Back to Journals » Therapeutics and Clinical Risk Management » Volume 18
Management Practice and Drug Related Problems and Its Contributing Factors Among Cervical Cancer Patients at Oncologic Center in Ethiopia: A Hospital-Based Retrospective Study
Authors Kefale B , Engidaw MT , Tesfa D , Molla M, Yismaw MB
Received 3 March 2022
Accepted for publication 4 June 2022
Published 10 June 2022 Volume 2022:18 Pages 643—655
DOI https://doi.org/10.2147/TCRM.S364923
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Belayneh Kefale,1 Melaku Tadege Engidaw,2 Desalegn Tesfa,2 Mulugeta Molla,3 Malede Berihun Yismaw1
1Clinical Pharmacy Unit and Research Team, Department of Pharmacy, College of Medicine and Health Sciences, Bahir Dar University, Bahir Dar, Amhara, Ethiopia; 2Department of Social and Public Health, College of Health Sciences, Debre Tabor University, Debre Tabor, Amhara, Ethiopia; 3Pharmacology and Toxicology Unit, Department of Pharmacy, College of Health Sciences, Debre Tabor University, Debre Tabor, Amhara, Ethiopia
Correspondence: Belayneh Kefale, Clinical Pharmacy and Research Unit, Department of Pharmacy, College of Medicine and Health Sciences, Bahir Dar University, Bahir Dar, Amhara, Ethiopia, Email [email protected]
Introduction: In cervical cancer therapy, there is a high prevalence of drug-related problems (DRPs) due to the high toxicity and complexity of most antineoplastic regimens. However, there is a paucity of data about DRPs among patients with cervical cancer in Ethiopia. Hence, the present study was aimed at investigating management practices and DRPs among patients diagnosed with cervical cancer.
Methods: A registry-based retrospective cohort study was employed among cervical cancer patients at the oncology center of Felege Hiwot Comprehensive Specialized Hospital (FHCSH). All patients with a histologically confirmed diagnosis of cervical cancer from January 2016 to December 2020 were included. Relevant information was recorded by reviewing medical records. The possibility of DRPs was evaluated by comparing with standard guidelines. Logistic regression analysis was employed.
Results: A total of 184 cervical cancer patients were included, with a mean age of 50.2± 10.7 years. A total of 216 DRPs were identified from 93 cervical cancer patients, translating to a prevalence of 50.5% and a mean of 2.32± 1.11 DRPs per patient. ADR (27.3%), DDI (25%), and the need for additional drug therapy (22.2%) were the most prevalent DRPs. DRPs were associated with the presence of co-morbidity (AOR = 4.23, 95% CI = 1.78– 10.05, p = 0.001), complications (AOR = 2.99, 95% CI = 1.28– 6.99, p = 0.011), being treated with ≥ 5 medications (AOR = 5.1, 95% CI = 2.38– 10.95, p < 0.001), being stage II (AOR = 0.14, 95% CI = 0.02– 0.90, p = 0.038), and stage III (AOR = 0.04, 95% CI = 0.01– 0.32, p = 0.003).
Conclusion: Cisplatin-based chemotherapy was the frequently used therapeutic option. Co-morbidity and complication status, number of medication and stage of cancer were significantly associated with DRPs. The study highlights the need of clinical pharmacy services to optimize drug therapy and reduce DRPs.
