Back to Journals » Journal of Asthma and Allergy » Volume 15
MAF bZIP Transcription Factor B (MAFB) Protected Against Ovalbumin-Induced Allergic Rhinitis via the Alleviation of Inflammation by Restoring the T Helper (Th) 1/Th2/Th17 Imbalance and Epithelial Barrier Dysfunction
Received 26 September 2021
Accepted for publication 14 February 2022
Published 25 February 2022 Volume 2022:15 Pages 267—280
DOI https://doi.org/10.2147/JAA.S335560
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Luis Garcia-Marcos
Yang Sun,* Tiancong Liu,* Weiliang Bai
Department of Otolaryngology Head and Neck Surgery, Shengjing Hospital of China Medical University, Shenyang, 110004, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Weiliang Bai, Tel +86-18940255758, Fax +86-24-62101966, Email [email protected]
Purpose: This work aimed to investigate the effects of MAF bZIP transcription factor B (MAFB) on the progression of allergic rhinitis (AR).
Patients and Methods: Nasal mucosa was isolated from AR patients and healthy individuals from Shengjing Hospital of China Medical University. The experimental procedures were approved by the Medical Ethics Committee of Shengjing Hospital of China Medical University (2019PS341K) in accordance with the Declaration of Helsinki. Informed consents were signed by participants or a parent/legal guardian of the participants under 18 years old of age. Then, an AR mouse model with MAFB overexpression was established with 25 μg ovalbumin (OVA) sensitization on day 0, 7, 14, followed by an injection with 1× 107 TU/mL lentivirus MAFB on day 19 and a nasal challenge with 500 μg OVA from day 21 to 27.
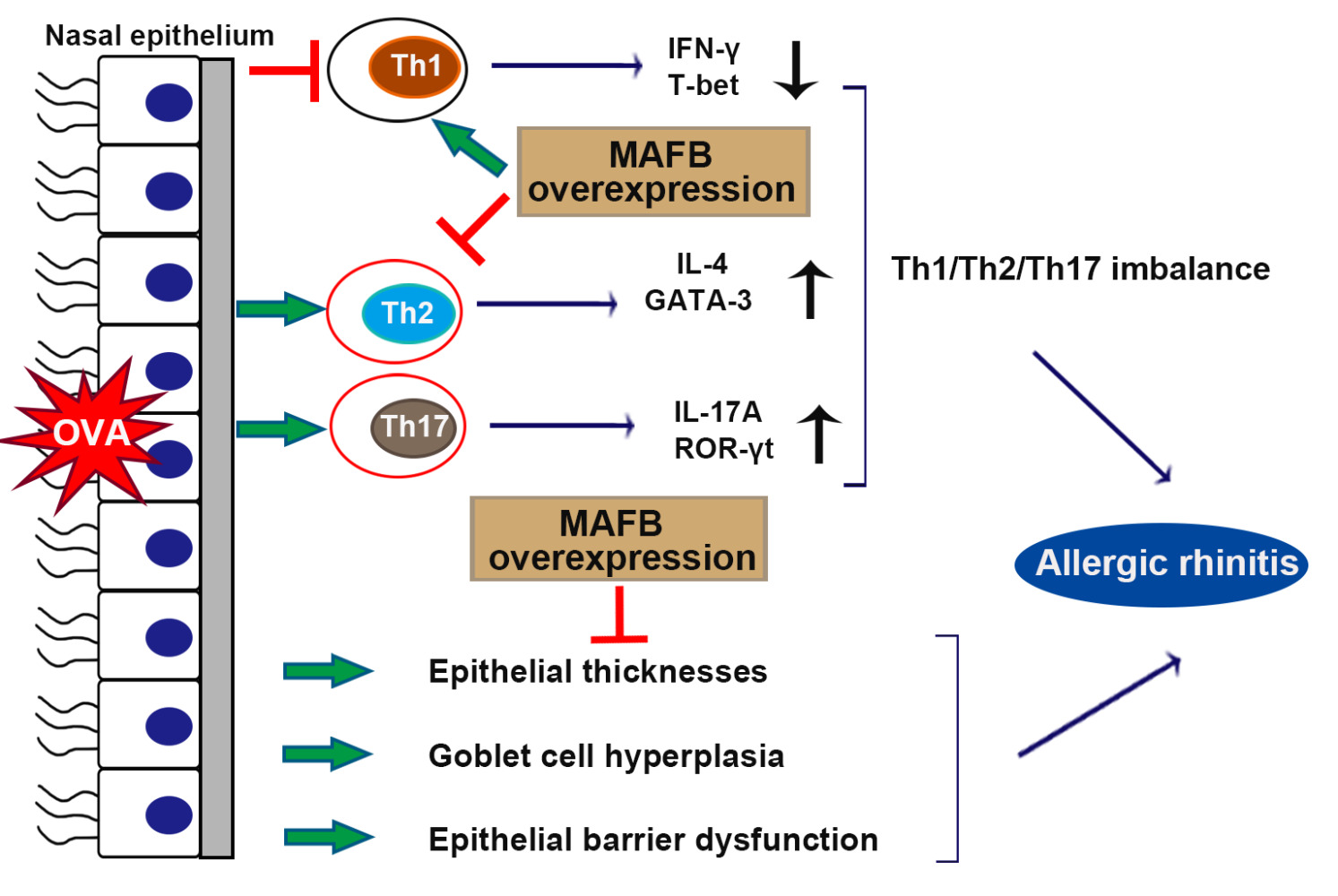
Results: The results revealed that MAFB was down-regulated in the nasal mucosa of AR patients. The up-regulation of MAFB protected the AR mice against the OVA-induced allergic symptoms (sneezing and nasal rubbing) by alleviating the OVA-induced epithelial thicknesses, goblet cell hyperplasia, and inflammation including the eosinophil and mast cell infiltration. Moreover, MAFB facilitated the T helper (Th) 1 response and inhibited the Th2 and Th17 responses by the down-regulation of T-box transcription factor 21 and the up-regulation of GATA binding protein-3 as well as retinoid-related orphan receptor-γt in the splenocytes of AR mice. MAFB was found to repress the differentiation of naive CD4+ T cells into Th2 cells. Subsequently, MAFB overexpression reversed the OVA-induced enhancement of epithelial permeability, downregulation of tight junctions, and upregulation of cadherin-26, indicating the protective role of MAFB on epithelial barrier integrity.
