Back to Journals » Cancer Management and Research » Volume 12
Malignant Ovarian Tumors During Pregnancy: A Multicenter Retrospective Analysis
Authors Wang L, Huang S, Sheng X , Ren C, Wang Q, Yang L, Zhao S, Xu T , Ma X , Guo R, Sun P , Lin Y , Li Y, Wang J, Wang Y
Received 29 July 2020
Accepted for publication 6 October 2020
Published 29 October 2020 Volume 2020:12 Pages 10841—10848
DOI https://doi.org/10.2147/CMAR.S271806
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xueqiong Zhu
Liya Wang,1 Shenjiao Huang,2 Xiujie Sheng,3 Chenchen Ren,4 Qiming Wang,5 Linqing Yang,6 Shuping Zhao,7 Tianmin Xu,8 Xiaoxin Ma,9 Ruixia Guo,10 Pengming Sun,11 Yang Lin,8 Yuhong Li,1 Jiandong Wang,12 Yudong Wang1
1Department of Gynecologic Oncology, International Peace Maternity and Child Health Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai Municipal Key Clinical Specialty, Shanghai Key Laboratory of Embryo Original Disease, Shanghai, 200030, People’s Republic of China; 2Department of Obstetrics and Gynecology, Guangzhou Women and Children’s Medical Centre, Guangzhou 510623, People’s Republic of China; 3Department of Obstetrics and Gynecology, The Third Affiliated Hospital of Guangzhou Medical University, Key Laboratory of Major Obstetric Diseases of Guangdong Province, Guangzhou 510150, People’s Republic of China; 4Department of Obstetrics and Gynecology, The Third Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, People’s Republic of China; 5Department of Obstetrics and Gynecology, Women and Children’s Hospital of Ningbo City, Ningbo 315012, People’s Republic of China; 6Department of Gynecology, Affiliated Hospital of Jining Medical University, Jining 272029, People’s Republic of China; 7Department of Gynecology, Women and Children’s Hospital of Qingdao, Qingdao 266000, People’s Republic of China; 8Department of Obstetrics and Gynecology, The Second Hospital of Jilin University, Changchun 130000, People’s Republic of China; 9Department of Obstetrics and Gynecology, Shengjing Hospital Affiliated of China Medical University, Shenyang 110004, People’s Republic of China; 10Department of Gynecology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, People’s Republic of China; 11Department of Obstetrics and Gynecology, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou 350001, People’s Republic of China; 12Department of Gynecologic Oncology, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing 100026, People’s Republic of China
Correspondence: Yudong Wang Department of Gynecologic Oncology
International Peace Maternity and Child Health Hospital, Shanghai Jiao Tong University School of Medicine, NO. 1961, Huashan Road, Shanghai 200030, People’s Republic of China
Email [email protected]
Jiandong Wang
Department of Gynecologic Oncology, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, NO. 251, Yao Jiayuan Road, Beijing 100026, People’s Republic of China
Email [email protected]
Purpose: The aim of this study was to investigate the clinical characteristics and management of malignant ovarian tumors during pregnancy, as well as the feto-maternal outcomes and analyze the influential factors on the pregnancy outcomes.
Patients and Methods: Eighty-five patients with ovarian malignancies during pregnancy treated at 12 tertiary hospitals between 2009 and 2019 were analyzed in this study. The clinical features, histopathological characteristics, clinical management, and maternal and perinatal outcomes were retrospectively analyzed. The clinical features and managements were compared between abortion group and live birth group.
Results: The following diagnoses were made: 41 (48.24%) patients with borderline ovarian tumors, 18 (21.18%) patients with epithelial ovarian cancers, 17 (20.00%) patients with non-epithelial ovarian malignancies and 9 (10.59%) patients with metastatic ovarian tumors. Thirty-six (42.45%) patients underwent conservative surgical treatment. Thirty-four (40.00%) patients opted for fertility-sparing surgery, and fifteen (17.56%) patients received radical surgery. Chemotherapy was administered to 32.94% of the patients. The proportion of ovarian malignancies diagnosed in the first trimester in the abortion group was higher than that in the live birth group (P< 0.05). However, tumor diameter, reproductive history, stage and surgical indications showed no significant differences between groups. A total of 67 live babies were recorded in this study, including 19 premature babies and 1 full-term newborn who died of respiratory distress. All of the BOTs were diagnosed with stage I, among whom 38 (92.68%) patients exhibited disease-free survival. Twenty-eight ovarian cancers were in stage I–II and 26 of them had disease-free survival with the longest follow-up time of 10 years. Five of the sixteen patients in advanced stage (stage III–IV) died, four of whom had metastatic tumors.