Keywords: drug-related problems, cervical cancer, Felege Hiwot Comprehensive Specialized Hospital, Ethiopia
Introduction
Background
According to the Global Cancer Statistics report cervical cancer is the third most commonly diagnosed cancer worldwide,1 and more than three-fourth of cases occur in the developing countries.2 In low- and middle-income countries, including Ethiopia, cervical cancer is the commonest cancer affecting reproductive organs and also the leading cause of death from cancer among women. In spite of the fact that the greatest burden of disease in Ethiopia is infectious diseases, the incidence of cancer is growing in an alarming rate.3 Even though there is a paucity of data about the exact incidence of cervical cancer in Africa due to the absence of cancer registration, the incidence of cervical cancer in Sub-Saharan Africa has been rising, and yet many cases remain undetected.4 In Ethiopia, although population-based data do not exist in the country except for Addis Ababa, it was estimated that the annual incidence of cancer is around 60,960 cases and the annual mortality is over 44,000. In 2010, it was estimated that 20.9 million women were at risk of developing cervical cancer in Ethiopia with an estimated 4648 and 3235 annual numbers of new cases and deaths, respectively.5 Cervical cancer patients have a high incidence of co-existing chronic diseases and the treatment of cancer is complex and carries an inherent risk of DRPs which ultimately influence the treatment outcome.6 DRPs can result in increased morbidity and mortality in contrast to the intended effect of the drugs. DRPs can be defined as “an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes”, it constitute a frequent safety issue among hospitalized or ambulatory patients leading to patient harm and increased healthcare costs.7 Moreover, the development of secondary solid malignancies after long-term use of chemotherapy and radiotherapy is an emerging hurdle to the health care providers.8 Patients with advanced stage of solid cancer commonly suffer from debilitating toxicity associated with cancer treatment modalities that can have a significant negative impact on quality of life.9 The management practice of cervical cancer is generally complex and carry an inherent risk of DRPs that has a potential to hinder the attainment of the desired goals of therapy6,10 due to the concomitant use of several drugs in their treatment regimens and also a higher risk for organ failure or altered metabolism with progression of their disease.11 Despite the fact that cervical cancer patients are highly susceptible to DRPs due to cytotoxic attributes of chemotherapeutic agents, data are scarce due to the shortage of studies conducted on DRPs among those groups of patients. It is estimated that 5–12.4% of hospital admissions are due to DRPs of which half of them are preventable12,13 and have a considerable negative consequence on the health of cancer patients in terms of prolonged hospitalization and increased cost of healthcare.14 A previous study in Australia showed that the median inpatient and emergency department healthcare cost was $5,640.87 per neutropenic episode among chemotherapy associated febrile neutropenic cancer patients.15 Likewise, another study reported that the cost of drug-related hospitalization in cancer patients was very high due to prolonged hospital stay.16 Previous studies showed that potential DDIs, ADR and non-adherence17–19 were the most prevalent types of DRPs in cancer patients. The Netherlands11 and Indian20 studies reported the prevalence of chemotherapy induced DRPs in cancer patients were 49.8% and 58.6%, respectively. Study done in Kenya identified that 93.8% of DRPs, 69.1% of ADRs and 46.9% of drug interactions in patients with cervical cancer.21 Another studies in Ethiopia were depicted that ADR and DRPs observed in 52.86% and 74.7% patients.22,23 Nowadays, non-adherence to anticancer treatment is also a growing concern and associated with poor treatment outcomes in some cancers, but more research is clearly needed to assist clinicians in prioritizing areas for improvement.24,25 Different studies demonstrated that age, length of hospital stay, number of medications, stage of cancer, comorbidity and complications are the determinants of DRPs.16,25
An extensive study of management practice and DRPs would provide valuable perspicacity for healthcare providers to lessen the incidence of DRPs and improve treatment outcomes in cancer patients.26 However, DRPs of cancer patients have not been well studied and documented in Ethiopia, as prior studies have largely focused on communicable diseases, such as AIDS/HIV, malaria, and tuberculosis.27 Therefore, we designed this retrospective cohort study to assess management practices and DRPs of cervical cancer patients at the oncology centers of FHCSH in the Amhara region, Ethiopia.
Methodology
Study Setting and Period
The study was conducted at the oncology center of FHCSH. All medical records of cervical cancer patients who had been diagnosed and treated from January 1, 2016 to December 31, 2020 at the oncologic center were retrospectively reviewed. The hospital is located in Bahir Dar and serves as an oncologic center for Amhara Regional State. It also serves as a referral and a training center. Since FHCSH is the largest tertiary hospital in the Amhara region, it has a diversified patient population drawn from across the region. The oncologic center has oncologists, trained nurses, and clinical pharmacists. It provides treatment for different types of cancers such as cervical, breast, colorectal, head and neck, lung, and lymphomas and their complications. The data from medical cards was reviewed from February 2021 to May 2021.
Study Design
A registry-based retrospective cohort study of patients diagnosed with histologically verified cervical cancer and treated in the study setting was employed.
Study Population
All adult patients with a histologically confirmed diagnosis of cervical cancer who were treated as inpatients or ambulatory at the oncology centers of FHCSH and who fulfilled the inclusion criteria were targeted.
Eligibility Criteria
All adult patients (≥18 years) charts/registry logbooks with histologically confirmed diagnosis of cervical cancers and who had been on treatment in the oncologic center of FHCSH from January 1, 2016–December 31, 2020 were part of this study.
Operational Definition of Terms
Drug Related Problem
In this study, DRP refers to the occurrence of at least one of the following undesirable events: overdose, dosage too low, ADR, DDI, medication use without indication, and need for additional drug therapy.14
Sample Size and Sampling Techniques
All medical records of cervical patients who had been treated at FHSCH during 2016–2020 were eligible for the study. Based on the above eligibility criteria, 184 medical records with a confirmed case of cervical cancer were included in the study. A total survey sampling technique will be employed to select the study population since the present study includes all patients with a confirmed diagnosis of cervical cancer at the study setting. The relevant data was collected by using chart reviews from February 2021 to May 2021.
