Back to Journals » Clinical Interventions in Aging » Volume 16
Lower Plasma Melatonin in the Intervertebral Disk Degeneration Patients Was Associated with Increased Proinflammatory Cytokines
Authors Tian Y, Ji Y, Mei X, Pan J, He W, Sun J, Wan K, Yang H
Received 2 November 2020
Accepted for publication 26 December 2020
Published 4 February 2021 Volume 2021:16 Pages 215—224
DOI https://doi.org/10.2147/CIA.S290045
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Yixing Tian, Yiming Ji, Xin Mei, Jun Pan, Wenye He, Jiajia Sun, Kaichen Wan, Huilin Yang
Department of Orthopaedic Surgery, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, People’s Republic of China
Correspondence: Huilin Yang Email [email protected]
Background: Intervertebral disc degeneration (IDD) was considered to be the pathological basis of intervertebral disc herniation (IDH). However, the plasma melatonin in the IDD cases and healthy controls remained unclear.
Methods: In this case–control study, a total of 71 IDD cases and 54 healthy controls were enrolled between April 2020 and August 2020. The diagnostic effect of plasma melatonin for IDD was detected using receiver operating characteristic curve. The correlations between two continuous variables were detected with the Pearson linear analyses.
Results: It was found that lower melatonin concentration was detected in the IDD cases (1.906 ± 1.041 vs 3.072 ± 0.511 pg/mL, P< 0.001). Through receiver operating characteristic curve analyses, it was found that plasma melatonin could be used as a diagnostic biomarker for IDD (area under curve=0.808, P< 0.001). In advanced correlation analyses, it was found that plasma melatonin concentration was negatively associated with the age, symptom durations, IDD disease severity and proinflammatory factors, including IL-6 and TNF-α concentrations (P< 0.05). Comparing with the higher melatonin groups, significantly increased IL-6 (0.601 ± 0.085 vs 0.507 ± 0.167 pg/mL, P=0.028) and TNF-α (3.022 ± 0.286 vs 2.353 ± 0.641, P< 0.001) were detected in the patients with lower melatonin concentration.
Conclusion: The plasma melatonin concentration was significantly decreased in the IDD cases and plasma melatonin could be used as a diagnostic biomarker for IDD. Lower plasma melatonin was associated with longer disease durations, elevated disease severity and higher inflammatory cytokines levels in IDD patients.
Keywords: intervertebral disc degeneration, melatonin, biomarker, inflammatory factors
Introduction
Lumbar disc herniation (LDH) was the main cause of low back pain and sciatica. Intervertebral disc degeneration (IDD) was considered to be the pathological basis of intervertebral disc herniation,1 but its underlying exact mechanism remained unclear. The effective treatments for severe spinal degenerative diseases were still surgeries, however, both fusion and non-fusion surgeries would influence the normal physiological structures of the intervertebral disc, and thus cause internal fixation failure, prosthesis wear, and adjacent segment regression. Various complications, such as physiological anatomy change and re-revision surgery, would increase the therapeutic costs and then caused a great economic burden on society.2 The etiology of IDD was complex and previous studies revealed that increased age,3 unhealthy behaviors and inflammatory reactions were associated with IDD incidence.4 Considering the important therapeutic value for the early management of IDD, detecting a potential molecular biomarker would provide both advanced understanding and more potential treatments for the IDD cases.
Melatonin was a neuroendocrine hormone and it was currently clinically used to regulate the circadian rhythm, adjust the jet lag, and relieve insomnia. The synthesis and secretion of melatonin are mainly regulated by the intensity of light stimulation.5,6 However, it was found that melatonin would demonstrate a variety of biological functions, such as antioxidative, anti-inflammatory, immune regulation and anti-tumor effects. It played protective roles in the biological processes of ischemia, hypoxia and reperfusion damage.7–9 In an in-vitro study based on human nucleus pulposus (NP) cells, it was found that melatonin could affect the biological properties of IDD through activating the ERK1/2 signaling pathway.10 Another elegant work reported that IL-1β could promote its own expression through NLRP3 inflammasome activation, while melatonin could be regarded as a potential therapy for IDD through disrupting the IL-1β positive feedback loop.11 It has been reported that abnormal circulating melatonin concentration could be used as a biomarker of different disorders, including long-term survival in pulmonary arterial hypertension and hip fracture patients with and without delirium.12,13 Considering the diagnostic and prognostic effects of plasma melatonin in IDD, it could be used as an indicator for IDD development and progression. In this study, we conducted a case–control study to detect the diagnostic effects for IDD. In the advanced analyses, the relationship between plasma melatonin and proinflammatory factors were examined to detect the effects of melatonin on inflammatory reactions.
