Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Lymphatic Alterations Under Tattoos: Preliminary Reports of One Observational Study
Authors Bourgeois P, Roman MM, Schweicher J, Lavoisier P, Maquet P, Karler C, Lizewski M , Fouarge A, Cuylits N, del Marmol V, Leduc O
Received 27 October 2022
Accepted for publication 22 December 2022
Published 27 January 2023 Volume 2023:16 Pages 257—265
DOI https://doi.org/10.2147/CCID.S393038
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Pierre Bourgeois,1– 4 Mirela Mariana Roman,5 Justine Schweicher,6 Pauline Lavoisier,6 Philippe Maquet,6 Clarence Karler,7 Mateusz Lizewski,8 Alessandro Fouarge,8 Nicolas Cuylits,8 Véronique del Marmol,1 Olivier Leduc6
1Service of Dermatology, Hospital Erasme, Université Libre de Bruxelles, Brussels, Belgium; 2Services of Nuclear Medicine, HIS-IZZ Hospitals, Université Libre de Bruxelles, Brussels, Belgium; 3Multi-Disciplinary Clinic of Lymphology, Institute Jules Bordet, Université Libre de Bruxelles, Brussels, Belgium; 4Service of Vascular Surgery, HIS-IZZ Hospitals, Université Libre de Bruxelles, Brussels, Belgium; 5Department of Mammo-Pelvic Surgery, Institute Jules Bordet, Université Libre de Bruxelles, Brussels, Belgium; 6Unité de lympho-phlébologie, Haute Ecole Bruxelles-Brabant, Haute Ecole Robert Schuman, Brussels, Belgium; 7Department of Anesthesia-Algologia, Hospital Moliere, Université Libre de Bruxelles, Brussels, Belgium; 8Service of Plastic, Reconstructive and Aesthetic Surgery, Hospital Erasme, Université Libre de Bruxelles, Brussels, Belgium
Correspondence: Pierre Bourgeois, Department of Dermatology, University Hospital Erasme, Université Libre de Bruxelles, 808, route de Lennik, Brussels, 1070, Belgium, Tel +32495201906, Email [email protected]
Background: The number of people within the European population having at least one tattoo has increased notably and with it the number of tattoo-associated clinical complications. The injected inks are known to be removed by the lymphatic vessels and can be found in the draining lymph nodes.
Aim of the Study: To report our observations on the lymphatic drainages seen under tattoos using near infrared fluorescence imaging of these lymphatic vessels after the injection of indocyanine green.
Material and Methods: Indocyanine green was injected intradermally at the basis of the 20 tattooed area(s) in 19 subjects (nine women and ten men; mean age = 28.6). Ten subjects had only black tattoos (six upper limbs and four lower limbs), five (two upper limbs and three lower limbs) black and white tattoos and five multi-colored tattooed limbs (four lower limbs and one upper limb).
Results: The imaging exams revealed alterations in eight individuals, seven of whom had tattoos on their lower limbs. Furthermore, the imaging results showed that the abnormalities might be related to the tattooed limb, the tattoo extent and colour.
Conclusion: Alterations of the cutaneous lymphatic channels are frequently observed under tattooed territories. Their causal factors should be more precisely studied in future works and these lymphatic alterations should be considered in tattooed patients when using similar imaging techniques for therapeutic and surgical assessments.
Keywords: tattoo, near infra-red fluorescence lymphatic imaging, indocyanine green, lymphatic, lymph vessel
Introduction
Fourteen percent of the European population had at least one tattoos in 2016. This figure is doubled in the 18–35 age group and the number is increasing.1 This progression is accompanied by a rise in associated clinical complications, including itching, photosensitivity, infections, allergic reactions, autoimmune diseases, scars, keloids, and various pigment changes.2,3 Swelling occurs frequently but usually is transient and rarely permanent.4
If transient swelling incident may be related to tissular inflammation, permanent ones might be related to functional and/or morphological lesions on the lymphatic system of the tattooed. The presence of inks used for tattoos in the Lymph Nodes (LN) draining tattooed areas was described more than 35 years ago,5 and, since then, frequently reported.6–12
However, their potential morphological consequences on the Lymphatic Vessels (LV) and their functioning have not been yet described to our knowledge.