Data Collection Tool
The data abstraction format was used to collect pertinent information about each patient such as socio-demographic characteristics, disease-related characteristics (histological types of cancer, stage of cancer, presence and types of co-morbidity), treatment regimen and modalities, and DRPs. The possibility of DRPs was evaluated by comparing them to NCCN, European Society of Medical Oncology practice guidelines, and Ethiopian cancer treatment protocols. The possibility of DDIs was assessed using standard drug interaction checkers such as Lexicomp or Stockley’s Drug Interactions. The severity of the reported ADRs will be assessed using the Modified Hartwig and Siegel ADR Severity Assessment Scale.28 The English version of the instrument was used.
Data Collectors Recruitment and Training
Three nurses and three pharmacists were recruited as data collectors, and they were attending short-term training, including the pretest. They were trained in the proper use of data collection instruments, with a focus on uniform question interpretation, strict application of study criteria, explanation of study objectives, implementation of sampling technique, and confidentiality of collected data.
Data Quality Control
Before initiating the actual study, a pre-test was done on 5% of the sample size in the study setting to ensure the clarity of the data collection instruments. Based on the findings of the pre-testing, all necessary adjustments were made to the data collection instruments before implementing them in the main study. The principal investigator, throughout the data collection process, was closely supervised. The collected data was checked for completeness and consistency on a daily basis. Maximum effort was taken to maintain the quality of data through the different steps like data entry, analysis, interpretation, and representation.
Data Processing and Analysis
After checking for completeness and consistency of responses, the data was cleaned, verified, coded, and categorized. Then, the data was entered into EpiData 4.6 software for Windows and exported to Stata version 16/MP for Windows for further descriptive and analytical analysis. Analyses were stratified according to stage of cancer, comorbidity and complication status, and treatment modalities and were discussed in relation to the patient’s data with cervical cancer. Logistic regression analysis was employed to investigate the potential contributing factors of DRPs. A binary logistic regression model was used to assess the independent effects of each variable on the prevalence of DRPs. Those variables with a p-value of ≤ 0.25 during binary logistic regression analysis were fitted into a multivariable logistic regression model to identify the independent contribution of each variable. AORs, 95% CI, were built to see the strength of associations. A p value ≤ 0.05 was considered significant.
Results
Sociodemographic and Clinical Characteristics of Study Participants
A total of 184 cervical cancer patients were included in this study. The mean age of the study participants was 50.2±10.7 years, and the predominant portion of the study subjects (84, 45.6%) were aged greater than 50 years. More than half (56.5%) of the study participants were urban residents. As illustrated in Table 1, based on histological types, squamous cell carcinoma (88.6%) was the most common type, followed by adenocarcinoma (7.1%). The study showed that 38.6% and 40.2% of the study population had stage II and stage III cancer, respectively. Further, 10.9% and 16.3% of the patients had recurrence and metastasis status, respectively. In this study, the liver followed by the lung were the major metastatic sites. Regarding co-morbidity and complication status, 50 (27.2%) and 47 (25.5%) patients had co-existing co-morbidities and complications, respectively. Hydronephrosis was the most encountered co-morbid and complication condition (Table 1).
 |
Table 1 Socio-Demographic and Clinical Characteristics of Cervical Patients at FHCSH |
Management Practice for Cervical Cancer and Its Complications
Chemotherapy 124 (67.4%) alone, followed by chemotherapy in combination with surgery 20 (10.9%), were the predominantly used treatment modalities in the study settings. Metoclopramide and dexamethasone combination 58 (31.5%) was the most commonly used prophylactic antiemetic regimen, followed by a combination of ondansetron and dexamethasone 38 (20.7%). Conversely, 48 (26.1%) of the study participants did not receive any antiemetic medications. The findings of the study also showed that morphine 58 (28.3%), paracetamol 37 (20.1%) and tramadol 32 (17.4%) were the most commonly used analgesics among the study participants (Table 2).
 |
Table 2 Medication Regimens of Patients with Cervical Cancer |
Types of Regimens Used in the Management of Cervical Cancer
The combination of cisplatin and 5-FU (42.9%), followed by paclitaxel and cisplatin (39.9%), were the most widely used treatment regimens in the management of cervical cancer in FHCSH. Conversely, the combination of leucovorin, 5-FU, and oxaliplatin was the least prescribed treatment regimen used (Figure 1).
 |
Figure 1 Types of regimens used in the management of cervical cancer at FHSCH. |
Prevalence of Drug-Related Problems
A total of 216 DRPs were identified from 93 cervical cancer patients, translating to a prevalence of 50.5% and a mean of 2.32±1.11 DRPs per patient in FHCSH. ADRs, DDIs and the need for additional drug therapy were the most prevalent DRPs, which accounted for 59 (27.3%), 54 (25%) and 48 (22.2%) cases, respectively, in FHCSH (Table 3).
 |
Table 3 Categories of Drug-Related Problems Among Patients with Cervical Cancer at FHCSH |
In terms of severity, 35% of the DDIs were significant, which required modification or close monitoring of the outcome of the drug interactions. However, 9% of DDIs were serious, which necessitates the use of alternative medications in the treatment regimen (Figure 2).
