Back to Journals » Clinical Interventions in Aging » Volume 15
Lymph Nodes Dissection in Elderly Patients with T3-T4 Laryngeal Cancer
Authors Pan Y, Zhao X, Zhao D, Liu J
Received 23 September 2020
Accepted for publication 18 November 2020
Published 8 December 2020 Volume 2020:15 Pages 2321—2330
DOI https://doi.org/10.2147/CIA.S283600
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Zhi-Ying Wu
Yafeng Pan,1 Xuye Zhao,2 Dean Zhao,1 Junhua Liu1
1Department of Otolaryngology, Xiang’an Hospital of Xiamen University, Xiamen, Fujian, People’s Republic of China; 2Department of Breast and Thyroid Surgery, Xiang’an Hospital of Xiamen University, Xiamen, Fujian, People’s Republic of China
Correspondence: Junhua Liu
Department of Otolaryngology, Xiang’an Hospital of Xiamen University, Xiamen, Fujian 361101, People’s Republic of China
Email [email protected]
Objective: To explore the survival value of lymph node dissection (LND) in elderly patients with T3-T4 laryngeal cancer, analyze the risk factors of lymph node metastasis, and construct a preoperative prediction model.
Materials and Methods: The study included 996 patients aged ≥ 65 years with laryngectomy confirmed T3-T4 laryngeal cancer queried from Surveillance, Epidemiology and End Results (SEER) database between 2010 and 2017. Propensity score matching (PSM) was applied to balance the effects of confounding factors. Kaplan–Meier (K–M) analysis and competitive risk model were used to compare the overall survival (OS) and cancer-specific survival (CSS) between LND and no-LND (N-LND) group. Combined with risk factors of multivariate logistic regression, a nomogram was built to predict lymph node metastasis preoperatively. The performance was assessed in the training set and the validation set, and internal validation was assessed.
Results: Among the cohort, 822 patients underwent LND and 410 patients had positive lymph nodes. The OS and CSS of patients who underwent LND were not better than that of N-LND patients (P> 0.05). The prognosis of patients with lymph node metastases was significantly worse than that of negative patients (P< 0.05). On multivariate logistic regression, supraglottis cancer, tumor size > 5cm and grade 3– 4 classification were associated with significantly greater odds of lymph node metastasis. The nomogram showed favorable predictive efficacy and good calibration (in the training cohort C-index=0.700; in the validation cohort C-index=0.721).
Conclusion: For elderly patients with T3-T4 laryngeal cancer, LND did not bring significant survival values. Supraglottis cancer, tumor size > 5cm and grade 3– 4 classification were independent risk factors of lymph node metastasis, which means poor prognosis. The nomogram developed was an easy-to-use tool for lymph node prediction.
Keywords: elderly, T3-T4 laryngeal cancer, lymph node dissection, survival, nomogram
Introduction
With the globalization of population aging, the elderly has become a vulnerable group suffering head and neck cancers. From 2000 to 2014, the number of patients aged ≥65 years with head and neck cancers increased by nearly 45%, and the number of patients aged ≥80 years increased by 70%.1 There will be 12,370 newly diagnosed laryngeal cancers, one of the common malignant tumors of the head and neck, according to the analysis of data collected by the Surveillance, Epidemiology and End Results (SEER) database in 2016, which causes approximately 3750 deaths in the United States in 2020.2 Approximately 60% of patients present with advanced (III or IV classification) disease when they are first diagnosed.3
In the American Society of Clinical Oncology (ASCO) Clinical Practice Guideline, there are clear guidelines for diagnosis and treatment of laryngeal cancer: Patients with advanced lesions of the glottis and all patients with supraglottic lesions should have elective treatment of the neck, even if clinically N0.4 However, there is no individualized treatment for the special population of elderly patients. It is well known that the elderly is highly likely to suffer from respiratory and cardiovascular diseases. Studies have shown that for elderly patients, increased operation time and more invasive surgeries can increase the incidence of complications.5,6 There are many clinical studies on the prognosis of lymph node metastasis in laryngeal cancer, but there is no specific study on the special population of elderly advanced laryngeal cancer.
To the best of our knowledge, this article is the first to study the significance of lymph node dissection (LND) in elderly patients with advanced laryngeal cancer, to explore the population that really benefits from LND, and to provide help for the personalized treatment of this special population clinically.