Indocyanine green is used for the near infra-red fluorescence imaging (NIRFI) of the lymphatic vessels and lymph nodes in various situations, such as in patients with Breast Cancer (BC) and melanoma, for Reverse Mapping and for the evaluation and managements of oedematous situations.13–23 In our study, we report our observations regarding altered lymphatic vascular drainages under tattooed areas using near infra-red fluorescence imaging after the injection of ICG.
Materials and Methods
This prospective monocentric observational study was approved by the Investigational Review Board (IRB) of the Academic Ethical Committee Brussels Alliance for Research and Higher Education under the number (CE) B200-2020-143 and registered at ClinicalTrials.gov (NCT05515146). It complies with the declaration of Helsinki.
Between December 2021 and January 2022, nineteen healthy volunteers (nine women and ten men; mean age = 28.6, range 23–39 years) with tattoos at the level, either of the lower limb (n = 11), or of the upper limb (n = 9) were successively enrolled in the study after providing written and signed informed consent to publish their demographic data (see Table 1) and -if selected- their pictures. Except in 5 cases, tattoos were older than 2 years (mean = 52.5 months) and none had symptoms of edema at the level of the limb.
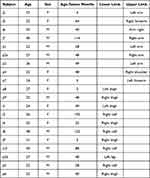 |
Table 1 Demographic Data with Localization of Their Tattoo-s |
Exclusion criteria were: (1) History of iodine allergy or anaphylactic reactions to insect bites or medications; (2) Hyperthyroidism; (3) Severe cardiac or pulmonary disease; (4) Significant renal failure (creatinine >400 μmole/l); (4) Pregnancy; (5) any known disease of the skin (such as psoriasis); (6) any disease known to affect the cutaneous tissues (such as diabetes); (7) any previous operation and/or trauma at the level of tattooed limb; (8) familial history of lower limb lymphedemas; (9) skin infection after the tattoo.
Ten were tattooed only with black inks and the others were tattooed with two or more colored inks (see Table 2).
Subjects were not limited in their normal behavior, diet, or medication intake before the study.
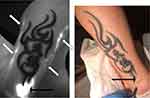
ICG 0.2 mL from 25mg of ICG diluted by 5.0 mL of sterile water for injection was injected intradermally at the basis of the tattooed cutaneous area. The injection site was chosen so that the tracer can flow under this area directly towards the LN at the root of the limb (see Figures 1 and 3).
The following figures were considered as normal (see Figures 1 and 2):
- Perfectly visible lymphatic vessels
- Fast lymphatic drainage (seen spontaneously within the 5 minutes after injection)
- Visible lymphatic drainage after stretching and massaging the injected site (3–5 minutes long)
- Direct lymphatic drainage between the injected site and the draining lymph node at the root of the limb (after 30 minutes of normal activities).
Six different patterns of lymphatic altered drainage were identified:
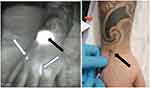
- No visualization of the lymphatic vessel(s) at the end of the imaging investigation (abnormality type 1: see Figure 3)
- Anarchic-tortuous drainage (abnormality type 2)
- Delayed drainage and/or fainter visualization of the LV when compared to the opposite limb (abnormality type 3)
- Lymphatic vessels not running under the tattooed area but seeming to bypass an obstacle and skirting the tattooed territory instead of crossing it (abnormality type 5: see Figure 4)
- Lymphatic lesion with reflux at the level of the LV draining the tattooed area (abnormality type 6)
- Downward lymphatic drainage-reflux from the injected site towards the distal part of the limb (abnormality type 4: see Figure 5).
When the situation was considered abnormal, the tracer (same characteristics in terms of volume and same intradermal route-administration) was injected the same day at the level of the opposite limb (or in case of subject j1, several weeks later and in her case, the tattooed limb was also injected with her consent).
Results: (See Table 2)
The most obvious alterations were observed in 3 cases (j1, j9 and p6) at the level of the thigh and one additional patient (p8) showed delayed drainage and/or fainter visualization of the LV when compared to the non-tattooed limb. Two subjects with tattoos at the level of the calf had lymphatic vessels not running under the tattooed area but seeming to bypass an obstacle and skirting the tattooed territory instead of crossing it (see Figure 4) and one subject (j3) had one lymphatic lesion with reflux at the level of the LV draining the tattooed area.
Eight of the twenty exams (40%) were considered as abnormal but 7 out of the 11 lower limbs and a single exam out the 9 upper limbs.