Methods
Study Designs
This current study was a prospective, case–control study. This study protocol was approved by the Ethical Committee of the First Affiliated Hospital of Soochow University. All the procedures in this current study were conducted in accordance with Declaration of Helsinki. The written informed consents were obtained by all IDD cases and healthy volunteers.
Study Populations
A total of 71 IDD cases and 54 healthy controls were enrolled between April 2020 and August 2020 in the Department of Orthopedics, the First Affiliated Hospital of Soochow University. In the IDD group, there were 39 females and 32 males, and there were 30 females and 24 males in the control group. The mean age was 59.0 ± 16.1 years in the IDD group and 55.6 ± 17.2 in the control group. There were no significant differences in the distribution in gender or age between the two included groups (P=0.545 and 0.259, respectively).
Inclusion and Exclusion Criteria of the Experimental Group Were Listed as Below
Inclusion criteria: (1) Symptoms: low back pain with unilateral or bilateral lower limb nerve root radiation pain; (2) Special nerve root stimulation test: straight leg improvement test, strengthening test or femoral nerve tension test positive; (3) muscle weakness, Numbness or lack of corresponding knee reflex or ankle reflex; (4) CT or magnetic resonance imaging (MRI) examination confirmed signs of disc herniation.
Exclusion criteria: (1) combined with spinal stenosis and spondylolisthesis; (2) with multi-segment disc herniation; (3) combined with spinal cord tumor; (4) history of spinal cord trauma; (5) combined with intervertebral space infection; (6) The corresponding segment of the intervertebral disc has a history of previous surgery; (7) with coronary heart disease, cerebrovascular disease, rheumatoid arthritis; (8) patients younger than 18 years old.
Control group inclusion and exclusion criteria: Inclusion criteria: imaging diagnosis of lower limb fractures (including femoral fractures, tibial fractures, ankle fractures, etc.), knee meniscus tear, cruciate ligament rupture, and confirmed by surgical treatment. Exclusion criteria: (1) combined with spinal cord tumors, previous history of spinal cord trauma; (2) combined with low back pain; (3) osteoarthritis, rheumatoid arthritis of the knee joint on the surgical side or the contralateral side; (4) previous lower limb surgery History; (5) Complicated with coronary heart disease, cerebrovascular disease, rheumatoid arthritis; (6) Patients younger than 18 years old.
Clinical Data Collection and Blood Samples Management
After the participants in both IDD and control groups being enrolled in this study, the basic clinical characteristics, including gender, age, smoking history, body mass index (BMI) value, back pain symptom durations as well as diabetic mellitus (DM) and hypertension history. The Pfirrmann grade was used to evaluate the severity of IDD and they were divided into five grades by two experienced spine surgeons (Tian Yixing and Ji Yiming). Previous studies have shown that the density, activity, and ability of the cells in the intervertebral disc to produce functional proteins gradually decrease with the Pfirrmann grading increases, and it confirmed that the Pfirrmann grading system could accurately reflect the degeneration of the intervertebral disc at the biological cell activity.14 The blood samples were collected between 9:00 am and 10:00 am after the clinical data being collected. A total of 2 mL vein blood was collected from the participants and then placed in the anticoagulated tubes with EDTA. The blood samples would be sent for centrifuge in 3000 rpm and 5 minutes as soon as possible. The plasma samples were obtained through aspirating the supernatant carefully and all the plasma samples were kept in −80°C until use.
