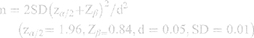Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 12
Magnesium Sulfate Improves Some Risk Factors for Atherosclerosis in Patients Suffering from One or Two Coronary Artery Diseases: A Double-blind Clinical Trial Study
Authors Sobhani AR, Farshidi H, Azarkish F , Eslami M, Eftekhar E, Keshavarz M , Soltani N
Received 12 May 2020
Accepted for publication 7 August 2020
Published 25 September 2020 Volume 2020:12 Pages 159—169
DOI https://doi.org/10.2147/CPAA.S261264
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Arthur E. Frankel
Ali Reza Sobhani,1 Hossein Farshidi,2 Fariba Azarkish,3 Mahdiya Eslami,2 Ebrahim Eftekhar,4 Mansoor Keshavarz,5 Nepton Soltani6
1Clinical Pathology Department, Faculty of Medicine, Hormozgan University of Medical Sciences, Bandar Abbas, Iran; 2Cardiovascular Research Center, Hormozgan University of Medical Sciences, Bandar Abbas, Iran; 3Molecular Medicine Research Center, Hormozgan Health Institute, Hormozgan University of Medical Sciences, Bandar Abbas, Iran; 4Endocrinology and Metabolism Research Center, Hormozgan University of Medical Sciences, Bandar Abbas, Iran; 5Physiology Department, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran; 6Physiology Department, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
Correspondence: Nepton Soltani
Physiology Department, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
Tel +98 9133251067
Fax +98 36688597
Email [email protected]
Purpose: Given the beneficial effect of MgSO4 on the cardiovascular system, this study was designed to investigate the effect of MgSO4 administration on suppressing some atherosclerotic risk factors in moderate coronary artery disease patients with one or two atherosclerotic vessels.
Patients and Methods: In a randomized double-blind placebo-controlled clinical trial study, 64 patients with moderate coronary artery disease (55– 69% stenosis) were selected according to angiography findings. Patients were divided into four groups including patients with one or two atherosclerotic vessels treated with MgSO4 (Mg-treated-VR1, Mg-treated-VR2, respectively), placebo treated patients with one or two atherosclerotic vessels (Control-VR1, Control-VR2, respectively). The patients received either placebo or MgSO4 supplement capsule containing 300 mg MgSO4 for six months on a daily basis. ESR, Ca/Mg ratio, urine Mg level, serum Mg, fibrinogen, homocysteine, uric acid, Na, K, Ca, CRP, T3, T4, TSH, BUN, and Cr concentrations were measured at baseline and every three months.
Results: Serum T3, Ca, K, homocysteine, CRP, and Mg concentrations were significantly improved in Mg-treated groups compared to placebo groups.
Conclusion: The results of this study showed that despite the slight change in serum magnesium level, oral administration of MgSO4for six months could slightly reduce the serum levels of some inflammatory and vascular factors in moderate coronary artery disease patients.
Keywords: atherosclerosis, MgSO4, fibrinogen, CRP, homocysteine, thyroid hormones
Introduction
Cardiovascular diseases are spreading rapidly among human population; hypertension and atherosclerosis are major causes of the disease.1 Cardiologists are eagerly looking for a way to prevent or stop the process of atherosclerosis. Many factors such as serum Ca and uric acid levels are involved in atherosclerosis induction.2 Intracellular Ca modulates LDL transport across endothelial cells3 and it can change to oxLDL. oxLDL is taken up by macrophages and vascular smooth muscle cells via a different kind of scavenger receptor and this is the first step in the development of atherosclerotic plaques.4 Studies have also shown that Ca is the most important cation that can increase catecholamine secretion from the adrenal gland, and catecholamine in turn increases the release of fatty acids from the adipose tissue.5,6 Burn et al reported that sympathetic stimulation to release catecholamine from adrenal gland is dependent on plasma Ca concentration and this action could be antagonized by Mg.7 Homocysteine is another risk factor for atherosclerosis induction and it is known to mediate cardiovascular problems through its opposing properties affecting the cardiovascular endothelium and smooth muscle cells with secondary alterations in subclinical arterial structure and function.8 It also might trigger the release of catecholamines.8 Fang et al demonstrated that homocysteine accelerated atherosclerosis in mice.9