Conclusion: Pregnant women with early-stage malignant ovarian tumors appear to have favorable outcomes. Conservative surgery is acceptable for early-stage borderline ovarian tumors during pregnancy. The gestational age of ovarian malignancy detection is key for pregnancy outcomes.
Keywords: malignant ovarian tumor, pregnancy, management, prognosis
Introduction
Malignant ovarian tumors are the second most frequent gynecological cancer diagnosed during pregnancy after cervical carcinoma. According to previous studies, the incidence of ovarian cancer is 0.02 to 0.38 per 10,000 pregnancies, and that of ovarian masses with low malignancy potential is 0.11 to 0.24 per 10,000 pregnancies.1,2 Although the incidence of gestational ovarian malignancy is low, it poses challenges in maternal and perinatal management.
When a malignant tumor is suspected, surgery treatment is indispensable and can be performed safely during pregnancy. According to the latest guidelines for gynecologic cancers in pregnancy,3 surgery staging should be performed for early-stage malignant disease (low malignant potential, invasive or germ cell). The current recommendations state that if surgical procedures are restricted during pregnancy because of an enlarged uterus and the limited possibility for manipulation, restaging should be planned postpartum. However, the adverse effects of a second surgery, such as new anesthesia and operative complications, must be considered. In previous studies, feto-maternal outcomes have been highly contingent on tumor stage at diagnosis and histological type.4–6 Herein, we evaluated the effects of surgery without staging on recurrence in pregnant women with malignant ovarian tumors, and compared the factors associated with different pregnancy outcomes through a multicenter review study. Our aim was to improve the knowledge of ovarian malignancies during pregnancy.
Patients and Methods
Study Population and Data Collection
This was a multicenter retrospective review of patients with malignant ovarian tumors during pregnancy who were diagnosed and treated at 12 independent hospitals between 2009 and 2019. A total of 91 patients with malignant ovarian tumors were treated during the 10 years period. The study was approved by each of the universities or hospitals involved. To better evaluate the therapeutic effects, we excluded six pregnant women with feto-maternal prognosis who were lost to follow-up after the initial treatment from further analysis. The collected data for the remaining 85 patients included the following: clinical and histopathological characteristics, stage (FIGO/2013), timing of diagnosis and surgery, indication of surgery, chemotherapy applied and feto-maternal outcomes. The clinical features and managements were compared between abortion group and live birth group. Adnexal masses were detected in routine pre-pregnancy or prenatal examination by ultrasound scans or MRI (magnetic resonance imaging). All patients underwent surgical resection for ovarian tumors. The pathological diagnoses were made by specialists from the department of pathology at each hospital. Patient treatment and follow-up were performed at the department of obstetrics and gynecology in each hospital. According to a previous study,7 conservative surgery was defined as cystectomy or unilateral salpingo-oophorectomy with no complementary surgical treatment. Fertility-sparing surgery was defined as at least one ovary being preserved with comprehensive staging (omentectomy, appendectomy, pelvic-peritoneal biopsies and/or lymphadenectomy). Radical surgery was defined as cytoreductive operation with hysterectomy.
Statistical Analysis
Quantitative data are shown as mean ± SD, and qualitative data are expressed as numbers (percentages). Independent samples t-tests were used to compare the quantitative data. The chi-square test and Fisher’s exact test were used to examine the differences in clinical features between the abortion group and live birth group. A P value <0.05 was considered statistically significant. SPSS software 17.0 (IBM Corporation, Armonk, NY, USA) was used for data analysis.