Determination of Melatonin and Proinflammatory Factors
Plasma melatonin concentrations of both IDD patients and healthy controls were measured using human melatonin ELISA Kit (Cat#: 10,698, Glory Science, USA). IL-1β, IL6 and TNF-α levels in the plasma were measured using IL-1β (Cat#:QLB00B, R&D, USA), IL-6 (Cat#: RJ11850, Renjie Bio, China) and TNF-α (589,201–480, Cayman, USA) ELISA kit. All the measurements were conducted according to the manufacturer’s protocols. Three repeats for all the samples measurement were adopted and the average value was used in final analyses.
Statistical Analysis
The continuous data were present as a mean ± standard deviation (SD) in this current study. The difference between two groups was analyzed with non-paired t-test, and the difference among more than two groups were analyzed with one-way analysis of variance (ANOVA) followed by the newman-keuls method for multiple comparison analysis test. The difference of grouped data was analyzed with Chi-square analysis. The diagnostic effect of plasma melatonin for IDD would be detected using receiver operating characteristic curve. The linear correlation between two descriptive data was detected with the Pearson linear analyses. The alpha significance value was < 0.05.
Results
Participant Characteristics
In this case–control study, the potential participants were screened for inclusions or exclusions based on the criteria for both IDD cases and healthy controls. As showed in Figure 1, the after excluding the inappropriate participants, a total of 71 IDD cases and 54 healthy controls were included in this study. The blood samples collections and laboratory examination were conducted for advanced analyses.
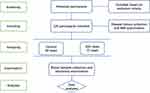 |
Figure 1 The flow chart of participants screening, including, grouping, examination and analyses in this study. |
After analyzing the clinical characteristics, plasma melatonin levels and the proinflammatory factors concentrations of the included participants in this study. The comparisons of these characteristics of the control and IDD cases were presented in Table 1. There were no significant differences in smoking status, BMI levels as well as DM and systemic hypertension histories between IDD cases and healthy controls (P=0.081, 0.164, 0.458 and 0.564, respectively). The mean symptom duration of the IDD cases was 30.00 ± 9.04 months and there were 7, 10, 28 and 26 IDD cases in Pfirmann Grade 2, 3, 4 and 5, respectively. In the advanced analyses on the melatonin levels and the proinflammatory factors concentrations, a higher IL-6 (0.601 ± 0.085 vs 0.507 ± 0.167 pg/mL, P<0.001) and a higher TNF-α (2.523 ± 0.642 vs 1.975 ± 0.078 pg/mL, P<0.001) levels were detected in the IDD cases comparing with the control group. No significant difference was detected in the IL-1β between IDD cases and healthy controls (P=0.850).
 |
Table 1 Baseline Characteristics of the Included Participants in This Study |
Decreased Plasma Melatonin in IDD Cases
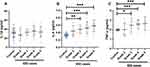
As shown in Figure 2A, a significant decreased melatonin concentration was detected in the IDD cases in Pfirmann Grade 3, 4 and 5, respectively (P=0.009, P<0.001 and P<0.001, respectively). In advance, the diagnostic effect of plasma melatonin for IDD were detected using receiver operating characteristic curve with the plasma melatonin data in 71 IDD cases and 54 controls. It was found that melatonin could be used as a diagnostic biomarker for IDD with an area under curve of 0.808 and P<0.001 (Figure 2B).
Melatonin Related Factors Detection
When the plasma melatonin in females and males were considered, it was 2.38 ± 1.04 pg/mL in the females and 2.45 ± 1.03 pg/mL in the males. No significant difference was detected in the plasma melatonin concentrations among females and males (P=0.692). To detect the clinical and experimental factors associated with the melatonin level, the correlation matrix was conducted in both normal controls and IDD cases. As showed in Figure 3A, melatonin level was negatively associated with increased age (r=−0.800, 95% CI: −0.8796 to −0.6779, P<0.001). No significant linear correlation was detected in melatonin level and other factors and the detailed data was presented in Figure 3A. In the IDD cases, significant negative correlation between melatonin level and age was detected (r=−0.380, 95% CI: −0.5633 to −0.1609, P=0.001). When the IDD symptom duration and disease severity considered, lower melatonin was associated with longer symptom duration and advanced disease severity (r=−0.487, P<0.001, and r=−0.740, P<0.001, respectively). Besides, significant linear between IL6 and TNF-α levels were detected (r= 0.720, 95% CI, 0.585 to 0.816, P<0.001). The detailed correlations were presented in Figure 3B.