Some studies considered fibrinogen as a major cardiovascular risk factor10,11 and they showed that the independent link between higher fibrinogen level and presence of coronary atherosclerosis.12 Moreover, there is a significant correlation between Mg deficiency and cardiovascular problems such as atherosclerosis by increasing plasma LDL level and promoting inflammation.13–15 Low serum Mg is associated with an increased risk of coronary heart disease mortality and sudden cardiac death.16 Studies have shown that low plasma Mg level causes endothelial dysfunction, which is another step for atherosclerotic plaque formation.14 Some researchers argued that Mg therapy could control BP better, improve endothelial function, and ameliorate subclinical atherosclerosis in hypertensive women.17 Orimoand et al found that Mg could suppress the development of atherosclerotic lesions in the aorta of cholesterol-fed rabbits without any changes on plasma total cholesterol and HDL-cholesterol levels.3 Hangyuan Guo et al showed the beneficial effect of Mg on the pathogenesis of coronary artery diseases by managing homocysteine-induced vessel problem.18 Our previous studies19–22 showed that in addition to the antidiabetic effect of MgSO4, it also could improve blood pressure, lipid profile, plasma oxLDL level and decrease LOX1 gene and protein expression in diabetic vessels in rats. Our previous findings also showed that MgSO4 could decrease the sensitivity of the diabetic vessels to catecholamine and improve vessel structure and function.
Given the beneficial effect of MgSO4 on the cardiovascular system as discussed above, this study attempted to investigate the effect of MgSO4 administration on suppressing some atherosclerotic risk factors in moderate coronary artery disease patients with one or two atherosclerotic vessels.
Patients and Methods
Present study is a randomized, double-blind, placebo-controlled trial study; and it was conducted in accordance with principles of good clinical practice and the ethical principles that have their origin in the Declaration of Helsinki. The Ethics Committee of Hormozgan University of Medical Sciences (ethic number IR.HUMS.REC.1397.019) has approved the study and it has been registered in the Iranian Registry of Clinical Trials (IRCT20151028024756N3 with registration number 29,097). All data of the present study have been filed in the angiography department of HUMS, furthermore, all our data is available in the Iranian Registry of Clinical Trials. All patients have completed an informed consent form and then entered the study; based on the completed form, all patients were allowed to publish the study results. This study has maintained the confidentiality of information and privacy of the participants. All participants and researchers were blinded to the study protocol, but the study predictor had access to the data and patients’ information. Sample randomization was allocated between groups using a computer-generated sequence of random numbers. The research flow diagram is shown in Figure 1.
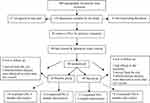 |
Figure 1 Study flow diagram. |
Participants
According to the SD for homocysteine ratio (which was determined in the pilot study) and the biostatistician suggestion, the sample size in this study was determined using following formula:
Angiography was done through femoral access and it evaluated epicardial coronary arteries including: (1) left anterior descending artery and major diagonal branches with larger than 2 mm in diameter; (2) left circumflex and large obtuse marginal or left PLV or left PDA if present; (3) right coronary artery and branches larger than 2 mm. According to angiography findings, patients with moderate coronary artery disease having one or two major coronary arteries with 55–69% stenosis were selected. Women over 55 and men over 45 were included and their medications were adjusted. The study exclusion criteria were as follows: pregnancy, taking Mg and Ca containing supplements, having renal insufficiency (serum Cr levels more than 1.3 mg/dL in women and more than 1.5 mg/dL in men), having increased hepatic enzymes (more than three folds over normal values), current infections (less than one month prior to study) and chronic inflammatory diseases, CVA, cancer and drug sensitivity. Atorvastatin, aspirin, clopidogrel, metoprolol, nitrate, losartan, and metformin were used for treatment. Based on randomization, patients were put into the following groups: (1) MgSO4 treated moderate coronary artery disease patients with one atherosclerotic vessel (Mg-treated-VR1); (2) MgSO4 treated moderate coronary artery disease patients with two atherosclerotic vessels (Mg-treated-VR2); (3) placebo treated moderate coronary artery disease patients with one atherosclerotic vessel (Control-VR1); (4) placebo treated moderate coronary artery disease patients with two atherosclerotic vessels (Control-VR2). Mg-treated groups received one capsule containing 300 mg of MgSO4 every morning for six months. The participants in placebo groups also received the same capsule containing wheat flour every morning for six months (Figure 1). Patients in all groups had the usual diet and performed physical activity during the study; only eight patients in each groups did not participate in the study completely. The patients were followed-up in person every month to see if they adhered to pharmacological treatment. The same cardiologist blinded to the patients grouping visited all patients every three months. Baseline and every three months fasting blood samples were taken. Serum was separated by centrifugation at 1500 g for 10 min and stored at −80°C until assay of biomedical markers.