Methods
Patients
A total of 20,357 cases of patients with laryngeal cancer were clearly diagnosed from 2010 to 2017 in the SEER database. Our research focused on patients aged ≥65 years diagnosed with T3-T4 laryngeal cancer, who received partial or total laryngectomy. Exclusion criteria: 1) unknown survival and incomplete follow-up time; 2) diagnosed autopsy/death certificate only; 3) unknown LND information.
The variables incorporated into the analysis included age at diagnosis, gender, race, income, tumor site (ICD-O-3 site code), tumor laterality, TNM Classification (AJCC, 7th edition, 2010), tumor size, histologic grade (Fuhrman grade) and surgery, including subsequent information such as survival status, survival period and cause of death were all extracted from the database.
The National Institute on Ageing classified the aging population into “young old” (65–74), “older old” (75–84) and “oldest old” (≥85). According to tumor location, patients were divided into four groups: “Glottis”, “Supraglottis”, “Subglottis” and “Other”. Among the code, “laryngeal cartilage”, “Overlapping lesion of larynx” and “Larynx, NOS” were not common in clinical classification with fewer cases, which were incorporated into the other items. Surgery treatment included partial laryngectomy (C30) and radical laryngectomy (C40).
Statistical Analysis
Firstly, the propensity score matching (PSM) was used to obtain matched data to reduce the influence of data deviation and confounding variables. Kaplan–Meier (K–M) analysis and the Log rank tests were applied to evaluate the survival differences of overall survival (OS) and cancer-specific survival (CSS) between LND and no-LND (N-LND) subgroups, and competitive risk model was used to further explore the differences in CSS between subgroups. Multivariable Logistic Regression was performed to assess the independent risk factors of lymph node metastasis in the elderly with T3-T4 laryngeal cancer, and establish a visual model that can predict lymph node metastasis preoperatively. The calibration of the nomogram was assessed with a calibration curve, and the AUC was calculated to quantify the discrimination performance of the nomogram.7
The statistical software used included SPSS version 24.0 software package (IBM Corporation, Armonk, NY, USA) and R language (version 3.6.0 R Foundation). All analyses were two-sided with statistical significance set at 0.05.
Results
Clinical Characteristics of Patients
There was a total of 969 elderly patients with T3-T4 laryngeal cancer eligible for screening in the SEER database from 2010 to 2017. Consistent with the epidemiological study of laryngeal cancer, male and squamous cell carcinoma were more common in the cohort. Among TNM Classification, T4 classification was more common than T3 classification, which were 612 cases (63.2%) and 357 cases (36.8%) respectively. There were 533 cases (55.0%) in N0 classification and only 20 cases of patients with distant metastasis (2.0%). In terms of treatment, the vast majority of advanced patients still choose total laryngectomy (890 cases, 91.8%), 822 cases (84.8%) also underwent LND at the same time, of which 410 cases (50%) clearly had lymph nodes metastases. In PSM analysis, variables other than LND were matched according to 1:3. The matching tolerance was set as 0.1 and 433 cases were matched (Table 1).
 |
Table 1 The Clinical Characteristics of Elderly Patients T3-T4 Laryngeal Cancer Before and After Propensity Score Matching |
Survival
Before PSM, although the median survival time (MST) of OS in patients having LND was 33.0 months (95% Confidence Interval [CI], 28.4–37.6; P=0.885), which was slightly longer than patients with N-LND (MST, 32.0 months; 95%, CI, 24.1–39.9; P=0.885). The MST of LND patients on CSS (MST, 51.0 months; 95% CI, 38.9–63.1; P=0.569) was lower than that of N-LND patients (MST, 58.0 months; 95% CI, 48.6–62.6; P=0.569). Whether in terms of OS or CSS, the difference between the two subgroups was not statistically significant (Figure 1A and C). In the competitive risk model, there was no significant difference between LND and N-LND patients, which yielded a P-value of 0.540 (Figure 2A).