In advanced study on the correlation between melatonin level and proinflammatory factors concentrations, it was found that melatonin was negatively associated with IL6 and TNF-α levels (P<0.001). As showed in Figure 4, no significant linear association between melatonin and IL-1β level in the IDD cases (R2=0.032, P=0.137, Figure 4A). Negative linear correlations between plasma melatonin and IL-6 (R2=0.407, P<0.001, Figure 4B) and TNF-α (R2=0.575, P<0.001, Figure 4C) were detected using a linear regression model.
Clinical and Experimental Characteristics in IDD Patients with Lower Melatonin Level
To detect the effect of melatonin in the clinical and experimental characteristics of IDD cases, the patients with IDD in 1st quartile of melatonin levels were compared with the 2nd to 4th quartiles. As showed in Table 2, no significant difference in age, gender, smoking history, BMI as well as DM or hypertension history was detected (P>0.05). The symptom duration was 39.78 ± 18.16 months in the lower melatonin group while it was 26.68 ± 18.17 months in the higher melatonin group (P=0.011). There were 3 and 15 cases in Pfirmann Grade 4 and 5 groups in the lower melatonin groups, while there were 7, 10, 25 and 11 cases in Pfirmann Grade 2, 3, 4 and 5 groups in the higher melatonin groups (P<0.001). Comparing with the higher melatonin groups, significantly increased IL-6 (0.601 ± 0.085 vs 0.507 ± 0.167 pg/mL, P=0.028) and TNF-α (3.022 ± 0.286 vs 2.353 ± 0.641, P<0.001) were detected. No significant difference in the IL-1β between lower and higher melatonin group was detected in this current case–control study (P=0.850).
 |
Table 2 Clinical and Experimental Characteristics in IDD Patients in and Above the 1st Quartile of Melatonin Levels |
Increased Plasma Pro-Inflammatory Factors in the IDD Cases
In advanced studies, the plasma pro-inflammatory factors, including IL-1β, IL-6 and TNF-α, in the healthy controls and IDD cases in different stages were presented in Figure 5. As demonstrated in Figure 5A, no significant difference among healthy controls and IDD cases in any stages were detected (P>0.05). Comparing with the control group, higher plasma IL-6 was detected in the IDD cases with Pfirrmann grade 3, 4 and 5, respectively (P=0.002, P<0.001, and P<0.001, respectively, Figure 5B). When the plasma TNF-α concentrations were considered, higher levels of plasma TNF-α would be detected in the IDD cases with Pfirrmann grade 3, 4 and 5, respectively (P=0.035, P<0.001, and P<0.001, respectively, Figure 5C).
Discussion
In this current study, we studied the plasma concentration of melatonin in the IDD cases. Through analyzing the melatonin concentration in 71 IDD cases and 54 controls, it was found that lower melatonin concentration was detected in the IDD cases. Through receiver operating characteristic curve analyses, it was found that plasma melatonin could be used as a diagnostic biomarker for IDD. In the advanced correlation analyses, plasma melatonin concentration was found to be negatively associated with the increased age, symptom durations, IDD disease severity and concentrations of proinflammatory factors, including IL-6 and TNF-α. This study highlighted the important roles of plasma melatonin in the development of IDD and provided deepened understanding of melatonin in the potential management of IDD.
