Back to Journals » Cancer Management and Research » Volume 10
Lymph node ratio as a valuable prognostic factor for patients with colorectal liver-only metastasis undergoing curative resection
Authors Deng YX , Peng JH, Zhao YJ, Sui QQ , Zhao RX, Lu ZH, Qiu MZ , Lin JZ, Pan ZZ
Received 22 March 2018
Accepted for publication 3 May 2018
Published 17 July 2018 Volume 2018:10 Pages 2083—2094
DOI https://doi.org/10.2147/CMAR.S169029
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Yuxiang Deng,1,* Jianhong Peng,1,* Yujie Zhao,1 Qiaoqi Sui,1 Ruixia Zhao,2 Zhenhai Lu,1 Miaozhen Qiu,3 Junzhong Lin,1 Zhizhong Pan1
1Department of Colorectal Surgery, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, People’s Republic of China; 2Department of Public Health, School of Public Health, Sun Yat-sen University, Guangzhou, People’s Republic of China; 3Department of Medical Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Background: Recent studies have suggested that the lymph node ratio (LNR) is a prognostic indicator for various malignancies. However, LNR has not been evaluated in colorectal liver-only metastasis (CRLM). This study aimed to investigate the prognostic value of LNR in patients with CRLM after curative resection.
Patients and methods: We retrospectively investigated the clinicopathologic features of 154 CRLM patients who underwent curative resection between 2005 and 2015. We classified patients into low and high groups based on their LNR by using the X-tile software. Survival curves were plotted through Kaplan–Meier method and compared by log-rank test. Cox proportional hazards analysis was performed to identify the factors associated with recurrence-free survival (RFS) and overall survival (OS).
Results: The patients were divided into two groups in which 124 patients were identified as LNR ≤0.33 and 30 patients as LNR >0.33. Compared to low LNR, high LNR was significantly associated with poor 3-year RFS (47.2% vs 16.7%, P=0.001) and OS (72.8% vs 45.3%, P=0.003) rates. Multivariate analysis indicated that the LNR was an independent predictor for 3-year RFS (hazard ratio, 2.124; 95% CI, 1.339–3.368; P=0.001) and OS (HR, 2.287; 95% CI, 1.282–4.079; P=0.005). However, the node (N) stage and lymph node distribution were not significantly associated with the 3-year RFS (P=0.071, P=0.226) or OS (P=0.452, P=0.791) in patients with CRLM.
Conclusion: This study demonstrated that LNR was an independent predictor for 3-year RFS and OS in patients with CRLM who underwent curative resection and that its prognostic value was superior to that of N stage and lymph node distribution.
Keywords: colorectal cancer, liver metastases, lymph node ratio, N stage, prognosis
Introduction
Hepatic resection is the main choice for the curative treatment of colorectal liver-only metastasis (CRLM).1 However, 75% of patients experience tumor recurrence after the first liver resection,2 and only 16% of these patients remain disease free for 10 years after hepatectomy.3 Therefore, the management of CRLM remains challenging. Understanding the prognostic factors affecting CRLM is important to individualize chemotherapy and to achieve maximum therapeutic effectiveness according to patient classification.
In recent years, the lymph node ratio (LNR), defined as the ratio between the number of metastatic lymph nodes (LNs) and surgically removed LNs, has been suggested as a prognostic factor in malignancies, including pancreatic, breast, and lung cancers.4–6 Moreover, the LNR has been recently demonstrated as a prognostic indicator for disease-free survival in patients with stage III colorectal cancer.7 However, the role of LNR in patients with CRLM who are undergoing curative resection has not been investigated until now. To date, the number of metastatic LNs is well accepted as one of the most important prognostic determinants for patients with stage IV colorectal cancer as acknowledged in the American Joint Committee on Cancer (AJCC) TNM classification of colorectal cancer.8,9 Nevertheless, the number of regional LNs retrieved from a surgical specimen can be affected by multiple factors, including surgical technique, patient age, immune response, and tumor grade and location.10–14 In addition, lymph node distribution (LND) has been shown to affect clinical outcomes in lung and esophageal cancer patients.15,16 Another study indicated LND as a prognostic index in patients with stage III colon cancer.17 However, LND is also affected by surgical technique and tumor grade.18–20 Therefore, the prognostic value of node (N) stage and LND is limited for cancer patients.