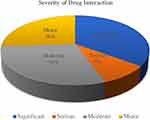 |
Figure 2 Severity of drug interactions among patients with cervical cancer at FHCSH (n = 54). |
Of the 59 ADRs identified in this study, the most common were vomiting (51.2%), nausea (43.7%), and leucopenia (37.1%). On the other hand, constipation and thrombocytopenia were the least prevailing ADRs (Table 4).
 |
Table 4 Types of Adverse Drug Reactions in Cervical Cancer Patients (n = 59) |
Factors Associated with Drug-Related Problems
In the univariable and multivariable binary logistic regression analysis, patients with cervical cancer with co-morbid conditions were 4 times (AOR = 4.23, 95% CI = 1.78–10.05, p = 0.001) more likely to have DRPs compared to their counterparts. In addition, patients with different complications were 3 times (AOR = 2.99, 95% CI = 1.28–6.99, p = 0.011) more likely to be present with DRPs. The number of medications and stage of cancer were also significantly associated with DRPs. Patients who have been treated with more than five drugs were 5 times (AOR = 5.1, 95% CI = 2.38–10.95, p < 0.001) more likely to have DRPs as compared to patients treated with less than five medications. Furthermore, stage II and III cancer patients were 0.86 (AOR = 0.14, 95% CI = 0.02–0.90, p = 0.038) and 0.96 times (AOR = 0. 04, 95% CI = 0.01–0.32, p = 0.003) less likely to have DRPs as compared to stage IV cancer patients, respectively (Table 5).
 |
Table 5 Univariable and Multivariable Binary Logistic Regression Analysis of Predictors of Drug-Related Problems |
Discussion
Cervical cancer is the third most common cancer worldwide, and more than three-fourths of cases occur in developing countries.2 Though drugs play an indispensable role in the treatment of cervical cancer, they are associated with DRPs. Hence, the present study was aimed at investigating management practices and DRPs among patients diagnosed with cervical cancer.
In the present study, the majority (45.6%) of participants are aged 50 years and above. Similarly, a study done in Kenya reported that 50% are aged above 50 years.21 A previous study conducted in Ethiopia also showed 80.2% are diagnosed above the age of 40.29 This is in line with the American Cancer Society report that cervical cancer is mostly diagnosed in women aged between 35 and 44, with the average age at diagnosis being 50.30 In developing countries, late diagnosis is common.31 Lack of awareness about cervical cancer disease and screening, practice in traditional healers and low-income level are the most responsible factors for high prevalence of delayed diagnosis of cervical cancer in Ethiopia.32–35 Hence, there is a need for public policies to improve the affordability of cancer care and enhance community awareness about the severity of the disease and referral system.29 To reduce morbidity and mortality from cervical cancer, primary prevention measures such as HPV vaccination and screening should be initiated and expanded.36 Clinical pharmacists can help prevent cervical cancer by providing and promoting HPV vaccination to the public.37,38
Squamous cell carcinoma accounts for 88.6% of study participants. Inconsistent with our study, a study done in Kenya reported that squamous cell carcinoma (91.4%) was the most common type, followed by adenocarcinoma (7.4%).21 Squamous cell carcinoma of the cervix is also the most prevalent histological cell type globally.39 The risk of squamous cells of the cervix to cancer is due to their intrinsic properties and the high mutation risk in this histologic type.
In the present study, 38.6%, 40.2%, and 16.3% of study subjects had clinical FIGO stage II, III, and IV cancers, respectively. This shows that delayed diagnosis is common. Similarly, a study done in one of the tertiary care teaching hospitals in Ethiopia reported that 86.3% of the women had delayed diagnosis of cervical cancer.35 A population-based study done in Ethiopia also showed nearly two-thirds (60.4%) of patients with cervical cancer were diagnosed at an advanced stage. Of which, stage IV patients account for 37.3%.29 Another study also reported the worst results, in which 69% of participants were diagnosed with stage IV cervical cancer.40 A study conducted at Kenyatta National Hospital also showed that 44.4% and 35.8% of the study population had stage II and stage III cervical cancer, respectively.21 On the contrary, studies done by Chen et al41 and Getahun et al33 reported that slightly more than half (̴̴ 53%) of participants were diagnosed with early stage (stage I) cervical cancer. The variation might be due to the availability of screening programs for cervical cancer among eligible women. The Getahun et al33 study, on the other hand, had a higher rate of early stage diagnosis because it was a community-based cross-sectional survey whose main goal was to assess women’s knowledge of cervical cancer. In Ethiopia, the percentage of cervical cancer screening among eligible women was much lower than the WHO recommendations, and only one in every seven women utilized cervical cancer screening.42 As a result, screening programs are critical for detecting cervical cancer early and reducing morbidity and mortality.
