Back to Journals » International Medical Case Reports Journal » Volume 13
May 2020: Is It Always COVID-19 No Matter What?
Authors Livrieri F, Ghidoni G, Piro R , Menzella F , Cavazza A, Lazzaretti C, Massari M, Montanari G, Fontana M, Facciolongo NC
Received 21 August 2020
Accepted for publication 8 September 2020
Published 2 November 2020 Volume 2020:13 Pages 563—567
DOI https://doi.org/10.2147/IMCRJ.S277474
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Francesco Livrieri,1 Giulia Ghidoni,1 Roberto Piro,1 Francesco Menzella,1 Alberto Cavazza,2 Claudia Lazzaretti,3 Marco Massari,3 Gloria Montanari,1 Matteo Fontana,1 Nicola Cosimo Facciolongo1
1Department of Medical Specialties, Pneumology Unit, Arcispedale Santa Maria Nuova, Azienda USL di Reggio Emilia- IRCCS, Reggio Emilia 42123, Italy; 2Pathology Unit, Azienda USL/IRCCS di Reggio Emilia, Reggio Emilia 42100, Italy; 3Infectious Diseases, Azienda Unità Sanitaria Locale, IRCCS di Reggio Emilia, Reggio Emilia, Italy
Correspondence: Francesco Livrieri
Department of Medical Specialties, Pneumology Unit, Arcispedale Santa Maria Nuova, Azienda USL di Reggio Emilia- IRCCS, Reggio Emilia 42123, Italy
Email [email protected]
Abstract: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is causing a massive outbreak throughout the world. In this period, diseases other than coronavirus disease (COVID-19) have not disappeared; however, it is hard for doctors to diagnose diseases that can mimic the clinical, radiological, and laboratory features of COVID-19, especially rare lung diseases such as acute eosinophilic pneumonia (AEP). We report the clinical case of a young patient who presented to the Emergency Department with respiratory failure and clinical symptoms, radiological aspects, and blood tests compatible with COVID-19; two swabs and a serology test for SARS-CoV-2 were performed, both resulted negative, but the respiratory failure worsened. Peripheral eosinophilia guided us to consider the possibility of a rare disease such as AEP, even if radiology findings were not pathognomonic. Therefore, we decided to perform a flexible bronchoscopy with bronchoalveolar lavage (BAL) at the lingula, which showed the presence of eosinophilia greater than 40%. As a consequence, we treated the patient with high-dose corticosteroids that completely resolved the respiratory symptoms. This case report highlights the difficulty of making alternative diagnoses during the COVID-19 pandemic, especially for rare lung diseases such as AEP, which may have initial characteristics similar to COVID-19.
Keywords: COVID-19, acute eosinophilic pneumonia, bronchoalveolar lavage, ground glass opacities
Introduction
As of August 31, 2020, 24,854,140 confirmed COVID-19 cases had been reported by the World Health Organization (WHO) worldwide, with 838,924 attributed deaths.1
Coronaviruses are enveloped, non-segmented, positive-sense, single-stranded RNA viruses. Significant human morbidity and mortality has been reported only with two beta coronaviruses before this pandemic: severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV).2 SARS-CoV-2 has the typical coronavirus structure with spike protein and other polyproteins, nucleoproteins, and membrane proteins, such as RNA polymerase, 3-chymotrypsin-like protease, papain-like protease, helicase, glycoprotein, and accessory proteins.3 SARS-CoV-2 uses the same ACE2 cell receptor and mechanism of entry in host cells as SARS-CoV.4
COVID-19 patients could be asymptomatic or show different clinical manifestations, including mild-to-moderate symptoms like fever, dry cough, and shortness of breath or, in other cases, a rapid deterioration and development of acute respiratory distress syndrome (ARDS). In patients with mild COVID-19, typical findings on chest computed tomography (CT) include predominance of ground glass opacities (GGO) in the early phase with an increase in the frequency of consolidations and linear opacities over 3 weeks post-onset of symptoms. It is possible to find these CT features in many different conditions: infections, drug toxicity, vaping-associated pulmonary injury, oxygen toxicity, toxic inhalants or pulmonary aspirations, acute eosinophilic pneumonia, alveolar hemorrhage, etc.5,6
This report has the goal to share our experience of a 23-year-old man, non-smoker, with no remote clinical history, presenting respiratory failure and fever, both suspected for COVID-19.
Case Report
In May 2020, during the outbreak of SARS-CoV-2 in Italy, a 23-year-old male, non-smoker, with no remote clinical history, presented to the Emergency Department; he had suffered from fever (up to 38°C), cough, and worsening shortness of breath for 3 days. At clinical examination, the most important clinical feature was bilateral wheezing; nevertheless, the patient did not have a history of asthma nor chronic sinusitis with nasal polyposis. During the visit, the patient did not refer to new smoking or second hand smoke habit, nor drugs or new antigen exposures.
