Back to Journals » Nutrition and Dietary Supplements » Volume 12
Magnitude and Its Predictors of Minimum Dietary Diversity Feeding Practice Among Mothers Having Children Aged 6–23 Months in Goba Town, Southeast Ethiopia, 2018: A Community-Based Cross-Sectional Study
Authors Gezahegn H , Tegegne M
Received 23 December 2019
Accepted for publication 30 September 2020
Published 27 October 2020 Volume 2020:12 Pages 215—222
DOI https://doi.org/10.2147/NDS.S243521
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Chandrika J Piyathilake
Habtamu Gezahegn,1 Mekonnen Tegegne2
1Medical Physiology Unit, Madda Walabu University Goba Referral Hospital, School of Medicine, Bale Goba, Ethiopia; 2Public Health Department, Madda Walabu University Goba Referral Hospital, School of Health Sciences, Bale Goba, Ethiopia
Correspondence: Habtamu Gezahegn Email [email protected]
Background: Diversified foods are considered key indicators of a balanced diet. Consumption of a minimum of four from the seven food groups is described as a minimum for dietary diversity. Nearly two-thirds of malnutrition-related child mortality is due to inappropriate feeding practice during the first two years of life. In Ethiopia, only five percent of children aged 6– 23 months received a minimum diversity diet. Therefore, this study was intended to assess the predictors of minimum diversified diet feeding practice among mothers having children aged 6– 23 months, in Goba Town, Southeast Ethiopia.
Methods: A community-based survey was employed in Bale-Goba town, Southeast Ethiopia from April to May 2018. A total of 517 study subjects were selected using systematic random sampling technique. A pretested interviewer administered questionnaire was used to collect the data. Ethical clearance was obtained from Madda Walabu University, Goba Referral Hospital. Data were entered to EpiData3.02 and analyzed using SPSS version 20, and the association between dependent and independent variables was assessed using bi-variable and multiple logistic regression. Statistical significance was considered with 95% confidence interval and p-value of less than 0.05.
Results: The proportion of children receiving minimum dietary diversity was 39.8% (95% CI 35.52, 44.08). Postnatal care visit (AOR=1.9 95% CI 1.3, 2.8) and attending growth-monitoring follow-up (AOR=1.5 95% CI 1.001, 2.2) were independent predictors, statically significant with dependent variable.
Conclusion: Almost forty percent received minimum dietary diversity among the study subjects. Attending postnatal visit and having growth-monitoring follow-up were factors associated with minimal meal frequency practice. Encouraging mothers to attend postnatal care visits and frequently bring their children to growth-monitoring follow-up is highly recommended.
Keywords: children, minimum dietary diversity, Goba Town
Background
Minimum dietary diversity is described as the proportion of children aged 6–23 months who, within 24 hours, consumed at least four of the seven food groups, to meet a balanced diet . These seven food groups include: grains, roots and tubers, legumes and nuts, dairy products, flesh foods, eggs, vitamin A-rich fruits, and vegetables, and other fruits and vegetables.1,2
According to the guidelines developed by the World Health Organization (WHO) regard Infant and Young Child Feeding (IYCF) practices for children aged 6–23 months a diversity diet is considered akey element.3 However, a comparison analysis of demographic and health survey data of Ethiopia showed that only 7% of children aged 6–23 months consumed a diversified diet.4
The magnitude of under-nutrition is higher in regions of Asia and sub-Saharan Africa.5 About one-third of child mortality globally, and more than ten percent of the total global disease burdens are due to child under nutrition.5,6 Malnutrition during the first two years of life is associated with physical, as well as cognitive problems into adulthood7 and not meeting minimum dietary diversity requirements is linked with under-nutrition.8–13
In Ethiopia, only five percent of children aged 6–23 months received minimum dietary diversity, with growth stunting, under-weight, and wasting at 38%, 24% and 10% respectively.14 Improving nutrition after the age of two years has no equally important impact on the growth and development of children like that of before two years.15
Feeding a diversified diet was significantly associated with level of education of mothers,16–20 income,16,18,21 post-natal care visit,19,21–25 age of child,23,26,27 media exposure,28,29 birth order,17,30 and child growth and monitoring follow-up.23,31
In the study area there was no research conducted on magnitude and predictors of minimum dietary diversity feeding practices. The unique feature of this study is that dietary diversity affectduring holidays and market days, data were not collected on these days and it helps to identify the usual practice of diversified diet. Therefore, this study was aimed at determining the magnitude of minimum dietary diversity feeding practices and its predictors among mothers of children aged 6–23 months residing in Goba town considering these days.