 |
Figure 2 Competing risk curve of CSS among elderly patients with T3-T4 laryngeal cancer: before PSM (A) and after PSM (B). |
After PSM, the MST of patients undergoing LND (OS: MST, 43.0 months; 95% CI, 31.4–54.6; P=0.055; CSS: MST, 73.0 months; 95% CI, 62.4–83.6; P=0.091) was better than N-LND patients (OS: MST, 31.0 months; 95% CI, 22.3–39.7; P=0.055; CSS: MST, 54.0 months; 95% CI, 44.1–57.6; P=0.091), but the difference was still not statistically significant (Figure 1B and D), and neither was the CSS difference between the two groups in risk model analysis (P=0.091). (Figure 2B)
For patients with clear lymph node metastasis, whether measured in OS (MST, 22.0 months; 95% CI, 18.4–25.6; P<0.001), or CSS (MST, 30.0 months; 95% CI, 24.5–35.5; P<0.001), were significantly lower than that of patients without lymph node metastases (OS: MST, 46.0 months; 95% CI, 34.6–57.4; P<0.001; CSS: MST, 80.0 months, 95% CI, 70.6–89.9, P<0.001). (Figure 3A and B)
 |
Figure 3 Kaplan–Meier curves of OS (A) and CSS (B) according to whether or not lymph node metastasis has. |
Predictive Factors for Lymph Node Metastasis
On multivariable logistic regression among the elderly with advanced laryngeal cancer, subglotitis (vs glottis: odds ratio [OR], 0.503; 95% CI, 0.239–1.059; P=0.070) and supraglottis (vs glottis: OR, 2.995; 95% CI, 2.029–4.422; P<0.001), tumor size 3–5cm (vs tumor size <3cm: OR, 1.683; 95% CI, 1.178–2.403; P=0.004) or tumor size >5cm (vs tumor size <3cm: OR, 3.010; 95% CI, 1.732–5.232; P<0.001), and grade 3–4 classification (vs grade 1–2 classification: OR, 2.101; 95% CI, 1.525–2.893; P<0.001) were associated with significantly greater odds of lymph nodes metastasis. Gender, income and race were not risk factors for lymph node metastasis on the multivariable model. Significant results are presented in Table 2.
 |
Table 2 Multivariable Cox Regression of Risk Factors for Lymph Node Metastasis Among Patients Who Underwent Lymph Node Dissection |
Generation of an Individualized Prediction Model
To provide a quantitative tool for clinicians, we attempted to construct an individualized nomogram model for the prediction of lymph node metastasis in the elderly with advanced laryngeal cancer. According to multivariable logistic regression, tumor site, tumor size and histological grade were significant predictive factors, which were analysis clinical candidate predictors in the prediction model (Figure 4A). We can calculate a risk score for each patient using the nomogram.
Good calibration was observed for the probability of lymph node metastasis in the training cohort (Figure 4B). The favorable calibration of the nomogram was confirmed with the validation set (Figure 4C). The nomogram showed favorable predictive efficacy, with an AUC of 0.705 (95% CI, 0.657–0.752) in the training set and 0.721 (95% CI, 0.664–0.777) in the validation set (Figure 4D and E).
The decision curve analysis for the nomogram is presented in Figure 5, which indicated that when the high-risk threshold for a doctor or a patient is within a range from 0.1 to 0.7, the prediction nomogram adds more net benefit than intervening either allor no patients.
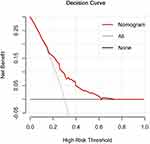 |
Figure 5 Decision curve analysis for the prediction nomogram. |
Discussion
Due to dysfunction of cardiopulmonary reserve, poor tolerance to drug therapy, short natural life span and other factors, elderly patients are inclined to accept minimally invasive surgery or radiotherapy and chemotherapy. The guideline for laryngeal cancer clearly provided that patients with advanced classifications must have elective LND.4 Owing to the particularity of the elderly population, LND means prolonging operation time and increasing surgical trauma. There is still a lack of large-scale multi-institution retrospective or prospective studies on the effect of LND on the survival and prognosis of elderly patients with advanced laryngeal cancer. This study was the first to explore the significance of LND for elderly patients with T3-T4 laryngeal cancer and constructed the first preoperative individualized prediction to evaluate lymph node metastasis.