Conclusion: MAFB protected against OVA-induced AR via the alleviation of inflammation by restoring the Th1/Th2/Th17 imbalance and epithelial barrier dysfunction.
Keywords: allergic rhinitis, MAF bZIP transcription factor B, inflammation, T helper cell differentiation, epithelial barrier dysfunction
Graphical Abstract:
Introduction
Allergic rhinitis (AR) is the most common case of nasal mucosal inflammation and negatively affects the life quality of approximately 10–20% of global population.1 The prevalence of AR has been reported to steadily elevate.2 AR is triggered by specific immunoglobulin E (IgE)-mediated reactions against allergens and is characterized by T helper (Th) 2 immunological pattern with eosinophil and mast cell infiltration.3 After exposure to upper respiratory tract, allergens are processed and transferred.4 A complex containing allergen is recognized by Th0 receptor, leading to the differentiation of naive T cells into CD4+ Th2 cells.4 The imbalance of Th1/Th2/Th17 contributes to the AR pathogenesis.5 Th2 cells act as an essential part in the development of AR, and the repression of Th2 dampens the AR progression.5 Moreover, cytokines secreted by Th2 cells including interleukin (IL)-4, IL-5, and IL-13 involve in the AR development by attracting the accumulation of inflammatory cells and destroying the epithelial barrier integrity to maintain the nasal mucosal inflammation.6,7 Then, barrier epithelial dysfunction lead to the increase of the sensitization and degranulation of mast cells, as well as the aggravation of the AR progression.8 Unfortunately, there is no satisfied cure for AR.9 Thus, there is an urgent need to explore novel target for the treatment of AR.
MAF bZIP transcription factor B (MAFB) is a member of Maf transcription factor family that encodes a leucine-zipper transcription factor.10 MAFB facilitates the anti-inflammatory M2 polarization of macrophages and plays a vital role in maintaining the systemic homeostasis.11,12 Singh et al13 have reported that the loss of MAFA and MAFB undermines the T cell function and abrogates the balance of peripheral immune responses against auto antigens, leading to the promotion of inflammation in pancreatic islets. Hashizume et al14 have found that MAFB is down-regulated in the early stages of Th-cell differentiation mediated by IL-4 and IL-10. Yin et al15 have testified that MAFB-up-regulated CD5low dendritic cells promote the IFN-interferon-γ (IFN-γ)-producing Th1 cell differentiation and suppress the IL-4 and IL-10-producing Th2 cell as well as the IL-17A-producing Th17 cell differentiation. These studies indicate the potential of MAFB on regulating the Th cell polarization and inflammatory responses. Thus, we considered whether MAFB could play a role in the AR progression via regulating the inflammation by regulating the T cell differentiation. However, there are no reports on it.
In this work, we aimed to investigate the function of MAFB in the AR progression. We found that MAFB was down-regulated in nasal mucosa of AR patients. Next, the effects of MAFB overexpression on allergic nasal symptoms of AR mice as well as the histological changes, Th cell responses, Th2 cell differentiation, epithelial barrier function were explored in the ovalbumin (OVA)-induced nasal mucosa. MAFB was expected to be a novel therapeutic target for the treatment of AR.
Patients and Methods
Human Nasal Mucosa Samples
Healthy patients (n= 10) were involved into the study as the control group. Healthy nasal mucosa was isolated from the inferior turbinate mucosa. Patients (n = 20) with persistent AR diagnosed had symptoms for at least 2 years were involved into the study. All patients did not show any clinical sign of infection in the nose at time of surgery. Patient information was listed in Table 1. The experimental procedures were approved by the Medical Ethics Committee of Shengjing Hospital of China Medical University (2019PS341K) in accordance with the Declaration of Helsinki. Informed consents were signed by participants or a parent/legal guardian of the participants under 18 years old of age.
 |
Table 1 Patient Characteristics |
The inclusion criteria were hypersensitivity to house dust mite diagnosed by skin prick test and allergen specific IgE test, no use of local or systemic glucocorticoids or other immunosuppressant drugs for at least 1 month before the study, no use of antihistamines for 1 week before the study and no respiratory infection for at least 1 month before the study. Serum total IgE was determined by the enzyme-linked immunosorbent assay method.