Our study identified that 10.9% of the patients had recurrence. This might be partly justified because of non-adherence to prescribed medications and/or interventions. Non-adherence to medications is partly due to stockouts of cytotoxic medications in an Ethiopian setting.43 In the present study, the majority of subjects were diagnosed lately and metastasis to the liver was seen in 16.3% of the patients. The late stage of cancer diagnosis is characterized by metastasis to adjacent and distant organs.31
The treatment options of cervical cancer in the study setting include surgery, radiation therapy and chemotherapy depending on the staging of cancer diagnosis, exact location of the cancer within the cervix, the type of cancer (squamous cell or adenocarcinoma), patient’s age and overall health. In this study, chemotherapy alone was used in about two-thirds of cases. The combination of cisplatin and 5-FU (42.9%), followed by paclitaxel and cisplatin (39.9%), were the most widely used treatment regimens. This finding is in line with a study done in Gondar, Ethiopia, which reported that chemotherapy was used in only 34.6% of participants, followed by surgery (33.4%) and radiation (27.2%).33 Chemo-radiation, which included weekly cisplatin and daily radiotherapy, was the most commonly used treatment regimen (50.6%) in the management of cervical cancer patients at Kenyatta National Hospital. Cisplatin and paclitaxel (9, 11.1%) were also the most commonly used combination anticancer agents.21 This is due to the fact that cisplatin-based chemotherapy is the first-line recommended regimen for cervical cancer treatment, which is associated with improvement in survival outcomes.
Antiemetics as prophylaxis and/or treatment were prescribed in 73.9% of patients, and the most commonly (52.2%) used regimen was metoclopramide OR ondansetron PLUS dexamethasone. This can be justified due to the use of highly emetogenic agents, such as cisplatin-based regimens in our study setting.
In the present study, 216 DRPs were identified among 93 (50.5%) of the included patients, giving an overall frequency of 1.17 DRPs per patient or an average of 2.32 DRPs in those patients with DRPs. The result was lower than studies done in Ethiopia (68.6%),43 Zaria (89.2%)44 and Kenya (93.8%).21 A multicenter prospective observational study done in Ethiopia among medical ward patients also showed a higher prevalence (67.7%) of DRPs among study participants.45 The difference might be attributed to the existing practice variation, healthcare professionals’ teamwork level, and availability of medication in the settings.
Despite the fact that the prevalence is lower than in many studies,21,43–45 further reduction of DRPs prevalence is required to improve the quality of health care in the setting. The integration of clinical pharmacists can better prevent and manage DRPs, thereby increasing the quality of patients’ care.43,46 Therefore, clinical pharmacy services should be established in hospitals to tackle the DRPs. ADRs (27.3%), followed by DDIs (25%), were the most prevalent DRPs identified. Similarly, a study done in Kenya reported that ADRs (69.1%) followed by DDIs (46.9%) were the most prevalent DRPs.21 A study done in Zaria also reported ADRs (29.02%) as the most prevalent DRPs, followed by treatment ineffectiveness (28.13%).44 ADRs are common among cancer patients due to the fact that the majority of these drug classes are non-selective and cause cellular toxicity to different organ systems. Nausea and vomiting accounted for the majority of ADRs. Similarly, nausea and vomiting account for the majority (46.15%) of ADRs among cervical cancer patients in Zaria.44 The cisplatin-based regimen is the first-line recommended regimen for cervical cancer treatment and is classified as a highly emetogenic agent and is associated with severe nausea and vomiting unless the patient is taking prophylactic antiemetics.47
Serious DDIs accounted for 9% of the drug interactions analyzed. Serious DDIs have been linked to higher healthcare costs, morbidity, and mortality.48–50 Once the serious drug interaction is found, the recommendation is either alternative medication use if available or discontinuation of the least vital agent. Hence, special emphasis should be given not only to the existence of drug interactions but also to the severity of the interaction.