If we consider the ink color itself, ten subjects had only black tattoos (6 upper limbs and 4 lower limbs) and three exams (1 upper limb and 2 lower limbs) were considered as abnormal. Five subjects (2 upper limbs and 3 lower limbs) had black and white ink tattoos and their exams were considered normal. Finally, five subjects presented 6 multi-colored tattoos (5 lower limbs and 1 upper limb) with five exams (4 lower limbs and 1 upper limb) considered abnormal.
Discussion
Interstitially injected colloidal particles are known to accumulate in the regional lymph nodes. This phenomenon is applied to find sentinel lymph nodes (SLN) in cancerous patients but after injection of radiocolloids and/or blue dyes (such as methylene blue) and/or fluorescent dyes (such as ICG).13–15,24 Several problems have been reported with inks used to tattoo. Some can indeed mimic metastases in LN in patients with melanoma.6–11 The presence of pigment was also observed in the axillary lymph nodes (LN) of women operated for breast cancer and with tattoos, imitating the appearance of a blue sentinel lymph node on visual inspection and causing the operator to either miss the true SLN or excise more than is needed which may be important for increasing the risk of arm morbidity from SLN biopsy.25 This potential problem was also raised in women affected by cervical cancer with skin tattoos located in the lower limbs, with at least one pelvic lymph node described on pathologic examination partially or totally occupied by the ink in 40% of women.26 False-positive Positron Emission Tomography after injection of 18-fluoro-deoxy-glucose (one exam which is now more and more frequently used in the pre-operative management of many cancers) have also been described suggesting the metastatic involvement of intra-abdominal lymph nodes but from a patho-physiological point of view are related to their inflammatory status secondary to the presence of tattoo ink. Benign, swellings after sun exposure are frequent and reported by 23% of the Tattooists (who are usually heavily tattooed with multiple extended coloured tattoos).4,27,28 Although these swellings were reported rarely permanent, such localized oedematous situations raise the question of their possible origin (among others) in morphological and/or functional alterations of the underlying lymphatic vessels induced by these tattoos.
Our series is small but some of our observations are interesting: differences between lower limbs and upper limbs (with alterations more frequent in case of the former than in the later), effect of the extent of the tattoo (reflux only observed in the case with the more extensive tattoo at the level of the upper limb) and problems more frequent when other than black inks are also used.
Lymphatic alterations under the tattooed areas were seen in six of eleven subjects at the level of the lower limbs and/but in one of the nine subjects with tattooed areas at the level of the upper limbs. Tattoos of the lower limbs can involve large skin surfaces, and this suggests that the extent of the tattooed area play a role (as for/in patient only black tattooed at the level of her upper limb: see Figure 1). A nation-wide survey in Germany revealed that about 28% of tattooed individuals have more than 3 tattoos and 36% have tattoos with 900 cm2 in size or even larger.29
These complications may be partly attributed to the mechanical aspect of tattooing, which has remained largely undefined.30,31 By repetitively inserting a needle into the skin, microtraumas may promote inflammation and create ports of entry for potential pathogens.32 Moreover, ink colours seem to induce lymphatic drainage alterations differently depending on ink’s composition. Based on our observations, proportionally, 83% of multicoloured tattoos presented alterations compared to 20% of black and black and white together. More precisely, red inks tend to provoke more allergic contact dermatitis (ACD) compared to other coloured or black inks.33 It must be pointed out here that Tattooists also mention that coloured inks are inserted at a deeper plane compared to conventional black inks.
Some of these lympho-fluoroscopic figures under these tattoos are similar to what is observed at the level of the upper limb with Axillary Web Syndrome (AWS).34 These AWS situations are frequent in patients operated for Breast Cancer but have also been reported in non-cancerous situations.35–37 In the framework of lymphoscintigraphic investigations after subcutaneous injection of radiocolloids, it has been demonstrated that they are secondary to lymph stasis, lymphangitis and lymphangio-obliteration.34 In BC patients with AWS, swelling at the onset of the syndrome is frequently seen. However, with the development of collaterals, clinically obvious edema remained in only one-third of the cases and was not observed in patients who presented no edema as part of their initial symptoms. These observations could explain why lymphedema of the limbs has not been reported so far and this despite the increasing number of tattooed subjects. However, the tattooed population is young, the fashion is relatively recent and the presence of tattoos should be taken into account as part of the clinical figure in the future by vascular surgeons, lymphologists, oncologists faced with patients with limb oedemas.