Accordingly, this study aimed to investigate the prognostic value of LNR, N stage, and LND in patients with CRLM who are undergoing curative resection and to determine an accurate prognostic prediction by comparing the prognostic values of these indexes.
Patients and methods
Patient population
We retrospectively examined data from consecutive patients with synchronous CRLM who underwent curative resection both for primary tumor and liver metastases at Sun Yat-sen University Cancer Center from January 2005 to July 2015. The enrolled patients met the following inclusion criteria: 1) histologically confirmed adenocarcinoma, 2) preoperative metastases confined to the liver, 3) R0 resection for primary lesions and metastases, and 4) synchronous primary tumor and liver metastases. Patients were excluded from the analysis if they died within 1 month of operation, had incomplete pathologic data, or were lost to follow-up within 3 months. Tumor stage was classified according to the 2010 AJCC staging system. The present study was undertaken according to the ethical standards of the World Medical Association Declaration of Helsinki. Institutional review board approval was obtained from independent ethics committees at the Sun Yat-sen University Cancer Center. Informed consent was waived because of the nature of retrospective study, and the patient data were kept confidentially.
Patient treatments
Treatment strategies including perioperative chemotherapy, radiofrequency ablation (RFA) therapy for hepatic metastases, and surgery were discussed by a multidisciplinary team. Patients whose tumors were deemed resectable with a low risk of recurrence were directly recommended for surgery, otherwise for preoperative chemotherapy. The chemotherapy regimens chosen included CapeOX (130 mg/m2 oxaliplatin injected intravenously on day 1 and 1000 mg/m2 capecitabine administered orally twice daily on days 1–14 for a 3-week cycle), FOLFOX (85 mg/m2 oxaliplatin and 400 mg/m2 leucovorin injected intravenously on day 1; 400 mg/m2 5-fluorouracil [5-FU] injected intravenously on day 1 and then 1200 mg/m2 5-FU injected intravenously for 2 days for a 2-week cycle), and FOLFIRI (180 mg/m2 irinotecan and 400 mg/m2 leucovorin injected intravenously on day 1; 400 mg/m2 5-FU injected intravenously on day 1 and then 1200 mg/m2 5-FU injected intravenously for 2 days for a 2-week cycle) regimens. All patients underwent standard complete mesocolic excision or total mesorectal excision with regional lymphadenectomy for the primary tumor according to the tumor location. Non-anatomical hepatectomy with R0 resection (tumor-free margin >1 mm) was also performed. The decision for the use of adjuvant chemotherapy was made by considering the patients’ tolerance to and preference of chemotherapy. For patients receiving preoperative chemotherapy, the adjuvant chemotherapy regimen was consistent with preoperative chemotherapy.
Definition
The LNR was defined as the number of metastatic LNs divided by the total number of retrieved LNs. The resected LNs were classified into three groups (pericolic, intermediate, and main) based on the Japanese classification of colorectal carcinoma.21 The pericolic group was defined based on LN metastasis adjacent to the colon and rectum or along the vascular arcades of marginal arteries, with no metastasis to the intermediate and main nodes; the intermediate group was defined based on metastasis along the course of major vessels supplying the colon and rectum, with no metastasis to the main nodes; and the main group was defined based on metastasis near the root of major vessels. The LN from the tumor tissue was separated by a surgeon. And then, the LNs’ dissection and wax block were performed by a technologist. Pathological diagnosis was made by a pathologist and validated by another experienced pathologist. For LND evaluation, we divided patients of our study into four groups according to the metastatic site of the surgical LNs, which were the pericolic group, intermediate group, main group, and no LNs metastasis group.
Follow-up
The follow-up protocol included evaluations every 3 months for the first 2 years after the completion of surgery, every 6 months from the third to fifth years, and once every year thereafter. Evaluations at each visit included obtaining complete blood count, evaluation of carcinoembryonic antigen and cancer antigen 19-9 levels, and physical examination. Chest radiography, abdominal and pelvic computed tomography, pelvic endoscopic ultrasonography, and magnetic resonance imaging were conducted every year; colonoscopy was performed on an annual basis. Overall survival (OS) was defined as the time from the date of liver resection to the date of death due to any cause or the date of last follow-up, and recurrence-free survival (RFS) was defined as the time from the date of liver resection to the date of postoperative recurrence or the last follow-up date. Patients without any event (recurrence or death) at the last follow-up date were regarded as randomly censored. Follow-up was terminated in June 2017.