Blood test showed an increase in peripheral blood eosinophilia (1.8x1,000/μL, 12.5% of the total leukocyte number) with leukocytosis (14.45x1,000/μL); in particular, neutrophils were 61.6% (8.91x1,000/μL), lymphocytes were 17.7 (2.56x1,000/μL), monocytes 7.6% (1.1x1,000/μL), and basophils were 0.6% (1.8x1,000/μL). A mild elevated C-reactive protein (CRP=1.53 mg/dL) was found, with negative procalcitonin, mild elevated Lactate dehydrogenase (LDH 384 U/L), mild elevated Interleukin-6 (IL-6=12.4 pg/mL), and negative D-dimer (230 ng/mL). Arterial blood gases showed: pH=7.39; pO2=59 mmHg; pCO2=40 mmHg; HCO3=25 mmol/l with a fraction of inspired oxygen of 35% in Venturi mask and arterial oxygen pressure on fraction of inspired oxygen ratio (PaO2/FiO2) of 168. Due to the persistence of respiratory symptoms and respiratory failure, the patient underwent a chest high-resolution CT scan, which reveal bilateral GGO localized in the upper left lobe (in particular in lingular segment) and in the right lower lobe (Figure 1).
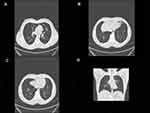 |
Figure 1 Chest high resolution CT scan: (A) GGO in the left upper lobe, (B) GGO in the lingular segments, (C) GGO in the right lower lobe, (D) coronal scan of the lung. |
For these reasons, he was hospitalized with suspected COVID-19 in the Infectious Diseases Unit in Santa Maria Nuova Hospital of Reggio Emilia.
As of the chest CT, the suspicious of COVID-19 was high, but we had to consider other possibilities in differential diagnosis, such as eosinophilic granulomatosis with polyangiitis (EGPA), alveolar hemorrhage, AEP, parasitic pneumonia, drug toxicity, or toxic inhalants.
In the Infectious Diseases Unit, on day one the patient was tested negative with nasopharyngeal swab for SARS-CoV-2, and the result was confirmed after 24 hours with a second nasopharyngeal swab that was negative as well. Moreover, serology test for SARS-CoV-2 was also negative.
In order to exclude a vasculitis, antineutrophil cytoplasmic antibodies (ANCA) were searched, and resulted asnegative.
After 24 hours from admission in hospital, patient’s respiratory conditions worsened with symptoms such as dyspnea and increase in respiratory rate (25 breaths per minute), so high flow oxygen therapy with a fraction of inspired oxygen of 60% was needed.
A therapy with two daily inhalations formoterol/beclomethasone metered-dose inhaler 100/6 μg was introduced to improve respiratory symptoms, with only partial improvement of symptoms.
In consideration of the increase in peripheral blood eosinophilia and bilateral GGO on chest CT, a flexible bronchoscopy with bronchoalveolar lavage (BAL) was performed; an amount of 150 mL of physiological liquid was introduced to perform BAL and 70 mL of clear liquid was collected, with no traces of blood in it. BAL revealed eosinophils alveolitis (40%) and mucous secretion with no evidence of bacteria or viruses, SARS-CoV-2 included. These findings were compatible with AEP (Figure 2). For this reason, we started a therapy with oral corticosteroid (prednisone 50 mg daily), with a rapid clinical improvement and no more fever.
 |
Figure 2 Bronchoalveolar lavage showing eosinophilia (about 40%). Papanicolau stain, 400x magnification. |
After 1 week of therapy, the initial respiratory failure was resolved and blood tests revealed a lower peripheral blood eosinophilia. The prednisone dose was halved in 14 days, and the patient was discharged with the dose of 25 mg. Before the discharging we obtained the informed consent for the publication of this case report.
Discussion
The clinical case we described highlights the high difficulty in differential diagnosis concerning rare lung diseases in COVID-19 pandemic period. AEP is the most frequently misdiagnosed of eosinophilic pneumonias because it mimics infectious pneumonia or acute respiratory distress syndrome in previously healthy individuals.7 In fact in this case, clinical features in the early stage were the same of SARS-CoV-2 related pneumonia (fever, cough, dyspnea) and chest CT was typical for COVID-19 as well, with bilateral GGO and a visual score (defined as the percentage of lung parenchyma occupied by the opacities) of 20%. The thoracic radiogram was not performed because, during the outbreak, the hospital internal protocol contemplates the execution of a chest CT-scan as the first radiological assessment in patients with respiratory symptoms suspected for COVID-19, due to its greater diagnostic yield for the signs of infection. Blood tests showed mild elevated levels of CRP, LDH, IL-6, also compatible with COVID-19. However, even if CT findings were not typical for AEP and the presence of peripheral eosinophilia itself was not significant, several repeated examinations confirmed high values, which were relevant in the context described.