Study Outcomes
The primary outcome was based on angiography findings, homocysteine, serum CRP and Mg levels, Ca/Mg ratio as well as urine Mg level measured prior to the intervention and at three-month intervals after the intervention. The secondary outcome was based on serum Cr, BUN, fibrinogen, ESR, uric acid, Na, K, Ca, T3, T4, and TSH concentrations measured during the abovementioned periods.
Measurements
The same weight and meter were used for measuring all participants’ in terms of height and weight.
The weight was measured while the participant was clothed scantily with no shoes. To determine BMI, weight in kilograms was divided by height in square meters.23 CLIA was applied to measure the thyroid function tests including serum total T3, total T4, and TSH. Serum Mg, Ca, K, Na, homocysteine, fibrinogen, uric acid, and urine Mg levels were measured using appropriate kits (Pars Azmun, Iran).
Statistical Analysis
The findings were shown as mean ±SD; the normality of all variables was studied using Kolmogorov–Smirnov test. Student’s t-test and one way ANOVA with Tukey's post hoc test were performed to analyze the likely variations between case and control group prior to intervention.
Student’s t-test and one way ANOVA with Tukey's post hoc test were done to compare the groups and study the likely differences between means of each group during the intervention. SPSS software, version 13, was applied to perform the analyses and P<0.05 was considered statistically significant.
Results
Sixty-four participants took part in the current study, and based on randomization, they were put into MgSO4 treated moderate coronary artery disease patients with one atherosclerotic vessel (Mg-treated-VR1, N=13), MgSO4 treated moderate coronary artery disease patients with two atherosclerotic vessels (Mg-treated-VR2, N=19), placebo treated moderate coronary artery disease patients with one atherosclerotic vessel (Control-VR1, N=13) and placebo treated moderate coronary artery disease patients with two atherosclerotic vessels (Control-VR2, N=19). The MgSO4-treated groups took 300 mg MgSO4 capsules daily. The placebo groups took placebo capsules. Eight participants in each MgSO4-treated and placebo group discontinued the study during the follow-up period because of migration or failing to fit eligibility criteria. The remaining participants in all groups underwent the complete six-month intervention successfully (Figure 1). Tables 1–3 show the study variables prior to the interventions.
In Table 4, all variables before intervention were compared in VR1 and VR2 patients. As shown in Table 4, our findings showed that ESR, serum Cr, uric acid, and T4 levels in VR1 participants were significantly less than VR2 patients before intervention, and serum T3 level was significantly greater than VR2 participants.
There were no statistical differences between Mg-treated-VR1 and control-VR1 groups before intervention (Tables 1 and 2 and Figure 2). No statistical differences were also seen between Mg-treated-VR2 and control-VR2 groups before intervention (Tables 1 and 2 and Figure 2). Nevertheless, significant differences were observed between ESR, serum homocysteine, and T4 concentrations between VR1 and VR2 in Mg-treated and control groups before intervention (Figures 3 and 4 respectively).
According to the results in Figure 2, MgSO4 administration for three months could significantly decrease Ca/Mg ratio, serum Ca, T3, and CRP levels in Mg-treated-VR1 compared to control-VR1 groups. MgSO4 therapy also could significantly increase serum K, Mg, and homocysteine levels in Mg-treated-VR1 compared to the control-VR1 groups. Serum homocysteine level was also significantly increased in Mg-treated-VR2 groups compared to control-VR2 patients (Figures 2–4).
According to Figures 2 and 3, three months after MgSO4 therapy in VR1 group, significant changes were observed in some data such as serum ESR, Ca/Mg ratio and serum CRP level if compared with their levels before intervention. Serum Ca level, ESR and Ca/Mg ratio in control-VR2 group was significantly decreased compared to the levels before intervention (Figures 2 and 3). Serum homocysteine levels in both VR1 control and magnesium treated groups before the intervention were less compared to VR2 patients in both groups (Figure 3).