Lymphatic alterations were shown years after the tattooing which suggest that they represent “long-standing” sequelae’s and established morphological alterations of the lymphatic vessels. A lymphoscintigraphic approach would have enabled us to see deep drainage not seen with ICG and would have been more physiological than with the intradermal injection of the fluorescent tracer used.38 However, it could have been less well accepted by some volunteers (due to the injection of radio-labelled particles) and a study is underway where the lymphatic network of the areas to be tattooed will be evaluated in a more physiological way before and after tattoos using fluorescence imaging but after subcutaneous injection of the ICG. The lymphatic drainages will be controlled using the same NIRFI approach two months after the tattooing and it is expected that we should observe the same lymphatic evolutions as in our lymphoscintigraphic evaluations of AWS.34
The case of the young woman with a multi-coloured tattoo from the wrist to the axilla and with lymphatic reflux in the hand (see Figure 5) may raise awareness of the problem raised by such tattoo. She can be considered as at risk for upper limb lymphedema if she had to be operated and to undergo one complete axillary node dissection for breast cancer. Such tattoos should therefore be discouraged for some subjects especially if they have other risk factors for such operations, as for instance when a familial history of Breast Cancer or of primary lymphedema is reported. In tattooed patients with surgery planned for a cancer at the level of one limb, their presence should also be considered as an indication for realization of a pre-operative evaluation of the lymphatic situation and function of their limbs and thereafter, either (if possible) for a sentinel lymph node selective lymphadenectomy, or for one LYMPHA approach if a complete axillary lymph node dissection is planned. An Axillary Reverse Mapping approach (with injection-s of ICG around and/or in the tattooed area) to identify the axillary lymph nodes draining the arm and the tattooed area could also be considered. For patients who would benefit from a CALND, especially if followed by a radiotherapy, their post-therapeutic management could also be adapted.39–43
Conclusion
Our work shows the presence of abnormal Lymphatic drainage under tattooed cutaneous areas in an unexpected high number of subjects. Consequently, further works are justified to define the causative factors of these lesions in a greater degree. Such alterations Such lymphatic alterations should also be considered in patients with limb edema or at risk for edema.
Data Sharing Statement
All relevant data are within the paper.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was received. This study was carried out on personal background.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Commission E, Centre JR, Senaldi C., et al. Safety of Tattoos and Permanent Make-Up: Final Report. Publications Office; 2017.
2. Kazandjieva J, Tsankov N. Tattoos: dermatological complications. Clin Dermatol. 2007;25(4):375–382. doi:10.1016/j.clindermatol.2007.05.012
3. Huisman S, van der Bent SAS, Maijer KI, Tio D, Rustemeyer T. Cutaneous non-allergic complications in tattoos: an overview of the literature. Presse Med. 2020;49(4):104049. doi:10.1016/j.lpm.2020.104049
4. Kluger N. Self-reported tattoo reactions in a cohort of 448 French tattooists. Int J Dermatol. 2016;55(7):764–768. doi:10.1111/ijd.13030
5. Yang TJ. Green coloration of superficial cervical lymph nodes in dogs tattooed in the ear. Zentralbl Veterinarmed A. 1986;33(10):788–790. doi:10.1111/j.1439-0442.1986.tb00592.x
6. Back L, Brown AS. Metastatic melanoma, or is it? Plast Reconstr Surg. 1986;77(1):138–140. doi:10.1097/00006534-198601000-00022