Methods
Study Design Time and Setting
A community-based cross-sectional study was conducted from April to May 2018 in Goba town, Southeast Ethiopia. Goba town is located in Bale zone, 444 km away from the capital city of Ethiopia, Addis Ababa. The climatic condition of the town is high land with the altitude of 2743 m above sea level. According to the 2018 Goba town health office report, the town has a total population of 46,845 of which 7697 are under-five children.32
Source Population, Sample Size and Sampling Technique
The source population was mothers with children aged 6–23 months, living in the study area. Sample size was calculated using single population proportion formula.33 The prevalence of minimum dietary diversity was taken as 18.8%,34 with the assumption of 95% CI and 5% margin of error. Then, the sample size calculated was 235. Using a design effect of 2 and multiplied by 2 and adding 10% non-response rate, then it became 517. Study subjects were selected from the gots (smaller administrative unit, smaller than kebele) of selected kebeles (smallest administrative unit) using systematic random sampling technique using health extension registration book as a frame. In cases where mother–child pairs were not available at home, again another visit was made.
Data Collection Tools and Procedure
The data were collected using interviewer administered questionnaire. The tools used comprised of socio-demographic characteristics of the child and the family, child and mother health service utilization, and feeding practice. Five clinical nurses and a public health officer were selected as data collectors and supervisor, respectively. Minimum dietary diversity score (MDDS) was assessed using 24-h recall method. Open recall method was to collect the data for all food groups and drinks consumed during the past 24 hours. Mothers were asked to mention food groups and drinks that she gave to her child during the previous day of the survey. Diversified diet score was calculated based on seven food groups which contain grains, legumes, dairy products, flesh foods, eggs, vitamin A rich fruits and vegetables, and other fruits and vegetables.
Operational Definition
Minimum Dietary Diversity
Receiving of at least four from the seven food groups by children of aged 6–23 months during the past 24 hours preceding the survey.35
Growth-Monitoring
A service provided to assess the nutritional status of the child monthly up to two years.
Antenatal Care
The care provided by health professionals to pregnant women in order to ensure the health of both mother and baby during pregnancy.
Postnatal Care
Includes services provided to women and newborns immediately after delivery and up to six weeks for both mother and baby after delivery.
Data Quality Issues
The tools were initially prepared in English then translated from English to Afan Oromo (local language) and then retranslated to English language. Data collectors were fluent speakers of Afan Oromo (local language). Training was given for data collectors and supervisor for two days on the objective, relevance of the study and confidentiality of information. A pretested data collection tool was used to collect data on 5% of the study subjecta out of the selected gots.
Data Processing and Analysis
The questionnaires were checked, cleaned, coded and entered to EpiData3.02 and analyzed by using SPSS version 20. A binary logistic regression model was primarily used to see the association of independent variables with minimum dietary diversity practice. Variables that had association with minimum dietary diversity feeding (at p-value of less than 0.25) in bi-variable logistic regression were entered in to the multivariable logistic regression model to control the effects of confounders.
Finally independent variables that had significant association were identified by using 95% confidence interval and calculating adjusted odds ratio, and p-value less than 0.05 was considered as statistically significant with dependent variable.
Results
Socio-Demographic and Economic Characteristics of Children and the Family
Out of 517 study subjects 503 participated, making a response rate of 97.3% (503/517). In this study, 264 (52.5%) children were males. The age categories of children were 255 (50.7%) in 6–11 months, 162 (32.2%) in 12–17 months and 86 (17.1) in 18–23 months. The highest dietary recall 365 (70.4%) was grains, roots and tubers and the least 89 (17.7%) was eggs.