Melatonin, as an important endocrine hormone, demonstrated important physiological functions including regulating the rhythm of the biological clock, metabolism regulation, anti-oxidation, and modify reproductive functions.15,16 As lower plasma melatonin was associated with increased age,17 thus plasma melatonin was associated with different degenerative disorders, including neurodegenerative disease and retinal degeneration.18,19 IDD was also an age-related disorder and it was natural to suppose that plasma melatonin was decreased in the IDD cases. In this current age-matched case–control study, it was found that plasma melatonin was lower in the IDD cases. However, it was noticed that a significant negative correlation between plasma melatonin and age was detected in both healthy controls and IDD cases, thus it provided us a clue that aging was a least one of the causes of the decreased melatonin. Besides, as the polymorphisms in melatonin synthesis pathways would influence the melatonin levels and was reported to be associated with the incidence with different diseases.20 Previous studies demonstrated that male gender could be regarded as a risk factor for IDD and IDH,1,21 it was interesting to detected the contribution of gender factor on the abnormal melatonin production in the IDD cases. As this is a gender-matched case–control study, no significant difference in the gender distribution between IDD cases and healthy controls. Considering the fact that lower melatonin was detected in the IDD cases, the gender was not a decisive factor for IDD incidence. This result was consistent with previous research about the melatonin level in the hip fractures cases,13 thus we can generate a preliminary conclusion that gender did not influence the melatonin level in the IDD cases. However, considering the complexed regulation factors and various functions of melatonin in different biological progresses, more pathophysiological regulations and genetic background of melatonin production would help to detect the effect of melatonin in the IDD development in the future.
In this current study, lower plasma melatonin was reported to be significantly associated with longer symptom durations and advanced IDD disease severity. As a biomarker associated with worsen IDD development, the melatonin supplementation would provide potential therapy effect for the IDD. In previous studies, it was reported that melatonin could promote the secretion of functional factors influencing the NP cell physiology and retard cell degeneration, thereby affecting the biological properties of the IDD.10 In another study based on cultured NP cells, it was found that melatonin could protect nucleus pulposus cells against apoptosis via mitophagy induction and ameliorated disc degeneration, providing the potential therapy for IDD.22 Considering that Melatonin was approved by the US Food and Drug Administration (FDA) as a raw material for dietary supplements, the clinical studies on the effects of melatonin on the IDD cases would help us to understand the biological effects of melatonin. It was also important to start the well-designed clinical trials after the protective effects and potential harmful effects were detected in more and more reliable animal models.
The anti-inflammatory effects of melatonin had been reported in various in-vitro studies and animal models.23–25 Chronic inflammation was also a risk factor for IDD and has been reported to be related with the development of IDD and the back pain.26,27 In this study, higher plasma melatonin was associated lower IL6 and TNF-α concentrations, and it provided us a highlighted information that melatonin supplementation, which would increase the plasma melatonin, might provide a significant therapy effect through down-regulation of the inflammation. The anti-inflammation effect of melatonin was also approved in IDD animal and cell models in previous studies,11,28 more studies on the melatonin supplementation on the proinflammatory factors in IDD patients would help in the understanding of the therapy effects of melatonin on IDD.
There were several strengths and limitations in this current study. The strengths included the relative comprehensive clinical and laboratory characteristics as well as a fixed time-point of the blood collection. While, there were also several limitations should be noted. First, several types, such as herniation, Schmorl’s node, Modic changes and so on, are included in the IDD, however, only the intervertebral herniation cases were included in the analyses. The melatonin concentrations in different IDD subtypes would help the understanding of the effects of melatonin on IDD incidence and we would like to presenting the melatonin levels in different IDD subtypes. Second, the absence of NP melatonin data weaken the mechanism detection on the effects of melatonin. Third, it was hard to detect the prognosis effect of plasma for the IDD progression without a long-term follow-up. In the advanced clinical studies, the melatonin levels in IDD subtypes, NP tissues and follow-up data would be included in the studies and thus provide more knowledge on the effects of melatonin on IDD incidence and progression.
Conclusions
In conclusion, the plasma melatonin was significantly decreased in the IDD cases and plasma melatonin could be used as a diagnostic biomarker for IDD. Advanced analyses demonstrated that lower plasma melatonin was associated with longer disease duration, elevated disease severity and higher inflammatory cytokines levels in IDD patients.