Results
Clinical Features
A total of 1,069,041 deliveries were recorded between 2009 and 2019, of which 91 were diagnosed with malignant tumors. The proportion of pregnant patients with malignant ovarian tumors in the 12 hospitals was 0.85 per 10,000 deliveries, ranging from 0.44 to 1.71 per 10,000 pregnancies. The clinical characteristics of the 85 patients are summarized in Table 1. The median age of the patients was 31.08 ± 5.25 years, with a range from 19 to 43 years, and there were 35 (41.18%) nulliparous patients. Four patients had a history of borderline ovarian tumors, one had a familial breast cancer history, and the remaining 80 (94.12%) had no previous history of cancer. Seventy-two (84.70%) tumors were unilateral, and 13 (15.29%) were bilateral. Patients mainly presented with tumors larger than 6 cm in diameter (82.35%; N=70). Tumors were diagnosed in the first, second, and third trimesters in 24, 21 and 35 patients, respectively. Adnexal masses were identified before pregnancy in five patients and were pathologically confirmed after surgery during pregnancy. Most patients were asymptomatic (88.24%; N=75), and ten patients had abdominal pain. Eighty-four patients underwent surgery during pregnancy: 13 (15.29%) during the first trimester, 29 (34.12%) underwent surgery during the second trimester, and 42 (49.41%) underwent surgery during the third trimester. One patient received surgical treatment in the postpartum period. Sixty (70.59%) women underwent cesarean section, 7 (8.24%) women delivered vaginally, and 18 (21.18%) women opted for elective abortion. A total of 67 (78.82%) live birth infants were delivered, including 48 (56.47%) full-term neonates (one newborn died of respiratory distress) and 19 (22.35%) premature (one with chromosome abnormality).
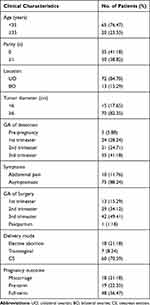 |
Table 1 Patient Characteristics and Obstetric Outcomes |
Tumor Histopathological Characteristics, Treatments and Feto-Maternal Outcomes
The distribution by histological type of borderline ovarian tumors and ovarian cancers are shown in Table 2. A total of 59 patients (69.41%) had epithelial ovarian tumors, including 41 (48.24%) borderline ovarian tumors (BOTs) and 18 (21.17%) epithelial ovarian cancers (EOCs). Other histopathological types were germ cell tumors (16.47%; N=14), sex cord-stromal tumors (2.35%; N=2), metastatic ovarian tumors (10.59%; N=9) and small cell carcinoma (1.18%; N=1).
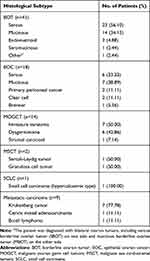 |
Table 2 Histological Subtypes Analysis of Ovarian Malignant Tumors |
Table 3 shows the patient distribution by stage, surgery, chemotherapy and feto-maternal outcomes. In this study, 67 cases (78.82%) were in stage I (FIGO 2013), including 41 BOTs and 26 ovarian cancers, 2 cases (2.35%) were in stage II (epithelial ovarian tumors), 7 cases (8.23%) were in stage III (6 epithelial ovarian cancer, 1 small cell carcinoma), and 9 metastatic ovarian tumors (10.59%) were in stage IV (7 Krukenberg tumors, 1 metastatic B-cell lymphoma tumor and 1 metastatic cervical tumor).
 |
Table 3 Patients’ Demography by Histological Type, Stage, Surgery, Chemotherapy, Maternal and Perinatal Outcome |
All patients underwent surgical treatment: 36 patients (42.35%) underwent conservative surgery without staging, including 26 patients with BOTs; 34 patients (40.00%) underwent fertility-sparing surgery with standard staging; and the other 15 patients (17.65%) were treated with radical surgery with complete staging. Most of the BOTs did not receive adjuvant chemotherapy, except for one case with pathological type as serous BOTs with invasive implants in stage Ic. Twenty-seven (61.36%; 27/44) patients with ovarian cancers received systematic chemotherapy after the initial surgery, three of whom received neoadjuvant chemotherapy during their pregnancies.
Eighteen (21.18%) pregnant women terminated their pregnancies to prioritize the treatment of the ovarian tumor, whereas 67 (78.82%) patients delivered live births. The birth weights ranged from 1295 g to 4180 g, and two neonates were classified as SGA. The median follow-up period was 28 months (ranging from 3 to 126 months). Information on maternal prognosis was available for 76 patients. At the time of review, five patients with stage III–IV cancer died (four metastatic ovarian tumors and one poorly differentiated adenocarcinoma in stage III). Tumor recurrence occurred in five patients (with 2 BOTs and 1 germ cell tumor, 1 adenocarcinoma in stage IIIc and 1 metastatic ovarian tumor). Thirty-eight (92.68%, 38/41) BOTs had complete remission. Twenty-eight (63.64%, 28/44) ovarian cancers had disease-free survival (26 ovarian cancers with stage I–II) with the longest follow-up of 10 years. Nine patients with maternal outcomes were lost to follow-up. The global survival rate was 83.53% (71/85).