Exclusion criteria were relevant hypersensitivity to other inhaled aeroallergens, systemic autoimmune or other diseases and treatment with allergen immunotherapy.
Lentivirus Infection
The cDNA of MAFB was cloned into the lentiviral vectorpLVX-IRES-puro (Fenghuishengwu, Changsha, China) to construct the MAFB over expression plasmids. Subsequently, HEK-293T cells were co-transfected with lentiviral vector and helper vector pSPAX2 and pMD2.G (Fenghuishengwu) by using the Lipofectamine 2000 (Invitrogen, Carlsbad, California, USA) in accordance with the manufacturers’ instructions. Following transfection for 48 h, lentiviral supernatant was collected using centrifugation (956 × g, 15 min) and further passed through a 45 μm filter (Costar, Cambridge, MA, USA) to obtain the lentiviral particles. Then, the optimal multiplicity of infection (MOI) was detected. 1 × 105CD4+ T cells were planted into 6-well plates of each well and then infected with the viral particles for 24 h at the optimal MOI of 100.
Murine AR Model and Treatment
The animal experimental procedures were approved by the Medical Ethics Committee of Shengjing Hospital of China Medical University following The Guideline for the Care and Use of Laboratory Animals. Four-week-old female BALB/c mice were purchased from HFK Bioscience (Beijing, China) and were randomly divided into four groups after one week of acclimation: Control, AR, AR + LV-NC, and AR + LV-MAFB (18 mice in each group: 6 for histological assessment, 6 for Western blot, and 6 for qRT-PCR). The murine AR model was established as previously described.16 In brief, mice were intraperitoneally injected with 25 μg OVA (Aladdin, Shanghai, China) and 1 mg aluminum hydroxide gel on days 0, 7, and 14. Next, mice were subjected to a nostril challenge with 500 μg OVA from day 21 to day 27. Selected groups (AR + LV-NC and AR + LV-MAFB) of mice were intravenously injected with 1×107 TU/mL LV-NCor LV-MAFB into the tail vein 48 h before the nostril challenge on day 19. Subsequently, 20 μL fluorescein isothiocyanate-dextran 4 kDa (FD4, 50 mg/mL) was applied to further evaluate the nasal mucosal permeability by the intranasal route. Mice were sacrificed 1 h after the FD4 administration. Murine serum, spleen tissues, and nasal mucosa were collected for further molecular experiments.
Isolation of Naive CD4+ T Cells and Induction of Th2 Differentiation
To obtain the naive CD4+ T cells, the spleen tissues of four-week old female BLAB/c were collected and then homogenized to single cells. Murine lymphocytes were obtained from splenocyte suspension by lymphocyte separating solution (Solarbio), followed by a centrifugation of 250 g for 10 min. CD4+ T cells were isolated from lymphocyte by the magnetic-activated cell sorting (MiltenyiBiotec, Cologne, Germany). Next, the induction of Th2 differentiation from CD4+ T cells was performed as previously described.17 CD4+ T cells were incubated with 75 ng/mL phorbol 12-myristate 13-acetate (Aladdin), 1 µg/mL ionomycin (Aladdin), and 1 µL BD GolgiPlug containing Brefeldin A (Aladdin) for 4 h, followed by an infection with LV-MAFB or LV-NC for 24 h. Subsequently, the induction of Th2 differentiation from naive CD4+ T cells were obtained by an incubation with 10 μg/mL anti-CD3 (Biolegend, San Diego, California, USA), 1 μg/mL anti-CD28 (Biolegend), 10 μg/mL IL-2 (Biolegend), 10 μg/mL IL-4 (Biolegend), and 10 μg/mL anti-IFN-γ (Biolegend) for 72 h.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from nasal mucosa tissues or cells by employing an RNA Simple Total RNA Kit (Tiangen, Beijing, China). The cDNA synthesis was performed by a BeyoRT II M-MLV reverse transcriptase (Beyotime, Shanghai, China). 2×Taq PCR MasterMix (Solarbio) and SYBR Green (Solarbio) were used to quantify the expression of genes. The relative expression of mRNA was calculated by 2 –ΔΔCtmethod. GAPDH was used as the internal control. The sequences of primer were shown in Table 2.
 |
Table 2 The Sequences of Primer Used in qRT-PCR |
Western Blot
Total protein was extracted from nasal mucosa tissues or cells by 1 mM PMSF mixed with RIPA. The concentration of the protein was detected by BCA Protein Assay Kit (Solarbio, Beijing, China). Equal content of protein samples was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes, followed by the block with 5% skimmed milk. Next, protein bands were incubated with primary antibodies at 4°C overnight and horseradish-peroxidase (HRP)-conjugated secondary antibodies (1:3000; Solarbio) at 37°C for 1 h. The bands were visualized by employing WD-9413B Gel imaging system (Liuyi Biotechnology, Beijing, China). The primary antibodies were anti-MAFB (1:2000; Santa Cruz, CA, USA), anti-T-box transcription factor 21 (T-bet; 1:1000; ABclonal, Wuhan, China), anti-GATA binding protein-3 (GATA-3; 1:1000; ABclonal), and anti-retinoid-related orphan receptor-γt (ROR-γt; 1:1000; Bioss, Beijing, China), and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH;1:10,000; proteintech).