Statistical analysis
Continuous variables were represented as the median (range) or the mean (SD), and categorical variables as percentages. The cutoff value for the LNR was assessed using the X-tile 3.6.1 software (Yale University, New Haven, CT, USA), identified from the minimum P value according to OS. Statistical analyses were performed using the SPSS 20.0 software (IBM Corporation, Armonk, NY, USA) and the GraphPad Prism 7 software (GraphPad Software, Inc., La Jolla, CA, USA). We compared continuous variables that were normally distributed using Student’s t-test and categorical variables using the chi-square (χ2) test, Fisher’s test, or nonparametric Mann–Whitney U test. The Kaplan–Meier method was used to estimate the survival rates for the different groups, and differences in survival were compared with the log-rank test. Variables that were statistically significant with a P<0.05 in univariate Cox models were further assessed with multivariate Cox models using a forward stepwise method. Hazard ratios (HRs) and 95% CIs were calculated. P<0.05 was considered statistically significant.
Results
Association between clinicopathologic features and LNR
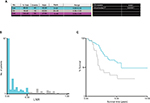
Among the 279 consecutive patients with CRLM undergoing curative resection, we excluded 90 patients with metachronous liver metastasis, 11 patients who were lost to follow-up, 2 patients who died within 30 days, and 22 patients without complete data. The final cohort thus consisted of 154 patients. The clinicopathologic characteristics of the 154 patients included in this study are shown in Table 1. The mean age of all patients was 56±12 years, and 64.9% of the patients were male. The median number of LNs retrieved was 12 (range 1–44), and the median number of metastatic LNs was 1 (range 0–19). X-tile software was used to determine 0.33 as the optimal cutoff value for the LNR at the maximum χ2 value of 8.807 (Figure 1). Totally, 124 patients were classified into the low-LNR (LNR ≤0.33) group, while 30 patients were classified into the high-LNR (LNR >0.33) group. The demographic and tumor characteristics with respect to the LNR status of the patients are presented in Table 2. Compared with the low-LNR group, the high-LNR group was significantly associated with more advanced N status (P<0.001), more LN metastasis (P<0.001), and poorer tumor differentiation (P=0.002). The differences in other evaluated factors were not statistically significant between the two groups.
Prognostic value of LNR, N stage, and LND
With a median follow-up time of 34 months (range 2–126 months) after liver resection, 58 (37.7%) patients experienced cancer-related mortality and 75 (48.7%) patients experienced disease recurrence; among these 75 patients with recurrence, 82.7% (62/75) had intrahepatic recurrence, 28.0% (21/75) had lung metastases, 14.7% (11/75) had abdominal pelvic metastases, and 1.3% (1/75) had other organ metastases. The median OS for patients in the high-LNR and low-LNR groups was 27 and 72 months, respectively. Kaplan–Meier analysis indicated that the 3-year RFS rate in the high-LNR group was significantly lower than that in the low-LNR group (16.7% vs 47.2%, P=0.001; Figure 2A). Similarly, the 3-year OS rate in the high-LNR group was also significantly lower than that in the low-LNR group (45.3% vs 72.8%, P=0.003; Figure 2B). Although the 3-year RFS rates in subgroups stratified by N stage were significantly different (49.9% for N0, 40.4% for N1, and 27.0% for N2, P=0.021; Figure 2C), the 3-year OS rate was comparable among the subgroups (68.4% vs 72.1% vs 59.2%, P=0.206; Figure 2D). However, there were no significant differences in the 3-year RFS (49.0% vs 35.6% vs 32.9% vs 35.7%, P=0.105; Figure 2E) or OS (66.4% vs 65.1% vs 70.0% vs 68.4%, P=0.589; Figure 2F) rate among the four groups (no metastasis, pericolic, intermediate, and main) stratified by LND. For patients with <12 resected LNs, the 3-year RFS rate in the high-LNR group was significantly lower than that in the low-LNR group (15.8% vs 38.4%, P=0.028; Figure 3A), while there was no difference in the 3-year OS rate (48.6% vs 57.4%, P=0.122; Figure 3B). For patients with at least 12 resected LNs examined, both 3-year RFS and OS rates in the high-LNR group were significantly lower than those in the low-LNR group (RFS, 18.2% vs 54.6%, P=0.046; Figure 3C and OS, 40.0% vs 85.9%, P=0.022; Figure 3D).
