 2013;7(3):209–218. doi:10.1007/s12281-013-0150-1
17. WHO. Guidelines for the management of corneal ulcer at primary, secondary, and tertiary care health facilities in the South-East Asia Region. 1–3SEA/Opthal/126; 2004.
18. Cao J, Yang Y, Yang W, et al. Prevalence of infectious keratitis in Central China. BMC Ophthalmol. 2014;14(1):43. doi:10.1186/1471-2415-14-43
19. O’Brien KS, Lietman TM, Keenan JD, Whitcher JP. Microbial keratitis: a community eye health approach. Community Eye Health. 2015;28(89):1.
20. Al-Mujaini A, Al-Kharusi N, Thakral A, Wali UK. Bacterial keratitis: perspective on epidemiology, clinico-pathogenesis, diagnosis and treatment. Sultan Qaboos Univ Med J. 2009;9(2):184.
21. Brown L. Resistance to ocular antibiotics: an overview. Clin Exp Optometry. 2007;90(4):258–262. doi:10.1111/j.1444-0938.2007.00154.x
22. Sharma S. Antibiotic resistance in ocular bacterial pathogens. Indian J Med Microbiol. 2011;29(3):218. doi:10.4103/0255-0857.83903
23. Team EE. CDC publishes report on antibiotic resistance threats in the United States for the first time. Euro surveillance bulletin Europeen sur les maladies transmissibles. 2013;18:38.
24. Anagaw B, Biadglegne F, Belyhun Y, Mulu A. Bacteriology of ocular infections and antibiotic susceptibility pattern in Gondar University Hospital, north west Ethiopia. Ethiop Med J. 2011;49(2):117–123.
25. Ibanga A, Asana U, Nkanga D, Duke R, Etim B, Oworu O. Indications for eye removal in southern Nigeria. Int Ophthalmol. 2013;33(4):355–360. doi:10.1007/s10792-012-9700-8
26. Saeed N, Khan MD. The challenge of microbial keratitis in Pakistan. Pak J Ophthalmol. 2010;26:3.
27. Titiyal JS, Negi S, Anand A, Tandon R, Sharma N, Vajpayee RB. Risk factors for perforation in microbial corneal ulcers in north India. Br J Ophthalmol. 2006;90(6):686–689. doi:10.1136/bjo.2005.079533
28. Aklilu A, Bitew A, Dessie W, et al. Prevalence and drug susceptibility pattern of bacterial pathogens from ocular infection in St. Paul’s Hospital Millennium Medical College, Ethiopia. J Bacteriol Mycol. 2018;5(8):1085.
29. Amsalu A, Abebe T, Mihret A, Delelegne D, Tadesse E. Potential bacterial pathogens of external ocular infections and their antibiotic susceptibility pattern at Hawassa University teaching and referral Hospital, Southern Ethiopia. Afr J Microbiol Res. 2015;9(14):1012–1019. doi:10.5897/AJMR2014.7282
30. Shiferaw B, Gelaw B, Assefa A, Assefa Y, Addis Z. Bacterial isolates and their antimicrobial susceptibility pattern among patients with external ocular infections at Borumeda hospital, Northeast Ethiopia. BMC Ophthalmol. 2015;15(1):103. doi:10.1186/s12886-015-0078-z
31. Tesfaye T, Beyene G, Gelaw Y, Bekele S, Saravanan M. Bacterial profile and antimicrobial susceptibility pattern of external ocular infections in Jimma University specialized hospital, Southwest Ethiopia. Am J Infect Dis Microbiol. 2013;1(1):13–20. doi:10.12691/ajidm-1-1-3
32. Teweldemedhin M, Gebreyesus H, Atsbaha AH, Asgedom SW, Saravanan M. Bacterial profile of ocular infections: a systematic review. BMC Ophthalmol. 2017;17(1):212. doi:10.1186/s12886-017-0612-2
33. Upadhyay MP, Karmacharya PC, Koirala S, et al. Epidemiologic characteristics, predisposing factors, and etiologic diagnosis of corneal ulceration in Nepal. Am J Ophthalmol. 1991;111(1):92–99. doi:10.1016/S0002-9394(14)76903-X
34. Kibret T, Bitew A. Fungal keratitis in patients with corneal ulcer attending Minilik II Memorial Hospital, Addis Ababa, Ethiopia. BMC Ophthalmol. 2016;16(1):148. doi:10.1186/s12886-016-0330-1
35. Anutarapongpan O, Brien TP. Update on management of fungal keratitis. Clin Microbiol Open Access. 2005.
36. Choudhary P, Chalisgaonkar C, Lakhtakia S, Dwivedi A, Khare V. A clinical study of pattern, complications, and visual outcome of viral keratitis. Int J Med Sci Public Health Online. 2017;6:6.
37. Gebremariam TT, Alemu TA, Daba KT. Bacteriology and risk factors of bacterial keratitis in Ethiopia. Health Sci J. 2015;9(5):1.