In K–M analysis, the MST of OS in elderly patients with T3-T4 laryngeal cancer was more than 30 months, and the MST of CSS was more than 50 months, regardless of whether LND was performed. This may be related to the particularity of the elderly population. It is known to us that elderly patients are at greater risk of cardiovascular and respiratory diseases.8,9 Besides, due to factors such as function degradation and reduced immunity, the natural life span of the elderly is relatively short. Therefore, laryngeal cancer is not the most important cause of death in elderly patients.10 Unexpectedly, although the prognosis of LND patients was better than that of N-LND patients, the difference was not statistically significant. It has been suggested that prolonged operative times and more invasive procedures will increase surgical complications in elderly patients with laryngeal cancer.5,6 In patients with advanced laryngeal cancer, greater surgical trauma and surgical complications increased the risk of 30-day readmission, which was associated with an increase in long-term and short-term mortality of patients.11
In summary, it is obvious that not all elderly patients with advanced laryngeal cancer will benefit from LND. For patients with laryngeal cancer, the more advanced the T classification gets, the greater possibility of lymph node metastasis there is.12–15 Positive cervical lymph nodes have a negative impact on the patient’s prognosis, and LND for such patients can improve survival significantly.16 Similarly, our study also showed that the survival of elderly patients with laryngeal cancer (including OS and CSS) with lymph node metastasis was significantly inferior to that of patients without metastasis. On the contrary, if the clinically over-assessment of lymph nodes was performed, LND for patients without lymph node metastasis will not bring a better prognosis.17,18
Therefore, whether LND is performed for patients with advanced laryngeal cancer depends on the preoperative assessment of lymph node metastasis. On multivariable logistic regression, tumor site, tumor size and histological grade were associated with significantly greater odds of lymph nodes metastasis. Consistent with most studies, supraglottic cancer was a risk factor for lymph node metastasis,15,19,20 whose relative risk of lymph node metastasis was close to 3 times that of glottic cancer. For poorly differentiated laryngeal cancer, the possibility of lymph node metastasis was significantly higher than that of well-differentiated cancer,14,20 and the relative risk of lymph node metastasis was twice that of well-differentiated laryngeal cancer. In addition, we also found a two-fold increase in lymph node metastasis in patients with tumor size >5cm compared with those with tumor size <3cm.
In order to better assist the clinical work, we constructed and validated a nomogram for the preoperative individualized prediction of lymph node metastasis in elderly patients with T3-T4 laryngeal cancer. The tumor site and tumor size can be easily obtained from the preoperative laryngoscope and CT test. Histological grade can be obtained through preoperative biopsy. Therefore, we recommended that the nomogram integrate tumor site, tumor size and histologic grade with satisfactory discrimination achieved in the primary cohort (C-index, 0.700), which was then improved in the validation cohort (C-index, 0.721).
Our study still had limitations that should be highlighted. It was a retrospective analysis based purely on SEER database. The incapacity to report physical function scores, comorbidity and some detailed information of the host in SEER, such as lymphocyte count, which may influence the treatment and survival. Some variables contained “unknown” category bringing statistical bias. Furthermore, patients included in our study were treated in different medical centers. Therefore, the quality of operations and the methods for diagnosing metastatic lymph nodes were not unified, which might influence the results of our study to some extent. Nevertheless, SEER database is an authoritative information source affiliated to the National Cancer Institute (NCI), whose statistical results are still representative. In the future, prospective clinical studies with larger cohorts are required to testify its real estimation capacity.
Conclusion
In this study, we found that: 1. The survival of elderly patients with T3-T4 laryngeal cancer underwent LND were not better than that for N-LND patients; 2. Supraglottis cancer, tumor size>5cm and histologic grade 3–4 classification were associated with significantly greater odds of lymph nodes metastasis; 3. A nomogram was constructed with favorable predictive efficacy to facilitate the preoperative individualized prediction of lymph node metastasis.
Compliance with Ethical Standards
All procedures performed in studies involving human participants conform to the standards of the institutional and national ethics committees, as well as to the 1964 Helsinki Declaration and subsequent relevant ethics. We obtained the authorization to access the SEER database, with the number 14,260-Nov2016. Patient informed consent is not required to extract data from the SEER database.