Six months after intervention, our results showed significant changes between Mg-treated-VR2 and control-VR2 groups in terms of Ca/Mg ratio, serum Ca, Mg and homocysteine levels (Figures 2 and 3). Significant changes in serum levels of magnesium and CRP were observed between Mg-treated-VR1 and control-VR1 six months after the intervention (Figures 2 and 3).
Discussion
The current study was a randomized double-blind clinical trial conducted on patients suffering from one or two coronary artery diseases. The results of the present study showed that ESR, homocysteine, and T4 concentrations were significantly lower in Mg-treated-VR1 and control-VR1 groups compared to Mg-treated-VR2 and control-VR2 groups before intervention, respectively. Administration of 300 mg/daily MgSO4 for six months significantly improved some atherosclerotic risk factors such as serum homocysteine, CRP, Ca, T3, K, and Mg levels compared to placebo received groups.
Based on our previous studies19,20 in chronic diabetic animal models, it was shown that oral administration of MgSO4 could dilate the vessels and improve atherosclerosis. It is not clear how Mg reduces atherosclerosis, but according to our previous findings, Mg is likely to improve atherosclerosis by reducing plasma oxLDL level and its receptor in endothelial cells.19 Moreover, hypomagnesemia increases the intracellular calcium level. Ca plays an important role in signaling pathways such as activation of NMDA receptor, which, in turn, causes the release vasoconstrictor agents, increase lipid peroxidation and NF-κB activation in smooth arteries. All these mechanisms can increase vessel atherosclerosis.24 We decided to design the present study to investigate the effect of MgSO4 administration on suppressing some atherosclerotic factors in moderate coronary artery disease patients with one or two atherosclerotic vessels.
Most of the cardiovascular patients were shown to be hypomagnesemic.17,23 The inverse relationships of the Mg levels in serum and urine with cardiovascular disease suggest a protective role of Mg in the process of atherosclerosis.25 Mg improves endothelial function and plays a role as a regulator of platelet aggregation. Mg also can prevent vasospasm and endothelial damage by stimulating endothelial proliferation and increase nitric oxide production.26 In the present study, serum Mg level in all participants in all groups was lower than the normal range and MgSO4 therapy could increase serum Mg level. Clinically, 1% of Mg exists extracellularly and plasma Mg level does not always truly reflect total body Mg stores.23 Serum Mg levels in all groups increased almost equally during the study period, but in both Mg-treated groups during the first three months, the increase was larger. Nevertheless, according to our findings, the administration of Mg reduced some atherosclerosis risk factors. Some studies showed the relationships between serum and urine Mg levels and serum lipids.25 Many findings from epidemiological studies,27 randomized controlled trials,28 and meta-analyses29,30 have illustrated the opposite relationships between Mg consumption and CVD. The researchers concluded that high Mg consumption and higher levels of circulating Mg are related to lower risk of major cardiovascular risk factors such as metabolic syndrome, diabetes, hypertension, stroke, lower risk of CVD, mainly ischemic heart disease and coronary heart disease respectively, by improving glucose and insulin metabolism, enhancing endothelium-dependent vasodilation, and improving lipid profile.31 Mg deficiency promotes the generation of atherosclerotic plaques in patients with hypomagnesemia.26 Studies showed that fibrinogen represents a major cardiovascular risk factor.10,12 Fibrinogen also represents an acute phase protein and it can associate with atherosclerosis.11 According to our results, six months after MgSO4 therapy, serum fibrinogen levels significantly decreased compared to its level at baseline and three months after intervention. Wang et al, in their cohort study, illustrated that serum fibrinogen level was not associated with coronary vulnerability and they reported independent association between plasma fibrinogen levels and the risk of CAD.32 But Papageorgiouand et al showed that fibrinogen is an inflammatory biomarker having a stronger effect on CAD and they believed that in some cases fibrinogen has a stronger effect on CAD risk than other risk factors such as hypertension.33 Homocysteine could be used as a potential marker for atherosclerosis progression.34,35 The result of this study indicated that administration of Mg could decrease serum levels of homocysteine in our patients.