The mean age of mothers was 32 years, with nearly one third 167 (32.2%) of them in age group of 30–34 years. About 71% of mothers of infant and young children attended primary education. More than of half 272 (53.3%) of the respondents were governmental workers. (Table 1)
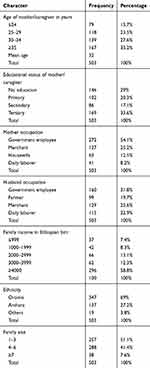 |
Table 1 Socio-Demographic Characteristics of Mothers Having Children Aged 6–23 Months Who Participated in The Study in Goba Town, Southeast Ethiopia, 2018 (N=503) |
Maternal Health Care Utilization Characteristics
More than half, 288 (57.3%) of the children’s mothers followed antenatal care (ANC). About 56.1% of the subjects were delivered at Health institution. Beside that 87.1% followed postnatal care. (Table 2)
 |
Table 2 Maternal Obstetric-Related Characteristics of Mothers Having Children 6–23 Months on Minimum Dietary Diversity Feeding Practices, Goba Town, Southeast Ethiopia, 2018 (N=503) |
Minimum Dietary Diversity Practice
Magnitude of children who receivedmet the diversified diet score was 39.8% (95% CI; 35.52, 44.08). Around 70.4% of children received food from roots, grains and tubers. About 18% of them received eggs in the previous 24h of the survey. (Table 3)
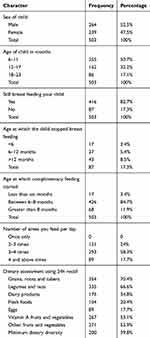 |
Table 3 Feeding Practice of Mothers Having Children Aged 6–23 Months Who Participated in the Study of Minimum Dietary Diversity Feeding Practice in Goba Town, Southeast Ethiopia, 2018 (N=503) |
Independent Predictors Statistically Significant with Diversified Feeding Practice
Owning a television, owning a radio, maternal occupation, occupation of father, place of delivery, following growth-monitoring, attending antenatal care and attending postnatal care visits were independent predictors significantly associated with dietary diversity score in bi-variable regression analysis. However, in multiple logistic analysis postnatal care visit 1.9 (1.3, 2.8) and attending growth-monitoring follow-up 1.5 (1.001, 2.22) were the only independent predictors statistically significant with the outcome variable. (Table 4)
 |
Table 4 Factors Associated with Minimum Dietary Diversity Feeding Practice Among Mothers Having Children Aged 6–23 Months in Goba Town, Southeast Ethiopia, 2018 (N=503) |
Discussion
The magnitude of children who received minimum diversified food was 38.9% (95% CI; 35.52, 44.08). The result of the present study is comparable with other studies done in Khalkhal city, North West Iran (42.3%); Tanzania (38.2%); Zambia (37%); and Wolaita Zone (43.2%).4,36–38
However, this result is higher than other studies done in Odisha in India (27.4%), Ghana (35.3%), Nepal (34%), Pakistan (22%), Benishangul Gumuz Region (23.7%), Damot Sore district in South Ethiopia (16.5%), Afar (30.8%), and slum areas around Bahir Dar city (7%).19,21–25,27,29,31,34,39–58 On the other hand the finding of this study is lower than in studies conducted in China (51.7%), Indonesia (50%), Ghana (46%), Sri Lanka (71%) and in Addis Ababa (59.9%).12,18,28,59–63 The possible explanation for the difference could be due to differences in socioeconomic status, variation in study settings and time gaps in the study.
After controlling the effect of confounding variables in multivariable regression analysis postnatal care service visit and child growth and monitoring follow-up were factors associated with dietary diversity practice.
Postnatal care service attendance was significantly associated with feeding practice of diversified foods. Odds of feeding diversified diet were 1.5 (AOR) times more likely among mothers who attended postnatal care service visits. The present study is in line with studies conducted in Goche district in South Ethiopia,22 Dabat district in Northwest Ethiopia23 and in Agrarian society of Bale Zone in Southeast Ethiopia.19
Mothers who took their child to a health facility for growth-monitoring follow-ups were 1.9 (AOR) times more likely to provide diversified diet than those who did not participate in growth-monitoring follow-up. The present study was comparable with a study conducted in Dabat district in Northwest Ethiopia.23
This could be due to the fact that growth monitoring follow-up has potential opportunity for getting health and nutritional knowledge.
Not all mothers used these services due to reasons such as lack of knowledge and attitude toward the service. Health workers, health extension workers and community volunteers play a great role in improving these services during thehome visits of these mothers.