Immunofluorescence Staining
After being deparaffinized and rehydrated, the sections (5 μm) of murine or human nasal mucosa were blocked with goat serum at room temperature for 15 min. Next, slides were incubated with anti-tryptase (1:100; Affinity, Cincinnati, OH, USA), anti-MAFB (1:400; CST, Danvers, MA, USA) or anti-cadherin-26 (CDH26; 1:400; Affinity) at 4°C overnight and then Cy3-conjugated IgG (1:200; Beyotime) at room temperature for an hour, while nuclei was counterstained with/without DAPI. Sections were viewed with BX53 fluorescence microscope (Olympus, Tokyo, Japan) at the magnification of 200 or 400.
Evaluation of Nasal Symptoms
Nasal allergy-like symptoms were assessed by the frequencies of sneezing and nasal rubbing movements. After the OVA challenge on day 27, mice were placed into an animal cage (one mouse per cage), and the frequencies of sneezing and nasal rubbing movements within 15 min were counted.
Enzyme-Linked Immunosorbent Assay (ELISA)
Nasal lavage fluid (NALF) was obtained following the method previously described.18 Mice were anesthetized, and a micropipette was inserted into one nostril, and 1 mL saline was laved into the nasal cavity. The NALF was collected and centrifuged. The supernatants were collected for further measurements. The concentration of IL-4, IL-17A, and IFN-γ in NALF and murine serum was detected by employing ELISA kits purchased from MultiScience (Lianke) Biotech (Hangzhou, China) as per the users’ protocols. The serum levels of IgE and OVA-specific IgE (sIgE) were assessed by ELISA kits purchased from MultiScience (Lianke) Biotech and Jianglai Bio-Technology (Shanghai, China).
Histological Assessment
Paraffin-embedded sections of murine nasal mucosa (5μm) were deparaffinized in xylene, and rehydrated in an ethanol gradient with distilled water. Next, sections were stained with hematoxylin and eosin (H&E; Solarbio) for general morphological observation to examine the epithelial thicknesses and eosinophil infiltration, periodic acid-Schiff (PAS; Leagene Biotech, Beijing, China) staining to evaluate the goblet cell hyperplasia (mucus hypersecretion), and Sirius red (Solarbio) staining to assess the eosinophil infiltration for general morphological observation. Two observers independently selected three sections of nasal mucosa of each mouse. Then, three visual fields of each section were randomly selected to image at the magnification of 200 or 400, and cells were further counted.
Flow Cytometry
1×106 CD4+ T cells were incubated with 0.25 μg APC-conjugated anti-mouse CD4+ at 4°C for 20 min in the dark, followed by the permeabilization at 4°C for 20 min. Next, CD4+ T cells were incubated with 1 μgfluorescein isothiocyanate-conjugated anti-mouse IFN-γ, 0.25 μg PE-conjugated anti-mouse IL-4, and 0.25 μgphycoerythrin-conjugated anti-mouse IL-17A at 4°C for 20 min, respectively. After wash and centrifugation, cells were resuspended with 500 μL buffer and analyzed with NovoCyte flow cytometer (ACEA Biosciences, San Diego, California, USA).
Statistical Analysis
Data was shown as mean ± SD. Statistical differences among multiple groups were evaluated using one-way ANOVA. Comparisons between two groups were assessed by unpaired t-test. Non-parametric analysis was performed by employing Kruskal-Wallis test with Dunn’ multiple comparisons test. All data was analyzed by GraphPad Prism 8.0 software. The difference at p<0.05 was considered statistically significant.
Results
MAFB Was Down-Regulated in the Nasal Mucosa of AR Patients and AR Mouse Model
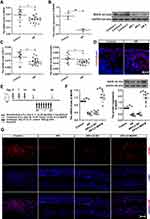
The relative expression of MAFB was investigated by using qRT-PCR and Western blot. As shown in Figure 1A and B, the mRNA and protein levels of MAFB were down-regulated in the nasal mucosa of AR patients compared to healthy individuals. Then, the expression of epithelial barrier dysfunction-related indicators (zona occludens-1 (ZO-1), occluding, and CDH26) was subsequently evaluated in AR patients. We found that ZO-1 and occludin exhibited a lower expression and CDH26 had a higher expression in the nasal mucosa of AR patients compared to control participants (Figure 1C and D). These results indicated the epithelial barrier dysfunction in AR patients. Next, to investigate the role of MAFB in the AR progression, we established an OVA-induced AR mouse model with MAFB overexpression (Figure 1E). The overexpression of MAFB reversed the down-regulated mRNA and protein levels of MAFB in nasal mucosa of mice induced by OVA (Figure 1F). Next, the results of immunofluorescence staining echoed the results of qRT-PCR and Western blot (Figure 1G). These results indicated that MAFB was down-regulated in the nasal mucosa of AR patients and AR mouse model.