Recently, CRP has attracted much attention, because its blood concentration increases even under chronic inflammatory conditions, such as the development of atherosclerosis.36,37 Mg has an anti-inflammatory effect by modulating the release of NF-κB, and decreased oxygen radicals production.26 Our results showed that MgSO4 therapy could decrease the serum level of CRP compared to the placebo group. ESR and Ca/Mg ratios are known as inflammatory indexes.
Mg controls the activity of some ionic channels, such as Na, K, and Ca.26 Reduced dietary K promoted atherosclerotic vascular calcification and increased aortic stiffness.38 Study also showed that low serum K is associated with stroke in people with cardiovascular disease, hypertension and diabetes mellitus.39 Our results showed that MgSO4 therapy could prevent hypokalemia in MgSO4-treated groups. There was a positive association between free thyroxine levels in middle-aged and elderly subjects and atherosclerosis throughout the whole disease spectrum, which was not dependent on cardiovascular risk factors.40 Moreover, there is a link between higher thyroid hormone levels and systolic hypertension and hypercoagulation, however, hyperlipidemia and inflammation could be initiated by lower circulating thyroid hormone concentration.40 Many epidemiological studies41–46 investigated the link between specific ranges of thyroid function and different atherosclerotic events and they found no link between thyroid function and atherosclerotic outcomes. Nevertheless, other studies observed an increased risk of atherosclerotic outcomes with either lower41,44 or higher thyroid function.43,45,47 Our results showed that three months after MgSO4 therapy serum level of T3 in the Mg-treated-VR2 group significantly decreased compared to the control-VR2 group.
ESR is a traditional biomarker for atherosclerosis because atherosclerosis is a chronic inflammation and the erythrocyte sedimentation rate is a suitable test for observing chronic inflammatory reactions.48 Our results showed a significant difference in ESR in both Mg-treated-VR1 and VR2 patients compared with control-VR1 and VR2 groups. In many studies, Mg has been introduced as an anti-inflammatory factor. In a study by Ige and Adewoye, oral Mg administration reduced the rate of inflammation in diabetic rats and reduced ESR levels.49 Researchers have also shown that elevated liver Mg levels may decrease ESR level in obese gastric bypass patients.50 However, chronic kidney disease due to atherosclerosis develops cardiovascular problems.51 Nevertheless, the results of the present study did not show the significant differences between both MgSo4 treated groups and all control groups regarding serum level of Cr and BUN. Our patients in all groups had no renal impairment and their plasma Cr and BUN levels were in the normal range. Therefore, following Mg administration, we observed no change in the serum levels of these parameters.
Uric acid acts as an antioxidant. However recently, some clinical and basic research have discovered close associations of hyperuricemia with inflammation.52 As our results indicate, plasma levels of uric acid in all participants were within the normal range, and MgSO4 treatment had no significant effect on serum levels. We did not observe a significant difference in urine Mg, serum T4, TSH, Na and Ca levels in both Mg-treated groups compared with all control groups.
Limitation
One of the present study limitations is the relatively small sample size, so future studies with a larger population are required to confirm our findings.
Conclusion
Overall, the results of this study showed that despite the slight change in serum Mg level, MgSO4 treatment slightly reduced the risk of inflammatory and vascular factors in moderate coronary artery disease patients. Therefore, according to our finding, MgSO4 treatment may have a protective effect against atherosclerosis.
Abbreviations
MgSO4, magnesium sulfate; LDL, low-density lipoprotein; oxLDL, oxidized LDL; LOX1 gene, lectin-like oxidized-LDL receptor 1; BP, blood pressure; CVA, cerebrovascular accident; CVD, cardiovascular diseases; Na, sodium; K, potassium; Ca, calcium; CRP, C-reactive protein; Cr, creatinine; BUN, blood urea nitrogen; T3, triiodothyronine; T4, Thyroxine; TSH, thyroid stimulating hormone; BMI, body mass index; CLIA, chemiluminescence immunoassays; NMDA, N-methyl-D-aspartate; CAD, coronary artery disease; ESR, erythrocyte sedimentation rat; EF, ejection fraction.