Study Strength and Limitation
The study strength was data were not collected during holidays and market days and its limitation was that as 24 hours recall of dietary feeding was used, recall biasmight exist.
Conclusion
Even though feeding of a diversified diet practice of infant and young children aged 6–23 months was slightly higher than previous studies done in Ethiopia, it is still lower than many African countries. Attending postnatal care visit and child growth-monitoring follow-up monthly at a health facility were factors associated with minimum dietary diversity feeding practice.
Abbreviations
ANC, antenatal care; AOR, adjusted odds ratio; CI, confidence interval; COR, crude odds ratio; IYCF, Infant and Young Child Feeding; MDD, minimum dietary diversity; PNC, postnatal care; WHO, World Health Organization.
Data Sharing Statement
The data sets of the current study are available from the corresponding author on reasonable request.
Ethical Approval and Consent to Participate
Verbal informed consent was obtained from all study participants and it is acceptable and approved by the Madda Walabu University, Goba Referral Hospital, this study was conducted in accordance with the Declaration of Helsinki.
Acknowledgment
We would like to thank study participants, data collectors, supervisors and Goba town health office.
Author Contributions
Both authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was obtained for this study.
Disclosure
The authors have no conflicts of interest.
References
1. WHO U, USAID A, UCDAVIS I. Indicators for Assessing Infant and Young Child Feeding Practices-Part I: Definitions; 2008. Geneva: WHO; 2008.
2. WHO. Indicators for Assessing Infant and Young Child Feeding Practices: Conclusions of a Consensus Meeting Held 6–8 November 2007. Washington DC, USA: World Health Organization (WHO); 2008.
3. WHO. Guiding Principles for Complementary Feeding of the Breastfed Child. Geneva: WHO; 2003.
4. Disha A, Rawat R, Subandoro A, Menon P. Infant and young child feeding (IYCF) practices in Ethiopia and Zambia and their association with child nutrition: analysis of demographic and health survey data. African J Food Agric Nutr Dev. 2012;12(2):5895–5914.
5. Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. lancet. 2008;371(9608):243–260. doi:10.1016/S0140-6736(07)61690-0
6. Ezzati M, Lopez AD, Rodgers A, Vander HS, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360(9343):1347–1360. doi:10.1016/S0140-6736(02)11403-6
7. Victora CG, Adair L, Fall C, et al. Maternal and child undernutrition: consequences for adult health and human capital. lancet. 2008;371(9609):340–357. doi:10.1016/S0140-6736(07)61692-4
8. Woldie H, Kebede Y, Tariku A. Factors associated with anemia among children aged 6–23 months attending growth monitoring at Tsitsika Health Center, Wag-Himra Zone, Northeast Ethiopia. J Nutr Metab. 2015;2015:1–9. doi:10.1155/2015/928632
9. Hirvonen K. Rural–urban differences in children’s dietary diversity in Ethiopia: a poisson decomposition analysis. Econ Lett. 2016;147:12–15. doi:10.1016/j.econlet.2016.08.003
10. Ahmad I, Khalique N, Khalil S, Maroof M. Dietary diversity and stunting among infants and young children: a cross-sectional study in Aligarh. Indian J Community Med. 2018;43(1):34. doi:10.4103/ijcm.IJCM_382_16
11. Zongrone A, Winskell K, Menon P. Infant and young child feeding practices and child undernutrition in Bangladesh: insights from nationally representative data. Public Health Nutr. 2012;15(9):1697–1704. doi:10.1017/S1368980012001073
12. Wang A, Scherpbier RW, Huang X, et al. The dietary diversity and stunting prevalence in minority children under 3 years old: a cross-sectional study in forty-two counties of Western China. Br J Nutr. 2017;118(10):840–848. doi:10.1017/S0007114517002720
13. Moges T, Birks KA, Samuel A, et al. Diet diversity is negatively associated with stunting among Ethiopian Children 6–35 months of age. Eur J Nutr Food Saf. 2015;5(5):1185–1186. doi:10.9734/EJNFS/2015/21313
14. Demographic E. Health Survey: Addis Ababa. Ethiopia and Calverton, Maryland, USA: Central Statistics Agency and ORC Macro. 2011.