The study also showed that the presence of comorbidities, complications, polypharmacy, and stage IV cervical cancer diagnosis were predictors for the occurrence of DRPs. Similarly, a study done by Mustapha et al showed that comorbidities and polypharmacy were determinants of DRP occurrence.44 In a multicenter prospective observational study conducted in Ethiopia, polypharmacy was also reported as a predictor of DRPs.45 Co-morbidities are associated with DRPs as individuals diagnosed with many clinical conditions will take many drugs that ultimately interact with one another. In a study conducted at Kenyatta National Hospital, advanced stage cervical cancer was also reported to be a predictor of DRPs.21 The association might be due to the complexity of the disease condition in terms of complications and the increased number of medications needed.
Conclusions
The majority of the patients had stage II and stage III cervical cancer. Chemotherapy was the most frequently used therapeutic option. More than half of cervical patients had one or more DRPs. One patient in four got ADRs and DDIs. Further, having co-morbid conditions and complications, patients taking many drugs/polypharmacy, and stage IV patients were significantly associated with the development of DRPs. Because the majority of cervical patients had one or more DRPs, clinical pharmacy services should be implemented to optimize drug therapy and reduce unwanted adverse events. In addition, the teamwork of healthcare professionals working in oncology wards should be strengthened. Furthermore, researchers and stakeholders ought to come up with relevant DRP mitigation strategies.
Abbreviations
ADR, adverse drug reaction; AOR, adjusted odd ratio; DDI, drug–drug interaction; DRP, drug-related problem; FHCSH, Felege Hiwot Comprehensive Specialized Hospital; NCCN, National Compressive Cancer Network; WHO, World Health Organization.
Data Sharing Statement
The datasets used during the current study are available in the main document.
Ethical Approval
The study was approved by Debre Tabor University’s Ethical Review Board (approval number: DTU/RE/1/3059/13), and subsequent permission was obtained from FHCSH’s Medical Record and Oncology Department. Due to the nature of the study, the Ethics Committee and the hospital waived informed written consent from study participants. Patients’ personal information and medication information were recorded while maintaining patient confidentiality and omitting their names and addresses. Our study was carried out in accordance with the Helsinki Declaration.
Acknowledgments
We would like to acknowledge the FHCSH oncology department for their invaluable assistance in providing information and other materials during the research process.
Author Contributions
All authors made a significant contribution to the work reported in the conception, study design, execution, acquisition of data, analysis, and interpretation; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Debre Tabor University (grant no. DTU/RE/1/3059/2013).
Disclosure
The authors declare that they have no conflicts of interest in relation to this work.
References
1. World Health Organization. Fact sheet N°297 Reviewed January 2013; 2013.
2. Denny L. The prevention of cervical cancer in developing countries. BJOG. 2005;112(9):1204–1212. doi:10.1111/j.1471-0528.2005.00713.x
3. Somi MH, Dolatkhah R, Sepahi S, Belalzadeh M, Naghashi S, Asghari Jafarabadi M. A 12-year trend analysis of the incidence of gastrointestinal cancers in East Azerbaijan: last updated results of an ongoing population-based cancer registry. BMC Cancer. 2019;19(1):782. doi:10.1186/s12885-019-6008-3
4. Cumbera SN, Nchanji KN, Tsoka-Gwegweni JM. Breast cancer among women in sub-Saharan Africa: prevalence and a situational analysis. South Afr J Gynaecol Oncol. 2017;9:35–37. doi:10.1080/20742835.2017.1391467
5. Federal Ministry of Health Ethiopia. National Cancer Control Plan 2016–2020. Available from: https://www.iccp-portal.org/sites/default/files/plans/CCP%20Ethiopia%20Final%20261015.pdf. Accessed June 18, 2020.
6. Bulsink A, Imholz ALT, Brouwers JRB, Jansman FGA. Characteristics of potential drug-related problems among oncology patients. Int J Clin Pharm. 2013;35(3):401–407. doi:10.1007/s11096-012-9747-7
7. Munenu K, Pierre Y, Munogo LT, et al. The prevalence and patterns of drug related problems associated with anti retroviral drugs in the management of HIV/AIDS patients at Ndola Central Hospital in 2016. J Pharm. 2016;6:8–23.
8. Iftikhar A, Jehanzeb K, Ullah A. Clinical pharmacy services in medical oncology unit, Peshawar, Pakistan. Pharmacologyonline. 2015;1:10–12.