Comparison of Clinical Characteristics Among Pregnancy Outcomes
The patients were divided into abortion group (N=18) and live birth group (N=67) according to pregnancy outcomes. The clinical features between groups are compared in Table 4. Thirty-five patients with tumors diagnosed during the third trimester (≥28 weeks) of pregnancy delivered live births. In the abortion group, the proportion of ovarian malignancies diagnosed in pre-pregnancy, and in the first and the second trimesters were 0 (0/18), 72.22% (13/18) and 27.78% (5/18), respectively. In live birth group, the proportions of ovarian malignancies diagnosed at different gestational ages were 15.62% (5/32), 34.37% (11/32) and 50% (16/32), and the difference between the groups was statistically significant (P<0.05). In live birth group, 65 patients underwent surgery during the second and the third trimester of pregnancy: 24 (35.82%) underwent surgery during the second trimester, and 41 (61.19%) underwent surgery during the third trimester. Whereas, in abortion group, more than half of the patients (66.67%, 12/18) underwent surgery during the first trimester. Moreover, tumor diameter, reproductive history, stage, surgical indication and operation type showed no significant differences (Table 4).
 |
Table 4 Comparison of Clinical Features Between Abortion Group and Live Birth Group |
Discussion
The occurrence of adnexal masses in pregnancy is reported in 1 per 76 to 1 per 2328 deliveries, and most are benign.8 The recently reported incidence of ovarian cancer in pregnancy varies from 0.31 to 0.67 per 10,000 pregnancies.9 Diagnosis of malignant ovarian tumors during pregnancy is increasing, owing to the tendency to delay of childbearing to later reproductive ages and the application of assisted reproductive technologies.10 We found that malignant ovarian tumors in pregnancy occurred in approximately 0.44 to 1.71 per 10,000 pregnancies, a rate is slightly higher than the incidence described previously. In the data presented here, in pregnant women, BOT was the most frequent ovarian malignancy (41 patients), followed by epithelial ovarian tumors (18 patients) and germ cell tumors (14 patients). This trend is consistent with those in other reports.4,8 Metastatic ovarian tumors are not commonly seen in pregnancy. Ten percent of ovarian cancers are estimated to be metastatic.11 In our study, nine patients with metastatic tumors were reported, accounting for 10.59%. Most maternal ovarian malignancies are in stage I (79.12%; N=72) at diagnosis, as previously reported.2,5
Management of ovarian masses during pregnancy is similar to that during non-pregnancy, with the consideration of maternal and fetal factors. Treatment should be individualized according to pathological type, stage, gestational age and maternal preference. Surgical treatment, a cornerstone of treatment of malignant ovarian tumors, is recommended in the second trimester of pregnancy to decrease the risks of miscarriage, torsion, rupture and delayed diagnosis of malignancy.12 The principles of management include comprehensive surgical staging. If the pelvic peritoneum and the pouch of Douglas cannot be reliably examined during surgery, restaging surgery at cesarean section or post-delivery may be important to determine the treatment plan.3,13
The most widely accepted surgical treatment of EOC in non-pregnant women is radical surgery, which includes bilateral adnexectomy and hysterectomy.14 For pregnant women with EOC who opt to preserve their pregnancies, the primary ovarian cancer treatment consists of appropriate surgical staging and debulking surgery followed by chemotherapy, timely delivery as well as neo-adjuvant chemotherapy with subsequent completing surgery.15 Previous reports have suggested that fertility-sparing surgery may be safe to stage IA/IC EOC.16,17 Most pregnant women with non-epithelial ovarian cancer (germ cell and sex cord-stromal tumors) are diagnosed with early-stage disease. Given the favorable prognosis of stage I tumors, fertility-sparing surgery with comprehensive staging is recommended. Another review has also stated that fertility-sparing surgery can be offered to patients with stage I epithelial ovarian tumors, germ cell ovarian or sex-cord stromal ovarian tumors.6 In our study, 16 of 28 patients with ovarian cancers at stage I/II received fertility-sparing operations. Although eight patients with ovarian cancers (stage IA/IC) received conservative treatment, such treatment remains strictly limited because of frequent relapse rates.