7. Anderson LL, Cardone JS, McCollough ML, Grabski WJ. Tattoo pigment mimicking metastatic malignant melanoma. Dermatol Surg. 1996;22(1):92–94. doi:10.1111/j.1524-4725.1996.tb00578.x
8. Moehrle M, Blaheta HJ, Ruck P. Tattoo pigment mimics positive sentinel lymph node in melanoma. Dermatology. 2001;203(4):342–344. doi:10.1159/000051787
9. Friedman T, Westreich M, Mozes SN, Dorenbaum A, Herman O. Tattoo pigment in lymph nodes mimicking metastatic malignant melanoma. Plast Reconstr Surg. 2003;111(6):2120–2122. doi:10.1097/01.PRS.0000057101.95872.A1
10. Jack CM, Adwani A, Krishnan H. Tattoo pigment in an axillary lymph node simulating metastatic malignant melanoma. Int Semin Surg Oncol. 2005;2:28. doi:10.1186/1477-7800-2-28
11. Chikkamuniyappa S, Sjuve-Scott R, Lancaster-Weiss K, Miller A, Yeh IT. Tattoo pigment in sentinel lymph nodes: a mimicker of metastatic malignant melanoma. Dermatol Online J. 2005;11(1):14. doi:10.5070/D31B43M26H
12. Soran A, Menekse E, Kanbour-Shakir A, et al. The importance of tattoo pigment in sentinel lymph nodes. Breast Dis. 2017;37(2):73–76. doi:10.3233/BD-170282
13. Murawa D, Hirche C, Dresel S, Hunerbein M. Sentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescence. Br J Surg. 2009;96(11):1289–1294. doi:10.1002/bjs.6721
14. Fujiwara M, Mizukami T, Suzuki A, Fukamizu H. Sentinel lymph node detection in skin cancer patients using real-time fluorescence navigation with indocyanine green: preliminary experience. J Plast Reconstr Aesthet Surg. 2009;62(10):e373–8. doi:10.1016/j.bjps.2007.12.074
15. Niebling MG, Pleijhuis RG, Bastiaannet E, Brouwers AH, van Dam GM, Hoekstra HJ. A systematic review and meta-analyses of sentinel lymph node identification in breast cancer and melanoma, a plea for tracer mapping. Eur J Surg Oncol. 2016;42(4):466–473. doi:10.1016/j.ejso.2015.12.007
16. Unno N, Nishiyama M, Suzuki M, et al. A novel method of measuring human lymphatic pumping using indocyanine green fluorescence lymphography. J Vasc Surg. 2010;52(4):946–952. doi:10.1016/j.jvs.2010.04.067
17. Unno N, Nishiyama M, Suzuki M, et al. Quantitative lymph imaging for assessment of lymph function using indocyanine green fluorescence lymphography. Eur J Vasc Endovasc Surg. 2008;36(2):230–236. doi:10.1016/j.ejvs.2008.04.013
18. Yamamoto T, Narushima M, Yoshimatsu H, et al. Dynamic Indocyanine Green (ICG) lymphography for breast cancer-related arm lymphedema. Ann Plast Surg. 2014;73(6):706–709. doi:10.1097/SAP.0b013e318285875f
19. Narushima M, Yamamoto T, Ogata F, Yoshimatsu H, Mihara M, Koshima I. Indocyanine Green Lymphography Findings in Limb Lymphedema. J Reconstr Microsurg. 2016;32(1):72–79. doi:10.1055/s-0035-1564608
20. Medina-Rodriguez ME, de-la-Casa-Almeida M, Martel-Almeida E, Ojeda-Cárdenes A, Medrano-Sánchez EM. Visualization of Accessory Lymphatic Pathways, before and after Manual Drainage, in Secondary Upper Limb Lymphedema Using Indocyanine Green Lymphography. J Clin Med. 2019;8(11):1917. doi:10.3390/jcm8111917
21. Tashiro K, Yamashita S, Saito T, Iida T, Koshima I. Proximal and distal patterns: different spreading patterns of indocyanine green lymphography in secondary lower extremity lymphedema. J Plast Reconstr Aesthet Surg. 2016;69(3):368–375. doi:10.1016/j.bjps.2015.10.042
22. Bell RD, Rahimi H, Kenney HM, et al. Altered lymphatic vessel anatomy and markedly diminished lymph clearance in affected hands of patients with active rheumatoid arthritis. Arthritis Rheumatol. 2020;72(9):1447–1455. doi:10.1002/art.41311
23. Yang JC, Wu SC, Chiang MH, Lin WC, Hsieh CH. Intraoperative identification and definition of “functional” lymphatic collecting vessels for supermicrosurgical lymphatico-venous anastomosis in treating lymphedema patients. J Surg Oncol. 2018;117(5):994–1000. doi:10.1002/jso.25014