MAFB Alleviated the Allergic Nasal Symptoms and Inhibited the Inflammation of Mice with AR
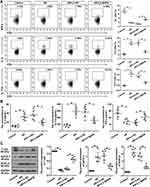
To explore the effects of MAFB on nasal symptoms, the frequencies of sneezing and nasal rubbing were counted within 15 min after the OVA intranasal challenge. As revealed in Figure 2A and B, there was a tendency that the up-regulation of MAFB reduced the increased frequencies of sneezing and nasal rubbing of mice induced by OVA. Next, the elevated concentration of IgE and sIgE in the serum of AR mice induced by OVA was restored by the MAFB up-regulation, suggesting the amelioration of the atopy status of AR mice (Figure 2C and D). Then, the immunocytologic analysis of nasal cells obtained from NALF was performed by Giemsa staining. The results exhibited that the high levels of MAFB reversed the increased count of total cells, eosinophils, neutrophils, lymphocytes, and monocyte macrophages induced by OVA (Figure 2E–I). Moreover, ELISA demonstrated that the decreased levels of IFN-γ and the elevated concentration of IL-4 and IL-17A in the serum of mice induced by OVA were reverted by the overexpression of MAFB (Figure 2J–L). Thus, we concluded that MAFB alleviated the allergic nasal symptoms and the inflammation of mice with AR.
MAFB Relieved the Histological Injuries and Dampened the Eosinophil and Mast Cell Infiltration of the Nasal Mucosa Induced by OVA
We further explored the OVA-induced histological changes of nasal mucosa by H&E staining. The OVA-induced nasal septum exhibited elevated epithelial thicknesses and eosinophil infiltration, which were relieved by the overexpression of MAFB (Figure 3A and B). The same changes of eosinophil infiltration were obtained by Sirius red staining (Figure 3C and D).Next, the results of PAS staining showed that the goblet cell hyperplasia (mucus hypersecretion) of the nasal mucosa epithelium induced by OVA was ameliorated by the up-regulation of MAFB (Figure 3E). Subsequently, Figure 3F and G revealed that the high expression of MFAB alleviated the OVA-induced mast cell infiltration of the nasal mucosa. The data indicated that MAFB mitigated the OVA-induced histological injury and suppressed the inflammatory infiltration of nasal mucosa in AR mice.
MAFB Facilitated the Th1 Response and Inhibited the Th2 and Th17 Responses
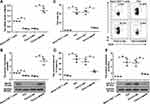
As the imbalance of Th1, Th2, and Th17 responses leads to the allergy inflammation, we considered whether MAFB could repair this imbalance. CD4+ IFN-γ+ Th1, CD4+ IL-4+ Th2, and CD4+ IL-17A+ Th17 cells were analyzed by flow cytometry. The results showed that MAFB overexpression reversed the reduced percentage of Th1 cells and the increased population of Th2 and Th17 cells in the splenocytes of AR mice (Figure 4A). Similarly, the same changes of the concentration of IFN-γ, IL-4, and IL-17A in NALF were obtained by ELISA (Figure 4B). Subsequently, we found that the infection of LV-MAFB restored the OVA-induced down-regulation of T-bet and the up-regulation of GATA-3 and ROR-γt in the nasal mucosa of AR mice (Figure 4C). These results suggested that MAFB facilitated the Th1 response and dampened the Th2 and Th17 responses.
MAFB Repressed the Th2 Differentiation
We further explored the effects of MAFB on Th2 differentiation. Figure 5A and B manifested that the down-regulation of MAFB levels in Th2 cells was up-regulated by the infection of LV-MAFB. Next, flow cytometry testified that the increased percentage of Th2 cells was decreased by the overexpression of MAFB (Figure 5C). Next, it was no surprise to know that the elevated concentration of IL-4 in the supernatant of Th2 cells was reduced by the MAFB up-regulation (Figure 5D). Subsequently, the high levels of MAFB decreased the up-regulation of GATA-3 in Th2 cells (Figure 5E). Therefore, we concluded that MAFB suppressed the Th2 differentiation.
MAFB Protected the Epithelial Barrier Integrity of AR Mouse Induced by OVA
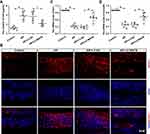
The epithelial barrier integrity of AR mice was further evaluated by FDA permeability, the expression of CDH26, ZO-1, and occludin. As displayed in Figure 6A, the OVA-induced increase of FDA in serum content was restored by the MAFB up-regulation. Immunofluorescence staining manifested that infection with LV-MAFB reverted the elevated levels of CDH26 in the nasal mucosa of AR mice (Figure 6B). Next, the results of qRT-PCR exhibited that the OVA-induced down-regulation of ZO-1 and occluding was reversed by the MAFB overexpression (Figure 6C and D). The data indicated that MAFB protected the epithelial barrier integrity of AR mice induced by OVA.