BOTs have excellent prognosis and in most patients are treated surgically without chemotherapy. Fertility-sparing surgery is preferred in women of childbearing age with stage I cancers.7 A meta-analysis has suggested that restaging surgery does not significantly decrease recurrence in patients with BOTs, and it exposes the patients to new anesthetic and operative complications.18 Zapardiel et al have indicated that restaging surgery does not affect the management of BOTs, particularly those with mucinous subtype and apparent FIGO stages above I.19 However, this treatment should be offered to patients with serous subtype and micropapillary patterns. This histological subtype has a high rate of occult extraovarian disease with invasive implants.20 A French multicenter study has indicated that pregnancy, compared with non-pregnancy, promotes borderline ovarian tumor progression, and complete staging surgeries are rarely performed initially. Up-front salpingo-oophorectomy should be considered, and restaging should be planned.21 In our study, 63.41% (26/41) of patients with BOTs underwent conservative surgery without staging; 2 of them relapsed, and 24 survived without tumors. Therefore, conservative surgery is acceptable for borderline ovarian tumors associated with pregnancy in early stage of tumor. Because of the lack of randomized controlled trials or prospective cohorts on this subject, our study has limitations, and the results should be interpreted with caution. Herein, we focused our analysis exclusively on the recurrence rates.
When necessary, adjuvant chemotherapy or neoadjuvant chemotherapy should be applied. Previous studies have indicated that chemotherapy is recommended at the 2nd or 3rd trimester of gestation and should be discontinued 3 to 4 weeks before delivery to prevent myelosuppression in the parturient and neonates.22,23 According to available data, chemotherapy during the first trimester poses a high risk of fetal malformation and may increase the risk of premature rupture of membranes, infants being small for their gestational ages, premature labor and NICU admission during the second or third trimesters.22,24–26 Moreover, the administration of chemotherapy increases maternal stress.27
In a previous study, pregnant women with ovarian cancer have been found to be more likely to terminate the pregnancy, whereas those with borderline tumors or non-epithelial tumors are able to successfully deliver live newborns.4 In patients with advanced-stage epithelial ovarian cancer, termination of the pregnancy should be considered when the diagnosis is made in early pregnancy stages.6 We found that the abortion rates were significantly higher with ovarian malignancy detection in the first trimester than that in later trimesters. Further analysis showed no significant relationships among tumor size, reproductive history, stage, surgical indications and type. These data indicate that pregnancy outcomes are associated with gestational age at the time of tumor diagnosis. Patients with ovarian tumors detected in early stages of pregnancy often chose to prioritize treatment of diseases at the sacrifice of their babies, whereas those in the second trimester had higher expectations for the fetus. This result may be associated with doctors’ guidance, and patients’ awareness of tumors and fertility desires.
Evidence from the literature has indicated that pregnancy does not significantly affect the prognosis of ovarian tumors.28,29 The overall survival and recurrence-free survival rates of malignancy patients during pregnancy are similar to or better than those of non-pregnant patients.2,20 Mortality due to ovarian cancer has been reported to occur in 4.7% of patients with either ovarian cancer or borderline ovarian tumors, and the 5-year survival rate is 72% to 90%.2 Tumor stage is the most important prognostic factor,5,28,30 because it may be associated with the early detection and treatment of ovarian tumors by regular antenatal examination, and most tumors are in early stages.
Our findings show overall good outcomes of pregnancies with complications of ovarian tumors. In the present cases, seven of nine metastatic tumors were Krukenberg tumors. More than half the pregnancies ended in live birth (6/9). The prognosis of Krukenberg tumors during pregnancies is overall very poor, and the reported median survival time is 6 months; however, prognosis may be improved if radical surgery is achievable.31 In this study, four patients with metastatic tumors died, one had recurrence, and five were not followed up, thus suggesting that the actual mortality rate might have been higher. Prematurity is a significant newborn risk factor in women with malignant ovarian tumors. Other associated risks are intrauterine growth restriction, preterm rupture of membranes and intrauterine death.1,15 In our study, there were 19 (22.35%) premature and 2 low birth weight infants, in agreement with previous findings.
Conclusion
The diagnosis of ovarian malignancy is extremely low during pregnancy, and most patients are in stage I. The overall feto-maternal prognosis for early stages of tumor is favorable. Patients with ovarian tumors detected in early stages of pregnancy chose to prioritize cancer treatment and to sacrifice their babies, thus indicating that the gestational age at ovarian malignancy diagnosis is a high-risk factor for pregnancy outcomes. Conservative surgery is acceptable for early-stage borderline ovarian tumors during pregnancy with a low recurrence rate, whereas staging surgery is recommended for other ovarian cancers in any stages. Our data reveal several clinical features that might be beneficial in future studies on malignant ovarian tumors in pregnant women.