Acknowledgments
Deputy of Research of Hormozgan University of Medical Sciences supported this study. We thank Niak Pharmaceuticals Co. for providing MgSO4 and placebo (Gorgan Province, Iran).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Torres N, Guevara-Cruz M, Velazquez-Villegas LA, Tovar AR. Nutrition and atherosclerosis. Arch Med Res. 2015;46(5):408–426. doi:10.1016/j.arcmed.2015.05.010
2. Lim DH, Lee Y, Park GM, et al. Serum uric acid level and subclinical coronary atherosclerosis in asymptomatic individuals: an observational cohort study. Atherosclerosis. 2019;288:112–117. doi:10.1016/j.atherosclerosis.2019.07.017
3. Orimo H, Ouchi Y. The role of calcium and magnesium in the development of atherosclerosis. Experimental and clinical evidence. Ann N Y Acad Sci. 1990;598:444–457. doi:10.1111/j.1749-6632.1990.tb42315.x
4. Brinkley TE, Kume N, Mitsuoka H, et al. Variation in the human lectin‐like oxidized low‐density lipoprotein receptor 1 (LOX‐1) gene is associated with plasma soluble LOX‐1 levels. Exp Physiol. 2008;93(9):1085–1090. doi:10.1113/expphysiol.2008.042267
5. Blum JW, Froehli D, Kunz P. Effects of catecholamines on plasma free fatty acids in fed and fasted cattle. Endocrinology. 1982;110(2):452–456. doi:10.1210/endo-110-2-452
6. Kvetnansky R, Lu X, Ziegler MG. Stress-triggered changes in peripheral catecholaminergic systems. Adv Pharmacol. 2013;68:359–397. doi:10.1016/B978-0-12-411512-5.00017-8
7. Burn J, Gibbons W. The part played by calcium in determining the response to stimulation of sympathetic postganglionic fibres. Br J Pharmacol Chemother. 1964;22(3):540–548. doi:10.1111/j.1476-5381.1964.tb01708.x
8. Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14(1):6. doi:10.1186/1475-2891-14-6
9. Fang P, Zhang D, Cheng Z, et al. Hyperhomocysteinemia potentiates hyperglycemia-induced inflammatory monocyte differentiation and atherosclerosis. Diabetes. 2014;63(12):4275–4290. doi:10.2337/db14-0809
10. Ernst E. The role of fibrinogen as a cardiovascular risk factor. Atherosclerosis. 1993;100(1):1–12. doi:10.1016/0021-9150(93)90062-y
11. Ernst E. Fibrinogen as a cardiovascular risk factor–interrelationship with infections and inflammation. Eur Heart J. 1993;14:82–87.
12. Zhang Y, Zhu C-G, Guo Y-L, et al. Higher fibrinogen level is independently linked with the presence and severity of new-onset coronary atherosclerosis among Han Chinese population. PLoS One. 2014;9(11):e113460. doi:10.1371/journal.pone.0113460
13. Reffelmann T, Ittermann T, Dörr M, et al. Low serum magnesium concentrations predict cardiovascular and all-cause mortality. Atherosclerosis. 2011;219(1):280–284. doi:10.1016/j.atherosclerosis.2011.05.038
14. Maier JA. Low magnesium and atherosclerosis: an evidence-based link. Mol Aspects Med. 2003;24(3–1):137–146. doi:10.1016/s0098-2997(02)00095-x
15. Kupetsky-Rincon EA, Uitto J. Magnesium: novel applications in cardiovascular disease–a review of the literature. Ann Nutr Metab. 2012;61(2):102–110. doi:10.1159/000339380
16. Kieboom BC, Niemeijer MN, Leening MJ, et al. Serum magnesium and the risk of death from coronary heart disease and sudden cardiac death. J Am Heart Assoc. 2016;5(1):e002707. doi:10.1161/JAHA.115.002707
17. Cunha AR, D’El-Rei J, Medeiros F, et al. Oral magnesium supplementation improves endothelial function and attenuates subclinical atherosclerosis in thiazide-treated hypertensive women. J Hypertens. 2017;35(1):89–97. doi:10.1097/HJH.0000000000001129
18. Guo H, Lee J-D, Uzui H, et al. Effects of folic acid and magnesium on the production of homocysteine-induced extracellular matrix metalloproteinase-2 in cultured rat vascular smooth muscle cells. Circ J. 2006;70(1):141–146. doi:10.1253/circj.70.141