Clinical and radiological features described could have been suitable for various diagnoses, however EGPA and COVID-19 were less probable diagnoses after the results of negative ANCA test and two negative swabs for SARS-COV-2. For these reasons, a fiber-optic bronchoscopy with BAL was necessary, confirming the presence of eosinophilia >40% and the absence of blood, excluding alveolar hemorrhage. Bronchoscopy is relatively contraindicated in patients with suspected and confirmed COVID-19 infections and its only role is when less invasive testing to confirm COVID-19 are inconclusive and there is a suspicion for an alternative diagnosis that would impact clinical management or an urgent life-saving intervention.8
Eosinophilic lung disease (ELD) is a group of lung pathologies characterized by an increase of eosinophils within the lung parenchyma. The diagnosis can be made with the presence of chest opacities associated with blood and parenchymal eosinophilia, confirmed by BAL (threshold of eosinophils >25%) and possibly a pulmonary biopsy. In the diagnostic management of ELD, a known etiology could be found (parasitic infection, allergic bronchopulmonary aspergillosis, drug toxicity), while also unknown causes (Loeffler syndrome, acute or chronic eosinophilic pneumonia, idiopathic hypereosinophilic syndrome) and eosinophilic vasculitis (EGPA, formerly known as Churg–Strauss syndrome) could cause these diseases.9
AEP is unusual in the group of ELD; the most frequent respiratory symptoms are dyspnea, cough, and fever, lasting more than 2 weeks, frequently followed by acute respiratory failure. Up to 50% of patients with AEP have a history of asthma, they are young, with a mean age of approximately 30 years, with male predominance. Alveolar eosinophilia is a main feature of AEP: more than 25% eosinophils in BAL are required to make the diagnosis. At the same time, blood hyper-eosinophilia is frequently found.7–10 BAL is required to confirm alveolar eosinophilia and also to exclude other diagnoses (ie, infectious diseases or malignancies). A typical radiologic feature of AEP is the localization in the upper lobes without basilar involvement of unilateral or bilateral opacities, typically with no segmental distribution.11
Acute eosinophilic diseases could be triggered by many different causes: vasculitis, infectious diseases (aspergillus, parasites, etc.), drugs, asthma, other rare lung diseases such as Langerhans cell histiocytosis, organizing pneumonia, idiopathic pulmonary fibrosis, and malignancies. However, they can be also idiopathic.7 In this particular case, we ruled out any potential exposure with a detailed medical history, the presence of vasculitis by performing serum ANCA level, other rare lung diseases with the radiological findings and, finally, the infectious diseases by performing a bronchoalveolar lavage.
In this clinical case there were some misleading characteristics: the absence of history of asthma, initial symptoms typical of COVID-19, and the absence of a peculiar imaging for AEP (typical combination on CT of poorly defined nodules of ground-glass attenuation, interlobular septal thickening, bilateral pleural effusion, and airspace consolidation).7 In this case, having ruled out any of the possible triggers, we hypothesized an idiopathic origin of the disease.
The case we described revealed the difficulty, during the pandemic of COVID-19, to diagnose other diseases with clinical symptoms and radiologic patterns similar to COVID-19, such as EGPA, parasitic pneumonia, AEP, drug toxicity, and others. Therefore, it is important not to stop at the initial hypothesis of COVID-19 if others differential diagnoses are reasonable, even if that means to perform a more invasive but conclusive examination.
Data Sharing Statement
All data generated or analyzed are included in this published article. Any additional data may be obtained from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
As this manuscript meets the definition of a case report, Institutional Review Board (IRB) review was not required for publication.
Consent for Publication
Consent for publication was obtained from the patient.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. WHO. Coronavirus disease (COVID-19) dashboard data. Available from: 2020/8/31 https://covid19.who.int/.
2. Guarner J. Three emerging coronaviruses in two decades. Am J Clin Pathol. 2020;153:420–421. doi:10.1093/ajcp/aqaa029.
3. Shereen MA, Khan S, Kazmi A, et al. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi:10.1016/j.jare.2020.03.005.
4. Gralinski LE, Menachery VD. Return of the coronavirus: 2019-nCoV. Viruses. 2020;12(2):135. doi:10.3390/v12020135.
5. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;396:497–506. doi:10.1016/S0140-6736(20)30183-5.
6. Hani C, Trieu NH, Saab I, et al. COVID-19 pneumonia: a review of typical CT findings and differential diagnosis. Diagn Interv Imaging. 2020;101(5):263–268. doi:10.1016/j.diii.2020.03.014.
7. Cottin V. Eosinophilic lung diseases. Clin Chest Med. 2016;37(3):535–556. doi:10.1016/j.ccm.2016.04.015.
8. Wahidi MM, Lamb C, Murgu S, et al. 2020 AABIP statement on COVID-19 infections. Available from: https://aabronchology.org/2020/03/12/2020-aabip-statement-on-bronchoscopy-covid-19-infection/.
9. Weissler JC. Eosinophilic lung disease. Am J Med Sci. 2017;354(4):339–349. doi:10.1016/j.amjms.2017.03.020.
10. Bartal C, Sagy I, Barski L. Drug-induced eosinophilic pneumonia: a review of 196 case reports. Medicine (Baltimore). 2018;97(4):e9688. doi:10.1097/MD.0000000000009688.
11. Price M, Gilman MD, Carter BW, Sabloff BS, Truong MT, Wu CC. Imaging of eosinophilic lung diseases. Radiol Clin North Am. 2016;54(6):1151–1164. doi:10.1016/j.rcl.2016.05.008.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