Ethical Statement
This study was approved by the Ethics Committee of International Peace Maternity and Child Health Hospital in Shanghai and conducted in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The ethical approval covered all the researches which were conducted in the 12 hospitals. Owing to the retrospective study design and analysis of clinical data, written informed consent was formally waived by the Ethics Committee of International Peace Maternity and Child Health Hospital. All clinical data were anonymously analyzed.
Acknowledgments
This study is supported by the Shanghai Municipal Key Clinical Specialty (No. shslczdzk06302), National Natural Science Foundation of China (No. 81172477, 81402135), the Project of the Science and Technology Commission of Shanghai Municipality (No. 17441907400) and Shanghai Jiao Tong University Medicine-Engineering Fund (No. YG2017MS41). Written informed consent was obtained from all the patients for publication of this manuscript.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nazer A, Czuzoj-Shulman N, Oddy L, Abenhaim HA. Incidence of maternal and neonatal outcomes in pregnancies complicated by ovarian masses. Arch Gynecol Obstet. 2015;292(5):1069–1074. doi:10.1007/s00404-015-3700-7
2. Leiserowitz GS, Xing G, Cress R, Brahmbhatt B, Dalrymple JL, Smith LH. Adnexal masses in pregnancy: how often are they malignant? Gynecol Oncol. 2006;101(2):315–321. doi:10.1016/j.ygyno.2005.10.022
3. Amant F, Berveiller P, Boere IA, et al. Gynecologic cancers in pregnancy: guidelines based on a third international consensus meeting. Ann Oncol. 2019;30(10):1601–1612. doi:10.1093/annonc/mdz228
4. Morikawa A, Ueda K, Takahashi K, et al. Pathology-oriented treatment strategy of malignant ovarian tumor in pregnant women: analysis of 41 cases in Japan. Int J Clin Oncol. 2014;19(6):1074–1079. doi:10.1007/s10147-014-0669-3
5. Blake EA, Kodama M, Yunokawa M, et al. Feto-maternal outcomes of pregnancy complicated by epithelial ovarian cancer: a systematic review of literature. Eur J Obstet Gynecol Reprod Biol. 2015;186:97–105. doi:10.1016/j.ejogrb.2015.01.010
6. Boussios S, Moschetta M, Tatsi K, Tsiouris AK, Pavlidis N. A review on pregnancy complicated by ovarian epithelial and non-epithelial malignant tumors: diagnostic and therapeutic perspectives. J Adv Res. 2018;12:1–9. doi:10.1016/j.jare.2018.02.006
7. Plett H, Harter P, Ataseven B, et al. Fertility-sparing surgery and reproductive-outcomes in patients with borderline ovarian tumors. Gynecol Oncol. 2020;157(2):411–417. doi:10.1016/j.ygyno.2020.02.007
8. Aggarwal P, Kehoe S. Ovarian tumours in pregnancy: a literature review. Eur J Obstet Gynecol Reprod Biol. 2011;155(2):119–124. doi:10.1016/j.ejogrb.2010.11.023
9. Mukhopadhyay A, Shinde A, Naik R. Ovarian cysts and cancer in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2016;33:58–72. doi:10.1016/j.bpobgyn.2015.10.015
10. Pavlidis N. Cancer and pregnancy: what should we know about the management with systemic treatment of pregnant women with cancer? Eur J Cancer. 2011;47(Suppl 3):S348–S352. doi:10.1016/S0959-8049(11)70199-X
11. Yacobozzi M, Nguyen D, Rakita D. Adnexal masses in pregnancy. Semin Ultrasound CT MR. 2012;33(1):55–64. doi:10.1053/j.sult.2011.10.004
12. Gasim T, Al Dakhiel SA, Al Ghamdi AA, et al. Ovarian tumors associated with pregnancy: a 20-year experience in a teaching hospital. Arch Gynecol Obstet. 2010;282(5):529–533. doi:10.1007/s00404-009-1346-z
13. Hasegawa T, Ishii Y, Yonezawa R, et al. Stage I ovarian cancer cases during early, mid and late pregnancy periods: three case reports and review of the literature. J Obstet Gynaecol Res. 2011;37(6):650–655. doi:10.1111/j.1447-0756.2010.01412.x