Abbreviations
IDD, intervertebral disc degeneration; LDH, lumbar disc herniation; MRI, magnetic resonance imaging; DM, diabetic mellitus; SD, stand difference; ANOVA, one-way analysis of variance; IL-1β, interleukin-1β; IL6, interleukin-6; TNF-α, tumor necrosis factor-α; BMI, body mass index.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study protocol was approved by the Ethical Committee of the First Affiliated Hospital of Soochow University (2020-186). The written informed consents were obtained by all IDD cases and healthy volunteers.
Consent for Publication
All authors have approved the final version of the manuscript and consented for publication.
Author Contributions
YXT, YMJ and HLY conceived of the study. YXT, XM, JP, WYH, KCW, HLY acquired samples and/or clinical data. YXT, YMJ, XM, JJS, KCW, HLY performed the statistical analysis. YXT, YMJ, XM, JP, HLY provided analysis and/or interpretation of data. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
No funding exists in this study.
Disclosure
The authors declare that they have no competing interests.
References
1. Hsu HT, Yue CT, Teng MS, et al. Immunohistochemical score of matrix metalloproteinase-1 may indicate the severity of symptomatic cervical and lumbar disc degeneration. Spine J. 2020;20(1):124–137. doi:10.1016/j.spinee.2019.08.004
2. Colella F, Garcia JP, Sorbona M, et al. Drug delivery in intervertebral disc degeneration and osteoarthritis: selecting the optimal platform for the delivery of disease-modifying agents. J Control Release. 2020;328:985–999. doi:10.1016/j.jconrel.2020.08.041
3. Tavakoli J, Diwan AD, Tipper JL. Advanced strategies for the regeneration of lumbar disc annulus fibrosus. Int J Mol Sci. 2020;21(14):4889. doi:10.3390/ijms21144889
4. Jain N, Brock JL, Phillips FM, Weaver T, Khan SN. Chronic preoperative opioid use is a risk factor for increased complications, resource use, and costs after cervical fusion. Spine J. 2018;18(11):1989–1998. doi:10.1016/j.spinee.2018.03.015
5. Li Y, Ma J, Yao K, et al. Circadian rhythms and obesity: timekeeping governs lipid metabolism. J Pineal Res. 2020;e12682.
6. Gurunathan S, Kang MH, Kim JH. Role and therapeutic potential of melatonin in the central nervous system and cancers. Cancers (Basel). 2020;12(6):1567. doi:10.3390/cancers12061567
7. Showell MG, Mackenzie-Proctor R, Jordan V, Hart RJ. Antioxidants for female subfertility. Cochrane Database Syst Rev. 2020;8:CD007807. doi:10.1002/14651858.CD007807.pub4
8. Ballester P, Richdale AL, Baker EK, Peiro AM. Sleep in autism: a biomolecular approach to aetiology and treatment. Sleep Med Rev. 2020;54:101357. doi:10.1016/j.smrv.2020.101357
9. Socaciu AI, Ionut R, Socaciu MA, et al. Melatonin, an ubiquitous metabolic regulator: functions, mechanisms and effects on circadian disruption and degenerative diseases. Rev Endocr Metab Disord. 2020;21:465–478. doi:10.1007/s11154-020-09570-9
10. Ge J, Zhou Q, Niu J, et al. Melatonin protects intervertebral disc from degeneration by improving cell survival and function via activation of the ERK1/2 signaling pathway. Oxid Med Cell Longev. 2019;2019:5120275. doi:10.1155/2019/5120275
11. Chen F, Jiang G, Liu H, et al. Melatonin alleviates intervertebral disc degeneration by disrupting the IL-1beta/NF-kappaB-NLRP3 inflammasome positive feedback loop. Bone Res. 2020;8:10. doi:10.1038/s41413-020-0087-2
12. Cai Z, Klein T, Geenen LW, et al. Lower plasma melatonin levels predict worse long-term survival in pulmonary arterial hypertension. J Clin Med. 2020;9(5):1248. doi:10.3390/jcm9051248
13. Scholtens RM, van Munster BC, van Faassen M, van Kempen MF, Kema IP, de Rooij SE. Plasma melatonin levels in hip fracture patients with and without delirium: a confirmation study. Mech Ageing Dev. 2017;167:1–4. doi:10.1016/j.mad.2017.08.016
14. Zhang C, Lin Y, Han Z, et al. Feasibility of T2 mapping and magnetic transfer ratio for diagnosis of intervertebral disc degeneration at the cervicothoracic junction: a pilot study. Biomed Res Int. 2019;2019:6396073.