Univariate and multivariate analyses
Univariate analysis revealed that an LNR >0.33 (HR, 2.072; 95% CI, 1.314–3.267; P=0.002), more liver metastases (HR, 2.062; 95% CI, 1.362–3.123; P=0.001), multiple metastatic segments in the liver (HR, 2.114; 95% CI, 1.376–3.246; P=0.001), and metachronous hepatic resection (HR, 1.788; 95% CI, 1.142–2.799; P=0.011) were associated with worse RFS rates in patients with CRLM (Table 3). Meanwhile, non-treatment before primary resection (HR, 0.460; 95% CI, 0.302–0.699; P<0.001) and non-RFA therapy within 3 months before or after hepatic resection (HR, 0.306; 95% CI, 0.182–0.515; P<0.001) were associated with a favorable RFS rate. In addition, an LNR >0.33 (HR, 2.301; 95% CI, 1.315–4.028; P=0.004), rectal tumors (HR, 1.961; 95% CI, 1.170–3.286; P=0.011), poorly differentiated tumors (HR, 2.245; 95% CI, 1.186–4.250; P=0.013), and no chemotherapy after liver resection (HR, 1.929; 95% CI, 1.069–3.484; P=0.029) were associated with worse OS in patients with CRLM. In contrast, no treatment before primary resection (HR, 0.526; 95% CI, 0.307–0.901; P=0.019) was associated with better OS rate. However, neither N stage nor LND was identified as a significantly prognostic factor for 3-year OS or RFS rate.
A multivariate Cox proportional hazards model was used to further analyze the significant prognostic factor in the univariate analysis. The multivariate analysis revealed that an LNR >0.33 (HR, 2.124; 95% CI, 1.339–3.368; P=0.001) and more liver metastases (HR, 1.724; 95% CI, 1.086–2.737; P=0.021) were associated with unfavorable RFS, whereas no treatment before primary resection (HR, 0.589; 95% CI, 0.381–0.912; P=0.018) and RFA therapy within 3 months before or after hepatic resection (HR, 0.488; 95% CI, 0.274–0.868; P=0.015) were associated with favorable RFS. In addition, an LNR >0.33 (HR, 2.287; 95% CI, 1.282–4.079; P=0.005), rectal tumors (HR, 1.929; 95% CI, 1.142–3.258; P=0.014), poorly differentiated tumors (HR, 2.041; 95% CI, 1.060–3.929; P=0.033), and no chemotherapy after liver resection (HR, 2.010; 95% CI, 1.106–3.653; P=0.022) were associated with unfavorable OS in patients with CRLM.
Discussion
Our findings demonstrate that the LNR is an independent predictor of RFS or OS in patients with CRLM who are undergoing curative surgical resection. We have also demonstrated that the LNR is superior to the N stage and LND as a predictor of RFS or OS in this cohort of patients.
Stage IV colorectal cancer is a heterogeneous group regarding the survival prognosis. The LNR is a continuous variable, but an optimal cutoff value makes it easier to define and identify the risk groups. For patients with high LNR, a worse differentiation grade was correlated. In another study, the authors also found that high LNR was also correlated to a higher risk of multiple metastasis locations and an often worse response to chemotherapy. All these factors add up toward a worse prognosis.22 Recent studies have proposed the use of the LNR instead of the N stage to predict survival.23 Ozawa et al reported that a higher LNR was associated with a worse 5-year OS, but not disease-free survival in patients with stage IV colorectal cancer who had undergone curative resection.24 Moreover, Ahmad et al reported that the LNR was an independent predictor of survival and was associated with the hepatic tumor burden.25 Furthermore, a meta-analysis of 33 studies suggested the LNR as an independent predictor of survival in colorectal cancer patients and recommended it to be regarded as a parameter in future oncologic staging systems.26 Although LNR cutoffs based on median values and quartiles have been proposed without consensus,24,27 multiple studies, including the ones mentioned above, have demonstrated significant association of the LNR with prognosis. In our study, the cutoff value for LNR was identified using the X-tile program. This tool is used to assess the biological relationships between indexes and outcomes of certain diseases and can generate corrected P values to evaluate the statistical significance of data assessed by cutoff values. This statistical method has been shown to be effective in a number of similar studies.28,29 The results of multivariate analysis in our study showed that the LNR was an independent indicator of 3-year RFS and OS. However, the N status did not influence the 3-year RFS or OS. These results were similar to the findings of the above studies.24,25,27 These results demonstrate that the extent of nodal involvement is affected by the aggressive biological behavior of tumors.