Discussion
AR, the IgE-mediated inflammation of nasal mucosa, is characterized by allergen Th2 immunological pattern with eosinophil and mast cell accumulation and the release of inflammatory factors.19 In this work, we found that MAFB was down-regulated in the nasal mucosa of AR patients. The up-regulation of MAFB protected the AR mice from the OVA-induced allergic symptoms by ameliorating the OVA-induced inflammation infiltration, epithelial thicknesses, goblet cell hyperplasia, and epithelial barrier dysfunction. Moreover, MAFB facilitated the Th1 response and inhibited the Th2 and Th17 responses, as well as the Th2 differentiation. MAFB is expected to be a novel therapeutic target for AR treatment.
MAFB is a bZIP transcription factor belonging to large Maf family.20 MAFB is expressed in macrophages and monocytes and acts as an essential part in their differentiation, development, and function maintenance.21 Significantly, MAFB is a novel regulator of inflammation in adipose, cerebral, and islet tissues.13,22,23 Liu et al24 have testified that MAFB represses type I interferon production by regulating CD14+ monocytes in patients with chronic hepatitis C. Soler Palacios et al25 have reported that MAFB involves in the alleviation of inflammation and mucosal repair in colitis model, as well as the reprogram of macrophages toward an anti-inflammatory and reparative profile. Shichita et al21 have found that MAFB suppresses the excess inflammation after ischemic stroke by promoting the clearance of damage signals upon the class A scavenger receptors. These studies indicate the potential role of MAFB on inflammatory regulation in various diseases. Herein, we found that the mRNA and protein levels of MAFB were lower in AR patients than that of healthy individuals. MAFB up-regulation exerts a protective effect against OVA-induced inflammation (including the eosinophil and mast cell infiltration, and the secretion of inflammatory factors) in the mouse model of AR as seen in the decreased frequencies of nasal rubbing and sneezing and in the reduced serum concentration of IgE.
Naive CD4+ T cells differentiate into at least three distinct lineages, Th1, Th2, and Th17 cells, with the fate of the cell at least partly determined by the transcription factors T-bet, GATA-3, and ROR-γt, respectively.26,27 The imbalance of Th1, Th2, and Th17 cells is regarded as the main induction factor of AR.28 The differentiation of naive CD4+ T cells into Th2 is markedly enhanced in AR, and the elevated release of Th2 cytokines promotes the expression of GATA-3.29 It has been reported that the nasal inflammation of AR is alleviated by the activation of Th1 and the repression of Th2 and Th17 responses.5 We considered whether the protective effects of MAFB on AR were mediated by the Th1/Th2/Th17 balance. Scholars have confirmed that MAFB is down-regulated in early differentiation of the IL-4-mediated Th1 and IL-12-mediated Th2 cells.14 The MAFB-up-regulated CD5low dendritic cells induce the differentiation of IFN-γ-producing Th1 cells and dampen the differentiation of IL-4-producing Th2 and IL-17A-producing Th17 cells.15 These studies suggest the potential of MAFB in T cell polarization. Herein, we found that MAFB overexpression facilitated the Th1 response and inhibited the Th2 and Th17 responses by the down-regulation of T-bet and the up-regulation of GATA-3 and ROR-γt in the splenocytes of AR mice. And MAFB repressed the differentiation from naive CD4+ cells to Th2 cells.
The cytokines secreted by Th2 cells involves in the AR development by attracting the accumulation of inflammatory cells and destroying the epithelial barrier integrity to maintain the nasal mucosal inflammation.30 The nasal epithelial barrier is primarily formed by tight junctions, which consists of membrane proteins including occluding, adhesion molecules, and scaffold adaptor proteins (ZO family proteins).31 Dysfunction of these tight junctions could enhance the exposure of nasal tissues to environmental antigens, leading to the elevation of the sensitization and the degranulation of mast cells that may contribute to the AR progression.32 Steelant et al33 reported the disruption of the mucosal epithelial barrier in patients with AR with the reduced levels of the tight junction proteins. Similarly, the same results were obtained in our study. The expression of ZO-1 and occluding was downregulated and CDH26 was upregulated in AR patients, which was consistent with the results of epithelial barrier dysfunction of AR mouse induced by OVA. Subsequently, MAFB overexpression rescued the OVA-induced disruption of the mucosal epithelial barrier, as evidenced by the reduced FDA content, upregulation of tight junctions and downregulation of CDH26. These results indicated the protective role of MAFB in epithelial barrier integrity of AR mice.
However, there are limitations in this study. First, we assessed the expression of tight junctions to indirectly evaluate the epithelial barrier integrity in AR clinical samples, whereas there lacks the measurement of transtissue resistance and permeability. Second, we found that MAFB exerts an excellent therapeutic effect against OVA-induced inflammation and epithelial barrier dysfunction in a mouse model of AR as seen in the decreased frequencies of nasal rubbing and sneezing and in the reduced serum concentration of IgE. But the underlying mechanism on how MAFB alleviates the inflammation and epithelial barrier disruption is still unclear. We attach much importance to the underlying mechanism of the study, which will be explored in our further investigation. Nevertheless, as far as we know, it is the first time to find the protective role of MAFB on ARprocess. MAFB was expected to be a novel therapeutic target for the treatment of AR. The present study may be valuable to elucidate the pathology of AR and provide therapeutic implications.