38. Constantinou M, Daniell M, Snibson GR, Vu HT, Taylor HR. Clinical efficacy of moxifloxacin in the treatment of bacterial keratitis: a randomized clinical trial. Ophthalmology. 2007;114(9):1622–1629. doi:10.1016/j.ophtha.2006.12.011
39. Morlet N, Minassian D, Butcher J, Group OS. Risk factors for treatment outcome of suspected microbial keratitis. Br J Ophthalmol. 1999;83(9):1027–1031. doi:10.1136/bjo.83.9.1027
40. Schaefer F, Bruttin O, Zografos L, Guex-Crosier Y. Bacterial keratitis: a prospective clinical and microbiological study. Br J Ophthalmol. 2001;85(7):842–847. doi:10.1136/bjo.85.7.842
41. Uchmanowicz B, Jankowska EA, Uchmanowicz I, Morisky DE. Self-reported medication adherence measured with morisky medication adherence scales and its determinants in hypertensive patients aged ≥60 years: a systematic review and meta-analysis. Front Pharmacol. 2019;10:10. doi:10.3389/fphar.2019.00168
42. Gopinathan U, Sharma S, Garg P, Rao GN. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: experience of over a decade. Indian J Ophthalmol. 2009;57(4):273. doi:10.4103/0301-4738.53051
43. Ng AL-K, To KK-W, Yuen LH, et al. Predisposing factors, microbial characteristics, and clinical outcome of microbial keratitis in a tertiary centre in Hong Kong: a 10-year experience. J Ophthalmol. 2015;2015.
44. Mun Y, Kim MK, Oh JY. Ten-year analysis of microbiological profile and antibiotic sensitivity for bacterial keratitis in Korea. PLoS One. 2019;14:3. doi:10.1371/journal.pone.0213103
45. Chidambaram JD, Venkatesh Prajna N, Srikanthi P, et al. Epidemiology, risk factors, and clinical outcomes in severe microbial keratitis in South India. Ophthalmic Epidemiol. 2018;25(4):297–305. doi:10.1080/09286586.2018.1454964
46. Kaye S, Tuft S, Neal T, et al. Bacterial susceptibility to topical antimicrobials and clinical outcome in bacterial keratitis. Invest Ophthalmol Vis Sci. 2010;51(1):362–368. doi:10.1167/iovs.09-3933
47. Khadka S, Chaudhary M, Thapa M. Evaluation of risk factors and treatment outcome of microbial keratitis in a tertiary eye center. Sudanese J Ophthalmol. 2018;10(1):25. doi:10.4103/sjopthal.sjopthal_5_18
48. Shah SIA, Shah SA, Rai P, Katpar NA, Abbasi SA, Soomro AA. Visual outcome in patients of keratomycosis, at a tertiary care centre in Larkana, Pakistan. J Pak Med Assoc. 2017;67(7):1035–1038.
49. Lavinsky F, Avni-Zauberman N, Barequet IS. Clinical characteristics and outcomes of patients admitted with presumed microbial keratitis to a tertiary medical center in Israel. Arq Bras Oftalmol. 2013;76(3):175–179. doi:10.1590/S0004-27492013000300009
50. Lalitha P, Prajna NV, Kabra A, Mahadevan K, Srinivasan M. Risk factors for treatment outcome in fungal keratitis. Ophthalmology. 2006;113(4):526–530. doi:10.1016/j.ophtha.2005.10.063
51. Tananuvat N, Punyakhum O, Ausayakhun S, Chaidaroon W. Etiology and clinical outcomes of microbial keratitis at a tertiary eye-care center in northern Thailand. J Med Assoc Thai. 2012;95(Suppl 4):S8–17.
52. Dhakwa K, Sharma M, Bajimaya S, Dwivedi A, Rai SK. Causative organisms in microbial keratitis, their sensitivity pattern and treatment outcome in western Nepal. Nepalese J Ophthalmol. 2012;4(1):119–127. doi:10.3126/nepjoph.v4i1.5863
53. Poole T, Hunter D, Maliwa E, Ramsay A. Aetiology of microbial keratitis in northern Tanzania. Br J Ophthalmol. 2002;86(8):941–942. doi:10.1136/bjo.86.8.941
54. Prajna NV, Mascarenhas J, Krishnan T, et al. Comparison of natamycin and voriconazole for the treatment of fungal keratitis. Arch Ophthalmol. 2010;128(6):672–678. doi:10.1001/archophthalmol.2010.102
55. Prajna NV, Krishnan T, Mascarenhas J, et al. Predictors of outcome in fungal keratitis. Eye. 2012;26(9):1226. doi:10.1038/eye.2012.99
56. Zapp D, Loos D, Feucht N, et al. Microbial keratitis-induced endophthalmitis: incidence, symptoms, therapy, visual prognosis and outcomes. BMC Ophthalmol. 2018;18(1):112. doi:10.1186/s12886-018-0777-3
57. Arunga S, Asiimwe A, Olet EA, et al. Traditional eye medicine use in microbial keratitis in Uganda: a mixed methods study. Wellcome Open Res. 2019;4.
58. Patwardhan A, Khan M. Topical ciprofloxacin can delay recovery from viral ocular surface infection. J R Soc Med. 2005;98(6):274–275. doi:10.1177/014107680509800610
59. Wilhelmus KR, Abshire RL. Corneal ciprofloxacin precipitation during bacterial keratitis. Am J Ophthalmol. 2003;136(6):1032–1037. doi:10.1016/S0002-9394(03)00636-6
60. Pinazo-Durán MD, Zanón-Moreno V, García-Medina JJ, Arévalo JF, Gallego-Pinazo R, Nucci C. Eclectic ocular comorbidities and systemic diseases with eye involvement: a review. Biomed Res Int. 2016.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.