Acknowledgments
The authors would like to thank SEER program for public access to the database.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Halmos GB, Bras L, Siesling S, van der Laan BFAM, Langendijk JA, van Dijk BAC. Age-specific incidence and treatment patterns of head and neck cancer in the Netherlands-a cohort study. Clin Otolaryngol. 2018;43:317–324. doi:10.1111/coa.12991
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi:10.3322/caac.21590
3. Groome PA, O’Sullivan B, Irish JC, et al. Management and outcome differences in supraglottic cancer between Ontario, Canada, and the surveillance, epidemiology, and end results areas of the United States. J Clin Oncol. 2003;21:496–505. doi:10.1200/JCO.2003.10.106
4. Forastiere AA, Ismaila N, Lewin JS, et al. Use of larynx-preservation strategies in the treatment of laryngeal cancer: American Society Of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36:1143–1169. doi:10.1200/JCO.2017.75.7385
5. Crosetti E, Caracciolo A, Molteni G, et al. Unravelling the risk factors that underlie laryngeal surgery in elderly. Acta Otorhinolaryngol Ital. 2016;36:185–193.
6. Teymoortash A, Bohne F, Kissing L, et al. Oncological and surgical outcome of total laryngectomy in combination with neck dissection in the elderly. Eur Arch Otorhinolaryngol. 2016;273:1825–1833. doi:10.1007/s00405-016-3970-0
7. Wu S, Zheng J, Li Y, et al. A radiomics nomogram for the preoperative prediction of lymph node metastasis in bladder cancer. Clin Cancer Res. 2017;23:6904–6911. doi:10.1158/1078-0432.CCR-17-1510
8. van de Schans SA, Janssen-Heijnen ML, Biesma B, et al. COPD in cancer patients: higher prevalence in the elderly, a different treatment strategy in case of primary tumours above the diaphragm, and a worse overall survival in the elderly patient. Eur J Cancer. 2007;43:2194–2202. doi:10.1016/j.ejca.2007.08.011
9. Mulcahy CF. Age-adjusted comorbidity and survival in locally advanced laryngeal cancer. Head Neck. 2018;40:2060–2069.
10. Chen AY, Matson LK, Roberts D, Goepfert H. The significance of comorbidity in advanced laryngeal cancer. Head Neck. 2001;23:566–572. doi:10.1002/hed.1079
11. Chaudhary H, Stewart CM, Webster K, et al. Readmission following primary surgery for larynx and oropharynx cancer in the elderly. Laryngoscope. 2017;127:631–641. doi:10.1002/lary.26311
12. Waldfahrer F, Hauptmann B, Iro H. [Lymph node metastasis of glottic laryngeal carcinoma]. Laryngorhinootologie. 2005;84:96–100. doi:10.1055/s-2004-826075.German.
13. Bulğurcu S, İdil M, Küçük Ü, Çukurova İ. The effect of extranodal extension on survival in laryngeal carcinoma. Ear Nose Throat J. 2020;99:305–308. doi:10.1177/0145561319862211
14. Chen LY, Weng WB, Wang W, Chen JF. Analyses of high-risk factors for cervical lymph node metastasis in laryngeal squamous cell carcinoma and establishment of nomogram prediction model. Ear Nose Throat J. 2020;145561320901613.
15. Sanabria A, Shah JP, Medina JE, et al. Incidence of occult lymph node metastasis in primary larynx squamous cell carcinoma, by subsite, T classification and neck level: a systematic review. Cancers (Basel). 2020;12:1059. doi:10.3390/cancers12041059
16. Rodrigo JP, Grilli G, Shah JP, et al. Selective neck dissection in surgically treated head and neck squamous cell carcinoma patients with a clinically positive neck: systematic review. Eur J Surg Oncol. 2018;44:395–403. doi:10.1016/j.ejso.2018.01.003
17. Ketterer MC, Lemus MLA, Beitinger U, Pfeiffer J, Knopf A, Becker C. Surgical nodal management in hypopharyngeal and laryngeal cancer. Eur Arch Otorhinolaryngol. 2020;277:1481–1489. doi:10.1007/s00405-020-05838-7
18. Fiorella R, Di NV, Fiorella ML, Russo C. “Conditional” neck dissection in management of laryngeal carcinoma. Acta Otorhinolaryngol Ital. 2006;26:356–359.
19. Petrović Z. Selective neck dissection for cervical node negative supraglottic carcinoma. J BUON. 2002;7:53–56.
20. Zhang Y, Xu S, Liu W, et al. Rational choice of neck dissection in clinically N0 patients with supraglottic cancer. Head Neck. 2020;42:365–373. doi:10.1002/hed.26014
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