Conclusion
MAFB was down-regulated in the nasal mucosa of AR patients. MAFB protected against OVA-induced AR via the alleviation of inflammation by restoring the Th1/Th2/Th17 imbalance and epithelial barrier dysfunction.
Abbreviations
AR, allergic rhinitis; CDH26, cadherin-26; ELISA, enzyme-linked immunosorbent assay; FD4, isothiocyanate-dextran 4; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GATA-3, GATA binding protein-3; H$E, hematoxylin and eosin; HRP, horseradish-peroxidase; IFN-γ, interferon-γ; IgE, immunoglobulin E; IL, interleukin; MAFB, MAF bZIP transcription factor B; NALF, nasal lavage fluid; OVA, ovalbumin; PAS, periodic acid-Schiff; qRT-PCR, quantitative real-time polymerase chain reaction; ROR-γt, retinoid-related orphan receptor-γt; sIgE, specific IgE; T-bet, T-box transcription factor 21; Th, T helper.
Data Sharing Statement
The data of this work is available on request to the corresponding author.
Ethics Approval and Informed Consent
The experimental procedures were approved by the Medical Ethics Committee of Shengjing Hospital of China Medical University (2019PS341K) in accordance with the Declaration of Helsinki. Informed consents were signed by all participants.
Acknowledgments
This work was supported by the National Natural Science Fund (NO: 81241083), the science and technology department project of Liaoning Province (NO:2013001010,2021JH2/10300087), the science plan program of Shenyang city (NO:F13-220-9-21, 20-205-4-029,21-172-9-08) and the 345 Talent Project of Shengjing Hospital of China Medical University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revised or critically reviewed the article; gave final approval of the version to be published; agree on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Campo P, Eguiluz-Gracia I, Bogas G, et al. Local allergic rhinitis: implications for management. Clin Exp Allergy. 2019;49(1):6–16. doi:10.1111/cea.13192
2. Meng Y, Wang C, Zhang L. Advances and novel developments in allergic rhinitis. Allergy. 2020;75(12):3069–3076. doi:10.1111/all.14586
3. Lam HY, Tergaonkar V, Ahn KS. Mechanisms of allergen-specific immunotherapy for allergic rhinitis and food allergies. Biosci Rep. 2020;40(4):BSR20200256. doi:10.1042/BSR20200256
4. Bernstein DI, Schwartz G, Bernstein JA. Allergic rhinitis: mechanisms and treatment. Immunol Allergy Clin North Am. 2016;36(2):261–278. doi:10.1016/j.iac.2015.12.004
5. Fan Y, Piao CH, Hyeon E, et al. Gallic acid alleviates nasal inflammation via activation of Th1 and inhibition of Th2 and Th17 in a mouse model of allergic rhinitis. Int Immunopharmacol. 2019;70:512–519. doi:10.1016/j.intimp.2019.02.025
6. Hellings PW, Steelant B. Epithelial barriers in allergy and asthma. J Allergy Clin Immunol. 2020;145(6):1499–1509. doi:10.1016/j.jaci.2020.04.010
7. Steelant B, Seys SF, Van Gerven L, et al. Histamine and T helper cytokine-driven epithelial barrier dysfunction in allergic rhinitis. J Allergy Clin Immunol. 2018;141(3):951–963. doi:10.1016/j.jaci.2017.08.039
8. KortekaasKrohn I, Seys SF, Lund G, et al. Nasal epithelial barrier dysfunction increases sensitization and mast cell degranulation in the absence of allergic inflammation. Allergy. 2020;75(5):1155–1164. doi:10.1111/all.14132
9. Kakli HA, Riley TD. Allergic rhinitis. Prim Care. 2016;43(3):465–475. doi:10.1016/j.pop.2016.04.009
10. Kong X, Luo J, Xiang H, et al. Expression of Mafb is down-regulated in the foreskin of children with hypospadias. J Pediatr Urol. 2021;17(1):
11. Kim H. The transcription factor MafB promotes anti-inflammatory M2 polarization and cholesterol efflux in macrophages. Sci Rep. 2017;7(1):75–91. doi:10.1038/s41598-017-00089-9
12. Hamada M, Tsunakawa Y, Jeon H, Yadav MK, Takahashi S. Role of MafB in macrophages. Exp Anim. 2020;69(1):1–10. doi:10.1538/expanim.19-0076
13. Singh T, Colberg JK, Sarmiento L, et al. Loss of MafA and MafB expression promotes islet inflammation. Sci Rep. 2019;9(1):9074. doi:10.1038/s41598-019-45528-x
14. Hashizume H, Hamalainen H, Sun Q, Sucharczuk A, Lahesmaa R. Downregulation of mafB expression in T-helper cells during early differentiation in vitro. Scand J Immunol. 2003;57(1):28–34. doi:10.1046/j.1365-3083.2003.01181.x
15. Yin X, Yu H, Jin X, et al. Human blood CD1c+ dendritic cells encompass CD5high and CD5low subsets that differ significantly in phenotype, gene expression, and functions. J Immunol. 2017;198(4):1553–1564. doi:10.4049/jimmunol.1600193
16. Mo JH, Kang EK, Quan SH, Rhee CS, Lee CH, Kim DY. Anti-tumor necrosis factor-alpha treatment reduces allergic responses in an allergic rhinitis mouse model. Allergy. 2011;66(2):279–286. doi:10.1111/j.1398-9995.2010.02476.x
17. Read KA, Powell MD, Sreekumar BK, Oestreich KJ. In vitro differentiation of effector CD4(+) T helper cell subsets. Methods Molecular Biol. 2019;1960:75–84.