For the clinical management of cancer, the TNM staging system is a standard that provides useful information for the choices of treatment and predicts patient prognosis.8 In our study, Kaplan–Meier analysis indicated that the 3-year RFS rate, but not the 3-year OS rate, was significantly different among subgroups stratified by the N stage. However, the Cox proportional hazards modeling indicated that the N stage was not associated with the 3-year OS or RFS. Thus, the N stage cannot be used as a prognostic indicator in patients with CRLM. Some investigators argue that this staging system is inevitably affected by the number of total LNs resected. As the number of removed LNs increases, the number of positive nodes might increase, which can result in a higher N stage.7 In addition, the number of regional LNs retrieved from a surgical specimen also varies with patient age and immune response and tumor grade or site.10–13 Therefore, because the N stage is affected by many factors, we conclude that it is a poor indicator of prognosis.
Our study further demonstrated that the stratification of patients according to LND cannot provide precise prognostic information in terms of OS or RFS. This is in contrast to the results reported by Leibold et al30 and Kobayashi et al17 who demonstrated that LND is an independent prognostic factor for OS or RFS. These discrepancies among our study and the studies by Leibold et al and Kobayashi et al might be due to the preference of choosing patients with early TNM stages. LND might play a significant role in the prognosis of patients with early and locally advanced colorectal cancer, but not in patients with CRLM because the outcomes in the latter group of patients are largely defined by the extent and nature of hematogenous metastatic disease.31 We speculate that distal metastasis is more important than local LN metastasis for predicting the survival of patients with CRLM. Thus, distal metastasis can affect patients’ survival irrespective of the LND determined after the radical resection of LNs. In addition, the National Comprehensive Cancer Network recommends the removal and pathologic examination of at least 12 LNs for resectable primary colorectal cancer. However, information on the location of the resected LNs is not required. Therefore, the resection of intermediate or main LNs cannot confer a survival benefit to patients.
The eighth edition of the AJCC staging manual recommends a minimum of 12 LNs for postoperative pathologic examination. In our study, we also performed Kaplan–Meier analysis on the survival of patients stratified by groups with at least 12 or <12 LNs examined. In the subgroup with <12 LNs examined, the LNR was associated with unfavorable RFS, but not OS. In addition, in the subgroups with 12 or more nodes examined, the LNR was associated with unfavorable RFS and OS. Thus, the examination of 12 or more LNs would result in a more accurate LNR, thereby strengthening the value of the LNR as a prognostic predictor. In addition, when patients with LNR ≤0.33 have two options for therapeutic regimens, the less-aggressive option may be the optimal one. In addition, these patients can consider undertaking a less-strict follow-up schedule, considering the cost of follow-up examinations.
It is now recognized that colorectal cancer is not a single disease. For example, prognosis as well as response to oncological treatment are different between colon cancers in different locations. It is a fact that left-sided colon cancer has a better prognosis and a more robust response toward antitumor therapy compared with right-sided colon cancer.32–34 In our study, there was no significant difference in LNR between left- and right-sided colon (data not shown), so here we have not discussed the impact of tumor location on the LNR value as well as prognosis. Besides, mismatch repair protein proficient (pMMR) and deficient (dMMR) patients are also different from each other in many aspects.35,36 But the number of cases in our study is relatively low. The different prognosis and the LNR values between pMMR and dMMR groups could be explored in future studies.
Here, we have confirmed that the LNR is a prognostic factor in patients with CRLM. However, the present study has several limitations. First, this study is limited by its retrospective design and single-institution focus; thus, a well-designed prospective study is needed to fully determine the value of LNR as a prognostic indicator. Second, some patients from our study were subsequently treated with chemotherapy and radiotherapy in different hospitals, but we do not have complete data regarding those cases. Third, we did not have access to the complete data of some patients. Nevertheless, the defined LNRs should be prospectively evaluated in multicenter studies.