15. Research NIoP, Training, Mitra, Associates, International M. Bangladesh Demographic and Health Survey, 2007. NIPORT; 2009.
16. Aemro M, Mesele M, Birhanu Z, Atenafu A. Dietary diversity and meal frequency practices among infant and young children aged 6–23 months in Ethiopia: a secondary analysis of Ethiopian demographic and health survey 2011. J Nutr Metab. 2013;2013:1–8. doi:10.1155/2013/782931
17. Beyene M, Worku AG, Wassie MM. Dietary diversity, meal frequency and associated factors among infant and young children in Northwest Ethiopia: a cross-sectional study. BMC Public Health. 2015;15(1):1007. doi:10.1186/s12889-015-2333-x
18. Solomon D, Aderaw Z, Tegegne TK. Minimum dietary diversity and associated factors among children aged 6–23 months in Addis Ababa, Ethiopia. Int J Equity Health. 2017;16(1):181. doi:10.1186/s12939-017-0680-1
19. Tegegne M, Sileshi S, Benti T, Teshome M, Woldie H. Factors associated with minimal meal frequency and dietary diversity practices among infants and young children in the predominantly agrarian society of bale zone, Southeast Ethiopia: a community based cross sectional study. Arch Public Health. 2017;75(1):53. doi:10.1186/s13690-017-0216-6
20. Ogbo FA, Ogeleka P, Awosemo AO. Trends and determinants of complementary feeding practices in Tanzania, 2004–2016. Trop Med Health. 2018;46(1):40. doi:10.1186/s41182-018-0121-x
21. Kumera G, Tsedal E, Ayana M. Dietary diversity and associated factors among children of orthodox christian mothers/caregivers during the fasting season in Dejen District, North West Ethiopia. Nutr Metab. 2018;15(1):16. doi:10.1186/s12986-018-0248-0
22. Dangura D, Gebremedhin S. Dietary diversity and associated factors among children 6–23 months of age in Gorche district, Southern Ethiopia: cross-sectional study. BMC Pediatr. 2017;17(1):6. doi:10.1186/s12887-016-0764-x
23. Belew AK, Ali BM, Abebe Z, Dachew BA. Dietary diversity and meal frequency among infant and young children: a community based study. Ital J Pediatr. 2017;43(1):73. doi:10.1186/s13052-017-0384-6
24. Temesgen H, Yeneabat T, Teshome M. Dietary diversity and associated factors among children aged 6–23 months in Sinan Woreda, Northwest Ethiopia: a cross-sectional study. BMC Nutr. 2018;4(1):5. doi:10.1186/s40795-018-0214-2
25. Liben M, Abuhay T, Haile Y. Factors associated with dietary diversity among children of agro pastoral households in Afar regional state. Northeastern Ethiopia. 2017;2017:5.
26. Gebremedhin S, Baye K, Bekele T, et al. Predictors of dietary diversity in children ages 6 to 23 mo in largely food-insecure area of south Wollo, Ethiopia. Nutrition. 2017;33:163–168. doi:10.1016/j.nut.2016.06.002
27. Mekonnen TC, Workie SB, Yimer TM, Mersha WF. Meal frequency and dietary diversity feeding practices among children 6–23 months of age in Wolaita Sodo town, Southern Ethiopia. J Health Popul Nutr. 2017;36(1):18. doi:10.1186/s41043-017-0097-x
28. Ahmad A, Madanijah S, Dwiriani CM, Kolopaking R. Complementary feeding practices and nutritional status of children 6–23 months old: formative study in Aceh, Indonesia. Nutr Res Pract. 2018;12(6):512–520. doi:10.4162/nrp.2018.12.6.512
29. Eshete T, Kumera G, Bazezew Y, Mihretie A, Marie T. Determinants of inadequate minimum dietary diversity among children aged 6–23 months in Ethiopia: secondary data analysis from Ethiopian demographic and health survey 2016. Agric Food Secur. 2018;7(1):66. doi:10.1186/s40066-018-0219-8
30. Ahmad I, Khalique N, Khalil S, Maroof M. Complementary feeding practices among children aged 6–23 months in Aligarh, Uttar Pradesh. J Family Med Prim Care. 2017;6(2):386. doi:10.4103/jfmpc.jfmpc_281_16
31. Gatahun A, Abyu M. Dietary diversity feeding practice and determinants among children aged 6–23 months in Kemba Woreda, southern Ethiopia implication for public health intervention. Nutr Food Sci. 2015;s13. doi:10.4172/2155-9600.S13-003