15. Kvietkauskas M, Zitkute V, Leber B, Strupas K, Stiegler P, Schemmer P. The role of melatonin in colorectal cancer treatment: a comprehensive review. Ther Adv Med Oncol. 2020;12:1758835920931714. doi:10.1177/1758835920931714
16. Wu ZY, Huang SD, Zou JJ, et al. Autism spectrum disorder (ASD): disturbance of the melatonin system and its implications. Biomed Pharmacother. 2020;130:110496. doi:10.1016/j.biopha.2020.110496
17. Jauhari A, Baranov SV, Suofu Y, et al. Melatonin inhibits cytosolic mitochondrial DNA-induced neuroinflammatory signaling in accelerated aging and neurodegeneration. J Clin Invest. 2020;130(6):3124–3136. doi:10.1172/JCI135026
18. Chang CC, Huang TY, Chen HY, et al. Protective effect of melatonin against oxidative stress-induced apoptosis and enhanced autophagy in human retinal pigment epithelium cells. Oxid Med Cell Longev. 2018;2018:9015765. doi:10.1155/2018/9015765
19. Zhao Y, Ren J, Hillier J, Jones M, Lu W, Jones EY. Structural characterization of melatonin as an inhibitor of the Wnt deacylase Notum. J Pineal Res. 2020;68(2):e12630. doi:10.1111/jpi.12630
20. Wang P, Liu L, Zhao LF, et al. Association of melatonin pathway gene’s single-nucleotide polymorphisms with systemic lupus erythematosus in a Chinese population. J Immunol Res. 2019;2019:2397698. doi:10.1155/2019/2397698
21. Yu C, Zhan X, Liu C, et al. Risk factors for recurrent L5-S1 disc herniation after percutaneous endoscopic transforaminal discectomy: a retrospective study. Med Sci Monit. 2020;26:e919888. doi:10.12659/MSM.919888
22. Chen Y, Wu Y, Shi H, et al. Melatonin ameliorates intervertebral disc degeneration via the potential mechanisms of mitophagy induction and apoptosis inhibition. J Cell Mol Med. 2019;23(3):2136–2148. doi:10.1111/jcmm.14125
23. Permpoonputtana K, Tangweerasing P, Mukda S, Boontem P, Nopparat C, Govitrapong P. Long-term administration of melatonin attenuates neuroinflammation in the aged mouse brain. EXCLI J. 2018;17:634–646. doi:10.17179/excli2017-654
24. Fu Z, Jiao Y, Wang J, et al. Cardioprotective role of melatonin in acute myocardial infarction. Front Physiol. 2020;11:366. doi:10.3389/fphys.2020.00366
25. Lin R, Wang Z, Cao J, Gao T, Dong Y, Chen Y. Role of melatonin in intestinal mucosal injury induced by restraint stress in mice. Pharm Biol. 2020;58(1):342–351. doi:10.1080/13880209.2020.1750659
26. Hernandez PA, Jacobsen TD, Chahine NO. Actomyosin contractility confers mechanoprotection against TNFalpha-induced disruption of the intervertebral disc. Sci Adv. 2020;6(34):eaba2368. doi:10.1126/sciadv.aba2368
27. Che H, Li J, Li Y, et al. p16 deficiency attenuates intervertebral disc degeneration by adjusting oxidative stress and nucleus pulposus cell cycle. Elife. 2020;9. doi:10.7554/eLife.52570
28. Zhang Y, He F, Chen Z, et al. Melatonin modulates IL-1beta-induced extracellular matrix remodeling in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration and inflammation. Aging (Albany NY). 2019;11(22):10499–10512. doi:10.18632/aging.102472
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.