The findings of our study suggest the need for the re-evaluation of the optimal use of the LN status. Many previous studies have shown that N stage and LND are effective prognostic factors. However, our study indicated that the LNR is superior to N stage and LND as a prognostic indicator in patients with CRLM. Therefore, surgeons can estimate the prognosis of patients with CRLM and formulate individualized treatment strategies based on the LNR.
Conclusion
Our study indicated that LNR is an independent prognostic indicator for OS and RFS in patients with CRLM after curative resection and that its prognostic value is better than that of N stage and LND.
Acknowledgments
We deeply appreciate the help from Diefei Liao, Shanshan Sun, and all colleagues of the Department of Colorectal Surgery at the Sun Yat-sen University Cancer Center, who were involved with performing the treatment in the current study. This work was funded by grants from the National Natural Science Foundation of China (No. 81772595), the Sun Yat-sen University Clinical Research 5010 Program (No. 2015024 and No. 2013013), and the Science and Technology Planning Project of Guangdong Province (No. 2016ZC0028). The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (http://www.researchdata.org.cn), with the approval number RDDA2017000437.
Disclosure
The authors report no conflicts of interest in this work.
References
Grundmann RT. Current state of surgical treatment of liver metastases from colorectal cancer. World J Gastrointest Surg. 2011;3(12):183–196. | ||
D’Angelica M, Kornprat P, Gonen M, et al. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol. 2011;18(4):1096–1103. | ||
Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25(29):4575–4580. | ||
La Torre M, Nigri G, Petrucciani N, et al. Prognostic assessment of different lymph node staging methods for pancreatic cancer with R0 resection: pN staging, lymph node ratio, log odds of positive lymph nodes. Pancreatology. 2014;14(4):289–294. | ||
Wang QX, Cai YF, Chen YY, et al. Additional prognostic value of Lymph Node Ratio (LNR) and Number of Negative Lymph Nodes (NLNs) in Chinese patients with triple negative breast cancer. Ann Clin Lab Sci. 2017;47(1):68–75. | ||
Zhao Y, Li G, Zheng D, et al. The prognostic value of lymph node ratio and log odds of positive lymph nodes in patients with lung adenocarcinoma. J Thorac Cardiovasc Surg. 2017;153(3):702–709.e1. | ||
Ren JQ, Liu JW, Chen ZT, et al. Prognostic value of the lymph node ratio in stage III colorectal cancer. Chin J Cancer. 2012;31(5):241–247. | ||
Gao P, Song YX, Wang ZN, et al. Is the prediction of prognosis not improved by the seventh edition of the TNM classification for colorectal cancer? Analysis of the surveillance, epidemiology, and end results (SEER) database. BMC Cancer. 2013;13:123. | ||
Fu J, Jiang M, Tan Y, et al. Synchronous resectable metastatic colorectal cancer: lymph node involvement predicts poor outcome. Medicine (Baltimore). 2015;94(30):e1215. | ||
Nedrebo BS, Soreide K, Nesbakken A, Eriksen MT, Søreide JA, Kørner H; Norwegian Colorectal Cancer Group. Risk factors associated with poor lymph node harvest after colon cancer surgery in a national cohort. Colorectal Dis. 2013;15(6):e301–e308. | ||
Wong SL. Lymph node evaluation in colon cancer: assessing the link between quality indicators and quality. JAMA. 2011;306(10):1139–1141. | ||
Sarli L, Bader G, Iusco D, et al. Number of lymph nodes examined and prognosis of TNM stage II colorectal cancer. Eur J Cancer. 2005;41(2):272–279. | ||
Bilimoria KY, Palis B, Stewart AK, et al. Impact of tumor location on nodal evaluation for colon cancer. Dis Colon Rectum. 2008;51(2):154–161. | ||
Wong SL, Ji H, Hollenbeck BK, Morris AM, Baser O, Birkmeyer JD. Hospital lymph node examination rates and survival after resection for colon cancer. JAMA. 2007;298(18):2149–2154. | ||
Guo D, Ni Y, Lv X, Zhang Z, Ye P. Distribution and prognosis of mediastinal lymph node metastases of nonsmall cell lung cancer. J Cancer Res Ther. 2016;12(Supplement):120–125. | ||