14. Stuart GC, Kitchener H, Bacon M, et al. 2010 Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: report from the fourth ovarian cancer consensus conference. Int J Gynecol Cancer. 2011;21(4):750–755. doi:10.1097/IGC.0b013e31821b2568
15. Grimm D, Woelber L, Trillsch F, Keller-v Amsberg G, Mahner S. Clinical management of epithelial ovarian cancer during pregnancy. Eur J Cancer. 2014;50(5):963–971. doi:10.1016/j.ejca.2013.12.020
16. Yin J, Wang Y, Shan Y, Li Y, Jin Y, Pan L. Pregnancy and oncologic outcomes of early stage low grade epithelial ovarian cancer after fertility sparing surgery: a retrospective study in one tertiary hospital of China. J Ovarian Res. 2019;12(1):44. doi:10.1186/s13048-019-0520-6
17. Schlaerth AC, Chi DS, Poynor EA, Barakat RR, Brown CL. Long-term survival after fertility-sparing surgery for epithelial ovarian cancer. Int J Gynecol Cancer. 2009;19(7):1199–1204. doi:10.1111/IGC.0b013e31819d82c3
18. Chevrot A, Hequet D, Fauconnier A, Huchon C. Impact of surgical restaging on recurrence in patients with borderline ovarian tumors: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;248:227–232. doi:10.1016/j.ejogrb.2020.03.023
19. Zapardiel I, Rosenberg P, Peiretti M, et al. The role of restaging borderline ovarian tumors: single institution experience and review of the literature. Gynecol Oncol. 2010;119(2):274–277. doi:10.1016/j.ygyno.2010.07.034
20. Morice P, Uzan C, Gouy S, Verschraegen C, Haie-Meder C. Gynaecological cancers in pregnancy. Lancet. 2012;379(9815):558–569. doi:10.1016/S0140-6736(11)60829-5
21. Fauvet R, Brzakowski M, Morice P, et al. Borderline ovarian tumors diagnosed during pregnancy exhibit a high incidence of aggressive features: results of a French multicenter study. Ann Oncol. 2012;23(6):1481–1487. doi:10.1093/annonc/mdr452
22. Ring AE, Smith IE, Jones A, Shannon C, Galani E, Ellis PA. Chemotherapy for breast cancer during pregnancy: an 18-year experience from five London teaching hospitals. J Clin Oncol. 2005;23(18):4192–4197. doi:10.1200/JCO.2005.03.038
23. Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy. Lancet Oncol. 2004;5(5):283–291. doi:10.1016/S1470-2045(04)01466-4
24. Azim HA
25. Azim HA
26. de Haan J, Verheecke M, Van Calsteren K, et al. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: a 20-year international cohort study of 1170 patients. Lancet Oncol. 2018;19(3):337–346. doi:10.1016/S1470-2045(18)30059-7
27. Mielcarek P, Nowicka-Sauer K, Kozaka J. Anxiety and depression in patients with advanced ovarian cancer: a prospective study. J Psychosom Obstet Gynaecol. 2016;37(2):57–67. doi:10.3109/0167482X.2016.1141891
28. Minig L, Otano L, Diaz-Padilla I, Alvarez Gallego R, Patrono MG, Valero de Bernabe J. Therapeutic management of epithelial ovarian cancer during pregnancy. Clin Transl Oncol. 2013;15(4):259–264. doi:10.1007/s12094-012-0963-3
29. Ferrandina G, Distefano M, Testa A, De Vincenzo R, Scambia G. Management of an advanced ovarian cancer at 15 weeks of gestation: case report and literature review. Gynecol Oncol. 2005;97(2):693–696. doi:10.1016/j.ygyno.2005.02.011
30. Behtash N, Karimi Zarchi M, Modares Gilani M, Ghaemmaghami F, Mousavi A, Ghotbizadeh F. Ovarian carcinoma associated with pregnancy: a clinicopathologic analysis of 23 cases and review of the literature. BMC Pregnancy Childbirth. 2008;8:3. doi:10.1186/1471-2393-8-3
31. Kodama M, Moeini A, Machida H, Blake EA, Grubbs BH, Matsuo K. Feto-maternal outcomes of pregnancy complicated by Krukenberg tumor: a systematic review of literature. Arch Gynecol Obstet. 2016;294(3):589–598. doi:10.1007/s00404-016-4048-3
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