24. Di Donna MC, Quartuccio N, Giallombardo V, et al. Detection of sentinel lymph node in vulvar cancer using (99m)Tc-labeled colloid lymphoscintigraphy, blue dye, and indocyanine-green fluorescence: a meta-analysis of studies published in 2010-2020. Arch Gynecol Obstet. 2022. doi:10.1007/s00404-022-06605-1
25. Soran A, Menekse E, Kanbour-Shakir A, et al. The importance of tattoo pigment in sentinel lymph nodes. Breast Dis. 2017;37(2):73. doi:10.3233/BD-170282
26. Kohler C, Foiato T, Marnitz S, et al. Potential surgical and oncologic consequences related to skin tattoos in the treatment of cervical cancer. J Minim Invasive Gynecol. 2016;23(7):1083–1087. doi:10.1016/j.jmig.2016.07.016
27. Grove N, Zheng M, Bristow RE, Eskander RN. Extensive tattoos mimicking lymphatic metastasis on positron emission tomography scan in a patient with cervical cancer. Obstet Gynecol. 2015;126(1):182–185. doi:10.1097/AOG.0000000000000701
28. Nam H, Smith S, Laing R. A pitfall of 18-fluorodeoxyglucose-PET in a patient with a tattoo. Lancet Oncol. 2007;8(12):1147–1148. doi:10.1016/S1470-2045(07)
29. Klugl I, Hiller KA, Landthaler M, Baumler W. Incidence of health problems associated with tattooed skin: a nationwide survey in German-speaking countries. Dermatology. 2010;221(1):43–50. doi:10.1159/000292627
30. Gálvez LO, Pérez MB, Rivas DF. High speed imaging of solid needle and liquid micro-jet injections. J Appl Phys. 2019;125(14):144504. doi:10.1063/1.5074176
31. Cu K, Bansal R, Mitragotri S, Fernandez Rivas D. Delivery strategies for skin: comparison of nanoliter jets, needles and topical solutions. Ann Biomed Eng. 2020;48(7):2028–2039. doi:10.1007/s10439-019-02383-1
32. Serup J, Carlsen KH, Sepehri M. Tattoo complaints and complications: diagnosis and clinical spectrum. Curr Probl Dermatol. 2015;48:48–60. doi:10.1159/000369645
33. Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82(2):73–82. doi:10.1111/cod.13423
34. Roman MM, Barbieux R, Eddy C, et al. Lymphoscintigraphic investigations for axillary web syndromes. Lymphat Res Biol. 2022;20(4):417–424. doi:10.1089/lrb.2021.0015
35. Welsh P, Gryfe D. Atypical presentation of axillary web syndrome (AWS) in a male squash player: a case report. J Can Chiropr Assoc. 2016;60(4):294–298.
36. Zhang Q, Tan C. Axillary web syndrome following granulomatous inflammation after folliculitis. Eur J Dermatol. 2016;26(3):314–315. doi:10.1684/ejd.2016.2762
37. Rashtak S, Gamble GL, Gibson LE, Pittelkow MR. From furuncle to axillary web syndrome: shedding light on histopathology and pathogenesis. Dermatology. 2012;224(2):110–114. doi:10.1159/000337210
38. Bourgeois P, Leduc O, Belgrado JP, Leduc A. Scintigraphic investigations of the superficial lymphatic system: quantitative differences between intradermal and subcutaneous injections. Nucl Med Commun. 2009;30(4):270–274. doi:10.1097/MNM.0b013e32831bec4d
39. Boccardo F, Valenzano M, Costantini S, et al. LYMPHA technique to prevent secondary lower limb lymphedema. Ann Surg Oncol. 2016;23(11):3558–3563. doi:10.1245/s10434-016-5282-4
40. Boccardo FM, Casabona F, Friedman D, et al. Surgical prevention of arm lymphedema after breast cancer treatment. Ann Surg Oncol. 2011;18(9):2500–2505. doi:10.1245/s10434-011-1624-4
41. Roman MM, Nogaret JM, Veys I, et al. The impact of temporal variation in ICG administration on axillary node identification during reverse mapping procedures. Chirurgia. 2022;117(3):305–311. doi:10.21614/chirurgia.2739
42. Foster D, Choy N, Porter C, et al. Axillary reverse mapping with indocyanine green or isosulfan blue demonstrate similar crossover rates to radiotracer identified sentinel nodes. J Surg Oncol. 2018;117(3):336–340. doi:10.1002/jso.24859
43. Schwarz GS, Grobmyer SR, Djohan RS, et al. Axillary reverse mapping and lymphaticovenous bypass: lymphedema prevention through enhanced lymphatic visualization and restoration of flow. J Surg Oncol. 2019;120(2):160–167. doi:10.1002/jso.25513
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.