Sugawara K, Yamashita H, Uemura Y, et al. Numeric pathologic lymph node classification shows prognostic superiority to topographic pN classification in esophageal squamous cell carcinoma. Surgery. 2017;162(4):846–856. | ||
Kobayashi H, Ueno H, Hashiguchi Y, Mochizuki H. Distribution of lymph node metastasis is a prognostic index in patients with stage III colon cancer. Surgery. 2006;139(4):516–522. | ||
Langman G, Patel A, Bowley DM. Size and distribution of lymph nodes in rectal cancer resection specimens. Dis Colon Rectum. 2015;58(4):406–414. | ||
Yao YF, Wang L, Liu YQ, Li JY, Gu J. Lymph node distribution and pattern of metastases in the mesorectum following total mesorectal excision using the modified fat clearing technique. J Clin Pathol. 2011;64(12):1073–1077. | ||
Merrie AE, Phillips LV, Yun K, McCall JL. Skip metastases in colon cancer: assessment by lymph node mapping using molecular detection. Surgery. 2001;129(6):684–691. | ||
Watanabe T, Itabashi M, Shimada Y, et al; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17(1):1–29. | ||
Derwinger K, Gustavsson B. A study of lymph node ratio in stage IV colorectal cancer. World J Surg Oncol. 2008;6:127. | ||
Peschaud F, Benoist S, Julie C, et al. The ratio of metastatic to examined lymph nodes is a powerful independent prognostic factor in rectal cancer. Ann Surg. 2008;248(6):1067–1073. | ||
Ozawa T, Ishihara S, Nishikawa T, et al. Prognostic significance of the lymph node ratio in stage IV colorectal cancer patients who have undergone curative resection. Ann Surg Oncol. 2015;22(5):1513–1519. | ||
Ahmad A, Reha J, Saied A, Espat NJ, Somasundar P, Katz SC. Association of primary tumor lymph node ratio with burden of liver metastases and survival in stage IV colorectal cancer. Hepatobiliary Surg Nutr. 2017;6(3):154–161. | ||
Zhang MR, Xie TH, Chi JL, et al. Prognostic role of the lymph node ratio in node positive colorectal cancer: a meta-analysis. Oncotarget. 2016;7(45):72898–72907. | ||
Jiang K, Zhu Y, Liu Y, et al. Lymph node ratio as an independent prognostic indicator in stage III colorectal cancer: especially for fewer than 12 lymph nodes examined. Tumour Biol. 2014;35(11):11685–11690. | ||
Wang W, Xu DZ, Li YF, et al. Tumor-ratio-metastasis staging system as an alternative to the 7th edition UICC TNM system in gastric cancer after D2 resection-results of a single-institution study of 1343 Chinese patients. Ann Oncol. 2011;22(9):2049–2056. | ||
Shi X, Hu WP, Ji QH. Development of comprehensive nomograms for evaluating overall and cancer-specific survival of laryngeal squamous cell carcinoma patients treated with neck dissection. Oncotarget. 2017;8(18):29722–29740. | ||
Leibold T, Shia J, Ruo L, et al. Prognostic implications of the distribution of lymph node metastases in rectal cancer after neoadjuvant chemoradiotherapy. J Clin Oncol. 2008;26(13):2106–2111. | ||
Stillwell AP, Ho YH, Veitch C. Systematic review of prognostic factors related to overall survival in patients with stage IV colorectal cancer and unresectable metastases. World J Surg. 2011;35(3):684–692. | ||
Petrelli F, Tomasello G, Borgonovo K, et al. Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review and meta-analysis. JAMA Oncol. Epub 2016 Oct 27. | ||
Creasy JM, Sadot E, Koerkamp BG, et al. The impact of primary tumor location on long-term survival in patients undergoing hepatic resection for metastatic colon cancer. Ann Surg Oncol. 2018;25(2):431–438. | ||
Brule SY, Jonker DJ, Karapetis CS, et al. Location of colon cancer (right-sided versus left-sided) as a prognostic factor and a predictor of benefit from cetuximab in NCIC CO.17. Eur J Cancer. 2015;51(11):1405–1414. | ||
Li P, Xiao ZT, Braciak TA, Ou QJ, Chen G, Oduncu FS. Impact of age and mismatch repair status on survival in colorectal cancer. Cancer Med. 2017;6(5):975–981. | ||
Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138(6):2073–2087.e3. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.