32. Goba town health office report 2018.
33. Daniel WW. Biostatistics; a Foundation for Analysis in the Health Sciences. 1974.
34. Kassa T, Meshesha B, Haji Y, Ebrahim J. Appropriate complementary feeding practices and associated factors among mothers of children age 6–23 months in Southern Ethiopia, 2015. BMC Pediatr. 2016;16(1):131. doi:10.1186/s12887-016-0675-x
35. WHO. Indicators for Assessing Infant and Young Child Feeding Practices: Part 2: Measurement. 2010.
36. Kamran A, Sharifirad G, Nasiri K, Soleymanifard P, Savadpour M, Akbar Haghighat M. Determinants of complementary feeding practices among children aged 6–23: a community based study. Int J Pediatr. 2017;5(3):4551–4560.
37. Victor R, Baines SK, Agho KE, Dibley MJ. Factors associated with inappropriate complementary feeding practices among children aged 6–23 months in T anzania. Matern Child Nutr. 2014;10(4):545–561. doi:10.1111/j.1740-8709.2012.00435.x
38. Sagaro GG, Alemayehu M. Dietary diversity and associated factors among infants and young children in Wolaita Zone, Southern Ethiopia. Sci J Clin Med. 2017;6(4):53. doi:10.11648/j.sjcm.20170604.12
39. Kar S, Bhattacharjee S, Samantaray P, Biswal S. Infant and young child feeding practices among marginalized populations of Odisha, state in India. J Epidemiol Res. 2015;2(1):39. doi:10.5430/jer.v2n1p39
40. Saaka M, Larbi A, Mutaru S, Hoeschle-Zeledon I. Magnitude and factors associated with appropriate complementary feeding among children 6–23 months in northern Ghana. BMC Nutr. 2016;2(1):2. doi:10.1186/s40795-015-0037-3
41. Saaka M, Wemakor A, Abizari A-R, Aryee P. How well do WHO complementary feeding indicators relate to nutritional status of children aged 6–23 months in rural Northern Ghana? BMC Public Health. 2015;15(1):1157. doi:10.1186/s12889-015-2494-7
42. Udoh EE, Amodu OK. Complementary feeding practices among mothers and nutritional status of infants in Akpabuyo Area, Cross River State Nigeria. SpringerPlus. 2016;5(1):2073. doi:10.1186/s40064-016-3751-7
43. Khanal V, Sauer K, Zhao Y. Determinants of complementary feeding practices among Nepalese children aged 6–23 months: findings from demographic and health survey 2011. BMC Pediatr. 2013;13(1):131. doi:10.1186/1471-2431-13-131
44. Joshi N, Agho KE, Dibley MJ, Senarath U, Tiwari K. Determinants of inappropriate complementary feeding practices in young children in Nepal: secondary data analysis of demographic and health survey 2006. Matern Child Nutr. 2012;8:45–59. doi:10.1111/j.1740-8709.2011.00384.x
45. Na M, Aguayo VM, Arimond M, Stewart CP. Risk factors of poor complementary feeding practices in Pakistani children aged 6–23 months: a multilevel analysis of the demographic and health survey 2012–2013. Matern Child Nutr. 2017;13:e12463. doi:10.1111/mcn.12463
46. Heidkamp RA, Ayoya MA, Teta IN, Stoltzfus RJ, Marhone JP. Complementary feeding practices and child growth outcomes in Haiti: an analysis of data from demographic and health surveys. Matern Child Nutr. 2015;11(4):815–828. doi:10.1111/mcn.12090
47. Gautam KP, Adhikari M, Khatri RB, Devkota MD. Determinants of infant and young child feeding practices in Rupandehi, Nepal. BMC Res Notes. 2016;9(1):135. doi:10.1186/s13104-016-1956-z
48. Aguayo VM. Complementary feeding practices for infants and young children in South Asia. A review of evidence for action post‐2015. Matern Child Nutr. 2017;13:e12439. doi:10.1111/mcn.12439
49. Rakotomanana H, Gates GE, Hildebrand D, Stoecker BJ. Situation and determinants of the infant and young child feeding (IYCF) indicators in Madagascar: analysis of the 2009 demographic and health survey. BMC Public Health. 2017;17(1):812. doi:10.1186/s12889-017-4835-1
50. Na M, Aguayo VM, Arimond M, Mustaphi P, Stewart CP. Predictors of complementary feeding practices in Afghanistan: analysis of the 2015 demographic and health survey. Matern Child Nutr. 2018;14(S4):e12696. doi:10.1111/mcn.12696
51. Ayana D, Tariku A, Feleke A, Woldie H. Complementary feeding practices among children in Benishangul Gumuz Region, Ethiopia. BMC Res Notes. 2017;10(1):335. doi:10.1186/s13104-017-2663-0
52. Roba KT, O’Connor TP, Belachew T, O’Brien NM. Infant and young child feeding (IYCF) practices among mothers of children aged 6–23 months in two agro-ecological zones of rural Ethiopia. Int J Nutr Food Sci. 2016;5(3):185–194.