9. Nelson KM, Talbert RL. Drug-related hospital admissions. Pharmacotherapy. 1996;16(4):701–707.
10. Livingston PM, Craike M, Slavin M. Clinical and economic burden of emergency department presentations for neutropenia following outpatient chemotherapy for cancer in Victoria, Australia. Oncologist. 2012;17(7):998–1004. doi:10.1634/theoncologist.2011-0456
11. Ko Y, Gwee YS, Huang YC, Chiang J, Chan A. Costs and length of stay of drug-related hospital admissions in cancer patients. Clin Ther. 2014;36(4):588–592. doi:10.1016/j.clinthera.2014.02.014
12. Vantard N, Ranchon F, Schwiertz V, et al. EPICC study: evaluation of pharmaceutical intervention in cancer care. J Clin Pharm Ther. 2015;40(2):196–203. doi:10.1111/jcpt.12242
13. Stoll P, Kopittke L. Potential drug–drug interactions in hospitalized patients undergoing systemic chemotherapy: a prospective cohort study. Int J Clin Pharm. 2015;37(3):475–484. doi:10.1007/s11096-015-0083-6
14. Chopra D, Rehan H, Sharma V, Mishra R. Chemotherapy-induced adverse drug reactions in oncology patients: a prospective observational survey. Indian J Med Paediatr Oncol. 2016;37(1):42. doi:10.4103/0971-5851.177015
15. Belachew SA, Erku DA, Mekuria AB, Gebresillassie BM. Pattern of chemotherapy-related adverse effects among adult cancer patients treated at Gondar University Referral Hospital, Ethiopia: a crosssectional study. Drug Healthc Patient Saf. 2016;8:83–90. doi:10.2147/DHPS.S116924
16. Sisay EA, Engidawork E, Yesuf TA, Ketema EB. Drug related problems in chemotherapy of cancer patients. J Cancer Sci Ther. 2015;07(02):55–59.
17. D’Amato S. Improving patient adherence with oral chemotherapy. Oncol Issues. 2008;23(4):42–45. doi:10.1080/10463356.2008.11884291
18. Yeoh TT, Tay XY, Si P, Chew L. Drug-related problems in elderly patients with cancer receiving outpatient chemotherapy. J Geriatr Oncol. 2015;6(4):280–287. doi:10.1016/j.jgo.2015.05.001
19. Parveen S, Sajjad R, Masood M, et al. Cervical cancer: outcome of treatment and causes of failure. JPMA. 2006;56(10):436.
20. Holub K, Biete A. Impact of systemic inflammation biomarkers on the survival outcomes of cervical cancer patients. Clin Transl Oncol. 2019;21(7):836–844. doi:10.1007/s12094-018-1991-4
21. Degu A, Njogu P, Weru I, Karimi P. Assessment of drug therapy problems among patients with cervical cancer at Kenyatta National Hospital, Kenya. Gynecol Oncol Res Pract. 2017;4(1):15. doi:10.1186/s40661-017-0054-9
22. Quinn BA, Deng X, Colton A, Bandyopadhyay D, Carter JS, Fields EC. Increasing age predicts poor cervical cancer prognosis with subsequent effect on treatment and overall survival. Brachytherapy. 2019;18(1):29–37. doi:10.1016/j.brachy.2018.08.016
23. Liu YM, Ni LQ, Wang SS, Lv QL, Chen WJ, Ying SP. Outcome and prognostic factors in cervical cancer patients treated with surgery and concurrent chemoradiotherapy: a retrospective study. World J Surg Oncol. 2018;16(1):1–7. doi:10.1186/s12957-017-1307-0
24. Wassie M, Argaw Z, Tsige Y, Abebe M, Kisa S. Survival status and associated factors of death among cervical cancer patients attending at Tikur Anbesa Specialized Hospital, Addis Ababa, Ethiopia: a retrospective cohort study. BMC Cancer. 2019;19(1):1–11. doi:10.1186/s12885-019-6447-x
25. Lund JL, Sanoff HK, Hinton SP, Muss HB, Pate V, Stürmer T. Potential medication-related problems in older breast, colon, and lung cancer patients in the United States. Cancer Epidemiol Biomark Prev. 2018;27(1):41–49. doi:10.1158/1055-9965.EPI-17-0523
26. Organization WH. Latest global cancer data: cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in 2018. International Agency for Research on Cancer. Geneva: World Health Organization; 2018.
27. World Health Organization. Preventing Chronic Diseases: A Vital Investment. Geneva: World Health Organization; 2005.
28. Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49:2229–2232.
29. Dereje N, Gebremariam A, Addissie A, et al. Factors associated with advanced stage at diagnosis of cervical cancer in Addis Ababa, Ethiopia: a population-based study. BMJ open. 2020;10(10):e040645. doi:10.1136/bmjopen-2020-040645
30. World Health Organization. Cancer key facts [Internet]; 2020. Available from: http://www.who.int/news-room/fact-sheets/detail/cancer.
31. Cancer Research UK. Worldwide cancer statistics [Internet]; 2020. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer.