18. Piao CH, Song CH, Lee EJ, Chai OH. Saikosaponin A ameliorates nasal inflammation by suppressing IL-6/ROR-γt/STAT3/IL-17/NF-κB pathway in OVA-induced allergic rhinitis. Chem Biol Interact. 2020;315:108874. doi:10.1016/j.cbi.2019.108874
19. Breiteneder H, Peng Y-Q, Agache I, et al. Biomarkers for diagnosis and prediction of therapy responses in allergic diseases and asthma. Allergy. 2020;75(12):3039–3068. doi:10.1111/all.14582
20. Dieterich LC, Tacconi C, Menzi F, et al. Lymphatic MAFB regulates vascular patterning during developmental and pathological lymphangiogenesis. Angiogenesis. 2020;23(3):411–423. doi:10.1007/s10456-020-09721-1
21. Shichita T, Ito M, Morita R, et al. MAFB prevents excess inflammation after ischemic stroke by accelerating clearance of damage signals through MSR1. Nat Med. 2017;23(6):723–732. doi:10.1038/nm.4312
22. Pettersson AML, Acosta JR, Björk C, et al. MAFB as a novel regulator of human adipose tissue inflammation. Diabetologia. 2015;58(9):2115–2123. doi:10.1007/s00125-015-3673-x
23. Zhang L, Liu C, Huang C, Xu X, Teng J. miR-155 knockdown protects against cerebral ischemia and reperfusion injury by targeting MafB. Biomed Res Int. 2020;2020:6458204. doi:10.1155/2020/6458204
24. Liu T-M, Wang H, Zhang D-N, Zhu G-Z. Transcription factor MafB suppresses type I interferon production by CD14 monocytes in patients with chronic hepatitis C. Front Microbiol. 2019;10:1814. doi:10.3389/fmicb.2019.01814
25. Soler Palacios B, Nieto C, Fajardo P, et al. Growth hormone reprograms macrophages toward an anti-inflammatory and reparative profile in an MAFB-dependent manner. J Immunol. 2020;205(3):776–788. doi:10.4049/jimmunol.1901330
26. Kardan M, Rafiei A, Ghaffari J, Valadan R, Morsaljahan Z, Haj-Ghorbani ST. Effect of ginger extract on expression of GATA3, T-bet and ROR-γt in peripheral blood mononuclear cells of patients with Allergic Asthma. Allergol Immunopathol (Madr). 2019;47(4):378–385. doi:10.1016/j.aller.2018.12.003
27. Mesquita D, Kirsztajn GM, Franco MF, et al. CD4 T helper cells and regulatory T cells in active lupus nephritis: an imbalance towards a predominant Th1 response? Clin Exp Immunol. 2018;191(1):50–59.
28. Xiang R, Xu Y, Zhang W, et al. Semaphorin 3A inhibits allergic inflammation by regulating immune responses in a mouse model of allergic rhinitis. Int Forum Allergy Rhinol. 2019;9(5):528–537. doi:10.1002/alr.22274
29. Ren M, Tang Q, Chen F, Xing X, Huang Y, Tan X. Decoction attenuates Th1 and Th2 responses in the treatment of ovalbumin-induced allergic inflammation in a rat model of allergic rhinitis. J Immunol Res. 2017;2017:8254324. doi:10.1155/2017/8254324
30. Siti Sarah CO, MdShukri N, MohdAshari NS, Wong KK. Zonula occludens and nasal epithelial barrier integrity in allergic rhinitis. PeerJ. 2020;8:e9834. doi:10.7717/peerj.9834
31. Beutel O, Maraspini R, Pombo-García K, Martin-Lemaitre C, Honigmann A. Phase separation of zonula occludens proteins drives formation of tight junctions. Cell. 2019;179(4):923–936.e11. doi:10.1016/j.cell.2019.10.011
32. Soyka MB, Wawrzyniak P, Eiwegger T, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-γ and IL-4. J Allergy Clin Immunol. 2012;130(5):1087–1096.e10. doi:10.1016/j.jaci.2012.05.052
33. Steelant B, Farré R, Wawrzyniak P, et al. Impaired barrier function in patients with house dust mite-induced allergic rhinitis is accompanied by decreased occludin and zonula occludens-1 expression. J Allergy Clin Immunol. 2016;137(4):1043–1053.e5. doi:10.1016/j.jaci.2015.10.050
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.