19. Fazlali M, Kharazmi F, Kamran M, et al. Effect of oral magnesium sulfate administration on lectin-like oxidized low-density lipoprotein receptor-1 gene expression to prevent atherosclerosis in diabetic rat vessels. J Diabetes Investig. 2019;10(3):650–658. doi:10.1111/jdi.12961
20. Soltani N, Keshavarz M, Dehpour AR. Effect of oral magnesium sulfate administration on blood pressure and lipid profile in streptozocin diabetic rat. Eur J Pharmacol. 2007;560(2–3):201–205. doi:10.1016/j.ejphar.2006.12.020
21. Soltani N, Keshavarz M, Minaii B, Mirershadi F, Zahedi Asl S, Dehpour AR. Effects of administration of oral magnesium on plasma glucose and pathological changes in the aorta and pancreas of diabetic rats. Clin Exp Pharmacol Physiol. 2005;32(8):604–610. doi:10.1111/j.0305-1870.2005.04238.x
22. Soltani N, Keshavarz M, Sohanaki H, Zahedi Asl S, Dehpour AR. Relaxatory effect of magnesium on mesenteric vascular beds differs from normal and streptozotocin induced diabetic rats. Eur J Pharmacol. 2005;508(1–3):177–181. doi:10.1016/j.ejphar.2004.12.003
23. Shahbah D, Hassan T, Morsy S, et al. Oral magnesium supplementation improves glycemic control and lipid profile in children with type 1 diabetes and hypomagnesaemia. Medicine. 2017;96(11):11. doi:10.1097/MD.0000000000006352
24. Shahi A, Aslani S, Ataollahi M, Mahmoudi M. The role of magnesium in different inflammatory diseases. Inflammopharmacology. 2019;27(4):649–661. doi:10.1007/s10787-019-00603-7
25. Cao Y, Wang C, Guan K, Xu Y, Su Y-X, Chen Y-M. Association of magnesium in serum and urine with carotid intima-media thickness and serum lipids in middle-aged and elderly Chinese: a community-based cross-sectional study. Eur J Nutr. 2016;55(1):219–226. doi:10.1007/s00394-015-0839-8
26. Severino P, Netti L, Mariani MV, et al. Prevention of cardiovascular disease: screening for magnesium deficiency. Cardiol Res Pract. 2019;2019:1–10. doi:10.1155/2019/4874921
27. Dong J-Y, Xun P, He K, Qin L-Q. Magnesium intake and risk of type 2 diabetes: meta-analysis of prospective cohort studies. Diabetes Care. 2011;34(9):2116–2122. doi:10.2337/dc11-0518
28. Guerrero‐Romero F, Rodríguez‐Morán M. Magnesium improves the beta‐cell function to compensate variation of insulin sensitivity: double‐blind, randomized clinical trial. Eur J Clin Invest. 2011;41(4):405–410. doi:10.1111/j.1365-2362.2010.02422.x
29. Fang X, Wang K, Han D, et al. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: a dose–response meta-analysis of prospective cohort studies. BMC Med. 2016;14(1):210. doi:10.1186/s12916-016-0742-z
30. Fang X, Han H, Li M, et al. Dose-response relationship between dietary magnesium intake and risk of type 2 diabetes mellitus: a systematic review and meta-regression analysis of prospective cohort studies. Nutrients. 2016;8(11):739. doi:10.3390/nu8110739
31. Rosique-Esteban N, Guasch-Ferré M, Hernández-Alonso P, Salas-Salvadó J. Dietary magnesium and cardiovascular disease: a review with emphasis in epidemiological studies. Nutrients. 2018;10(2):168. doi:10.3390/nu10020168
32. Wang J, Jia L, Li X, et al. New insights into the association between fibrinogen and coronary atherosclerotic plaque vulnerability: an intravascular optical coherence tomography study. Cardiovasc Ther. 2019;2019:1–12. doi:10.1155/2019/8563717
33. Papageorgiou N, Briasoulis A, Hatzis G, et al. Coronary artery atherosclerosis in hypertensive patients: the role of fibrinogen genetic variability. Rev Esp Cardiol (Engl Ed). 2017;70(1):34–41. doi:10.1016/j.rec.2016.05.024
34. Sreckovic B, Sreckovic VD, Soldatovic I, et al. Homocysteine is a marker for metabolic syndrome and atherosclerosis. Diabetes Metab Syndr. 2017;11(3):179–182. doi:10.1016/j.dsx.2016.08.026