32. Aweke YH, Ayanto SY, Ersado TL. Knowledge, attitude and practice for cervical cancer prevention and control among women of childbearing age in Hossana Town, Hadiya zone, Southern Ethiopia: community-based cross-sectional study. PLoS One. 2017;12(7):e0181415. doi:10.1371/journal.pone.0181415
33. Getahun F, Mazengia F, Abuhay M, Birhanu Z. Comprehensive knowledge about cervical cancer is low among women in Northwest Ethiopia. BMC Cancer. 2013;13(1):1–7. doi:10.1186/1471-2407-13-2
34. Birhanu Z, Abdissa A, Belachew T, et al. Health seeking behavior for cervical cancer in Ethiopia: a qualitative study. Int J Equity Health. 2012;11(1):1–8. doi:10.1186/1475-9276-11-83
35. Zeleke S, Anley M, Kefale D, Wassihun B. Factors associated with delayed diagnosis of cervical cancer in tikur anbesa specialized hospital, Ethiopia, 2019: cross-Sectional Study. Cancer Manag Res. 2021;13:579. doi:10.2147/CMAR.S285621
36. Hailu A, Mariam DH. Patient side cost and its predictors for cervical cancer in Ethiopia: a cross sectional hospital based study. BMC Cancer. 2013;13(1):1–8. doi:10.1186/1471-2407-13-69
37. Jennifer M, Sturpe DA, Khanna N. Human papillomavirus vaccine and cervical cancer prevention: practice and policy implications for pharmacists. J Am Pharm Assoc. 2008;48(1):e1–e16. doi:10.1331/JAPhA.2008.07032
38. Brewer NT, Chung JK, Baker HM, Rothholz MC, Smith JS. Pharmacist authority to provide HPV vaccine: novel partners in cervical cancer prevention. Gynecol Oncol. 2014;132:S3–S8. doi:10.1016/j.ygyno.2013.12.020
39. Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Global Health. 2020;8(2):e191–e203. doi:10.1016/S2214-109X(19)30482-6
40. Wassie M, Aemro A, Fentie B. Prevalence and associated factors of baseline anemia among cervical cancer patients in Tikur Anbesa Specialized Hospital, Ethiopia. BMC Womens Health. 2021;21(1):1–8. doi:10.1186/s12905-020-01152-w
41. Chen CP, Kung PT, Wang YH, Tsai WC. Effect of time interval from diagnosis to treatment for cervical cancer on survival: a nationwide cohort study. PLoS One. 2019;14(9):e0221946. doi:10.1371/journal.pone.0221946
42. Desta M, Getaneh T, Yeserah B, et al. Cervical cancer screening utilization and predictors among eligible women in Ethiopia: a systematic review and meta-analysis. PLoS One. 2021;16(11):e0259339. doi:10.1371/journal.pone.0259339
43. Yismaw MB, Adam H, Engidawork E. Identification and resolution of drug-related problems among childhood cancer patients in Ethiopia. J Oncol. 2020;2020. doi:10.1155/2020/6785835
44. Mustapha S, Mohammed M, Mustapha L, Yunusa I, Basgut B. A survey on drug related problems in cervical cancer patients receiving chemotherapy in ahmadu bello university teaching hospital zaria. Bayero J Pure Appl Sci. 2017;10(1):489–492. doi:10.4314/bajopas.v10i1.93S
45. Bekele F, Tsegaye T, Negash E, Fekadu G. Magnitude and determinants of drug-related problems among patients admitted to medical wards of southwestern Ethiopian hospitals: a multicenter prospective observational study. PLoS One. 2021;16(3):e0248575. doi:10.1371/journal.pone.0248575
46. Su YJ, Yan YD, Wang WJ, et al. Drug-related problems among hospitalized cancer pain patients: an investigative single-arm intervention trial. Annals of Palliative Medicine; 2020.
47. Percie du Sert N, Rudd JA, Apfel CC, Andrews PLR. Cisplatin-induced emesis: systematic review and meta-analysis of the ferret model and the effects of 5-HT3 receptor antagonists. Cancer Chemother Pharmacol. 2011;67(3):667–686. doi:10.1007/s00280-010-1339-4
48. Moura CS, Acurcio FA, Belo NO. Drug-drug interactions associated with length of stay and cost of hospitalization. J Pharm Pharm Sci. 2009;12(3):266–272. doi:10.18433/J35C7Z
49. Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc. 2001;41(2):192–199. doi:10.1016/S1086-5802(16)31229-3
50. Cohen IV, Makunts T, Abagyan R, Thomas K. Concomitant drugs associated with increased mortality for MDMA users reported in a drug safety surveillance database. Sci Rep. 2021;11(1):1–9. doi:10.1038/s41598-021-85389-x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