53. Areja A, Yohannes D, Yohannis M. Determinants of appropriate complementary feeding practice among mothers having children 6–23 months of age in rural Damot sore district, Southern Ethiopia; a community based cross sectional study. BMC Nutr. 2017;3(1):82. doi:10.1186/s40795-017-0202-y
54. Mekbib E, Shumey A, Ferede S, Haile F. Magnitude and factors associated with appropriate complementary feeding among mothers having children 6–23 months-of-age in northern Ethiopia; a community-based cross-sectional study. J Food Nutr Sci. 2014;2(2):36.
55. Ogbo FA, Page A, Idoko J, Claudio F, Agho KE. Trends in complementary feeding indicators in Nigeria, 2003–2013. BMJ Open. 2015;5(10):e008467. doi:10.1136/bmjopen-2015-008467
56. Wondu Garoma B, Yang N. Determinants of suboptimal complementary feeding practices among children aged 6–23 months in selected urban slums of Oromia Zones (Ethiopia). J Nutr Food Sci. 2017;7(593):2.
57. Gessese D, Bolka H, Alemu Abajobir A, Tegabu D. The practice of complementary feeding and associated factors among mothers of children 6–23 months of age in Enemay district, Northwest Ethiopia. Nutr Food Sci. 2014;44(3):230–240. doi:10.1108/NFS-07-2013-0079
58. Demilew YM, Tafere TE, Abitew DB. Infant and young child feeding practice among mothers with 0–24 months old children in slum areas of Bahir Dar City, Ethiopia. Int Breastfeed J. 2017;12(1):26. doi:10.1186/s13006-017-0117-x
59. Hipgrave D, Fu X, Zhou H, et al. Poor complementary feeding practices and high anaemia prevalence among infants and young children in rural central and western China. Eur J Clin Nutr. 2014;68(8):916. doi:10.1038/ejcn.2014.98
60. Issaka AI, Agho KE, Burns P, Page A, Dibley MJ. Determinants of inadequate complementary feeding practices among children aged 6–23 months in Ghana. Public Health Nutr. 2015;18(4):669–678. doi:10.1017/S1368980014000834
61. Senarath U, Godakandage SS, Jayawickrama H, Siriwardena I, Dibley MJ. Determinants of inappropriate complementary feeding practices in young children in Sri Lanka: secondary data analysis of demographic and health survey 2006–2007. Matern Child Nutr. 2012;8:60–77. doi:10.1111/j.1740-8709.2011.00375.x
62. Chakraborty B, Rumana J, Begum HA, Afroz A. Infant and young child feeding pattern in children attending in the outpatient department of an urban hospital. Bangladesh J Child Health. 2016;40(2):92–97. doi:10.3329/bjch.v40i2.31564
63. Olatona FA, Adenihun JO, Aderibigbe SA, Adeniyi OF. Complementary feeding knowledge, practices, and dietary diversity among mothers of under-five children in an urban community in Lagos State, Nigeria. Int J MCH AIDS. 2017;6(1):46. doi:10.21106/ijma.203
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