35. McCully KS. Homocysteine metabolism, atherosclerosis, and diseases of aging. Compr Physiol. 2011;6(4):471–505. doi:10.1002/cphy.c150021
36. Agrawal A. CRP after 2004. Mol Immunol. 2005;42(8):927–930. doi:10.1016/j.molimm.2004.09.028
37. Arici M, Walls J. End-stage renal disease, atherosclerosis, and cardiovascular mortality: is C-reactive protein the missing link? Kidney Int. 2001;59(2):407–414. doi:10.1046/j.1523-1755.2001.059002407.x
38. Sun Y, Byon CH, Yang Y, et al. Dietary potassium regulates vascular calcification and arterial stiffness. JCI Insight. 2017;2(19). doi:10.1172/jci.insight.94920
39. Johnson LS, Mattsson N, Sajadieh A, Wollmer P, Söderholm M. Serum potassium is positively associated with stroke and mortality in the large, population-based malmö preventive project cohort. Stroke. 2017;48(11):2973–2978. doi:10.1161/STROKEAHA.117.018148
40. Bano A, Chaker L, Mattace-Raso FU, et al. Thyroid function and the risk of atherosclerotic cardiovascular morbidity and mortality: the Rotterdam Study. Circ Res. 2017;121(12):1392–1400. doi:10.1161/CIRCRESAHA.117.311603
41. Åsvold BO, Bjøro T, Nilsen TIL, Gunnell D, Vatten LJ. Thyrotropin levels and risk of fatal coronary heart disease: the HUNT study. Arch Intern Med. 2008;168(8):855–860. doi:10.1001/archinte.168.8.855
42. Åsvold BO, Vatten LJ, Bjøro T, et al. Thyroid function within the normal range and risk of coronary heart disease: an individual participant data analysis of 14 cohorts. JAMA Intern Med. 2015;175(6):1037–1047. doi:10.1001/jamainternmed.2015.0930
43. Chaker L, Baumgartner C, Den Elzen WP, et al. Thyroid function within the reference range and the risk of stroke: an individual participant data analysis. J Clin Endocrinol Metab. 2016;101(11):4270–4282. doi:10.1210/jc.2016-2255
44. Rodondi N, Den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304(12):1365–1374. doi:10.1001/jama.2010.1361
45. Cappola AR, Arnold AM, Wulczyn K, Carlson M, Robbins J, Psaty BM. Thyroid function in the euthyroid range and adverse outcomes in older adults. J Clin Endocrinol Metab. 2015;100(3):1088–1096. doi:10.1210/jc.2014-3586
46. Cappola AR, Fried LP, Arnold AM, et al. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295(9):1033–1041. doi:10.1001/jama.295.9.1033
47. Collet T-H, Gussekloo J, Bauer DC, et al. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med. 2012;172(10):799–809. doi:10.1001/archinternmed.2012.402
48. Erikssen G, Liestøl K, Bjørnholt J, Stormorken H, Thaulow E, Erikssen J. Erythrocyte sedimentation rate: a possible marker of atherosclerosis and a strong predictor of coronary heart disease mortality. Eur Heart J. 2000;21(19):1614–1620. doi:10.1053/euhj.2000.2148
49. Ige A, Adewoye E. Oral magnesium treatment reduces anemia and levels of inflammatory markers in experimental diabetes. J Diet Suppl. 2017;14(1):76–88. doi:10.1080/19390211.2016.1205700
50. Johansson H-E, Haenni A, Zethelius B. Changes in erythrocyte sedimentation rate, white blood cell count, liver enzymes, and magnesium after gastric bypass surgery. J Obes. 2011;2011:1–6. doi:10.1155/2011/273105
51. Christoffersen C, Bartels ED, Aarup A, Nielsen LB, Pedersen TX. ApoB and apoM–new aspects of lipoprotein biology in uremia-induced atherosclerosis. Eur J Pharmacol. 2017;816:154–160. doi:10.1016/j.ejphar.2017.03.053
52. Kushiyama A, Nakatsu Y, Matsunaga Y, et al. Role of uric acid metabolism-related inflammation in the pathogenesis of metabolic syndrome components such as atherosclerosis and nonalcoholic steatohepatitis. Mediators Inflamm. 2016;2016:1–15. doi:10.1155/2016/8603164
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.