Back to Journals » International Medical Case Reports Journal » Volume 13
Lower Gastrointestinal Involvement in Multiple Myeloma: A Case Report
Authors Sadiq AM , Suleiman JM, Vuai HS, Sadiq AM, Mkwizu EW
Received 4 June 2020
Accepted for publication 1 August 2020
Published 21 August 2020 Volume 2020:13 Pages 353—357
DOI https://doi.org/10.2147/IMCRJ.S266203
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Abid M Sadiq,1,2 Jamil M Suleiman,1 Hassan S Vuai,1 Adnan M Sadiq,2,3 Elifuraha W Mkwizu1,2,4
1Department of Internal Medicine, Kilimanjaro Christian Medical Centre, Moshi, Tanzania; 2Kilimanjaro Christian Medical University College, Moshi, Tanzania; 3Department of Radiology, Kilimanjaro Christian Medical Centre, Moshi, Tanzania; 4Cancer Care Centre, Kilimanjaro Christian Medical Centre, Moshi, Tanzania
Correspondence: Abid M Sadiq
Department of Internal Medicine, Kilimanjaro Christian Medical Centre, PO Box 3010, Moshi, Tanzania
Email [email protected]
Abstract: Multiple myeloma (MM) commonly presents with anemia and bone pain. Besides bone marrow involvement, MM has a tendency to involve other organs with gastrointestinal (GI) tract, especially lower GI tract involvement being uncommon. Our case shows an elderly male with anemia due to suspected GI malignancy. Investigations revealed anemia, thrombocytopenia, positive stool occult blood and inconclusive diagnostic endoscopic findings. An elevated serum total protein, serum calcium and serum creatinine gave rise to the suspicion of MM which was later supported by the fulfilment of the CRAB criteria, expressed as hypercalcemia, renal failure, anemia and lytic lesions on skull X-ray. The relationship between multiple myeloma and GI bleeding is rare and indicates extramedullary disease which is a sign of poor prognosis. Early detection may alter the prognostic course of MM.
Keywords: multiple myeloma, anemia, gastrointestinal, bleeding
Introduction
Plasma cell malignancy arises from monoclonal proliferation of plasma cells, and these include multiple myeloma (MM), primary plasmacytoma and extramedullary plasmacytoma. Extramedullary plasmacytoma can occur in the presence of another plasma cell malignancy such as MM.1
Neoplastic proliferation of plasma cells in the bone marrow resulting in extensive bone destruction with systemic features suggestive of malignancy is characteristic for MM.2 Occasionally they can infiltrate other organ systems such as the kidney causing renal impairment. Most MM patients show features related to the infiltration of plasma cells into the bone or involved organ. The main cause of morbidity is bone involvement as osteolytic bone lesions show no new bone formation.2 The common clinical features patients present with are anemia, bone pain, elevated serum creatinine, generalized weakness, elevated serum calcium and weight loss.3
Diagnosis of MM is based on clonal bone marrow plasma cells ≥10% or bone or extra-medullary plasmacytoma histologically proven, and with any one or more myeloma defining events;2 end-organ damage due to plasma cell infiltration (hypercalcemia, renal insufficiency, anemia and bone lesions, meeting the CRAB criteria), clonal bone marrow plasma cell percent ≥60%, free light chain (FLC) level ≥100mg/L and/or more than 1 focal lesion on magnetic resonance imaging.
Anemia mainly occurs due to the replacement of bone marrow by plasma cells. But other possible causes include erythropoietin deficiency, renal failure, chemotherapy, dysregulated cell apoptosis and vitamin B12 or folate deficiency.4 Myeloma bone complications include bone pain, pathologic fractures, cord compression and hypercalcemia. This is characterized by increased bone resorption and reduced bone formation leading to osteoporosis and lytic lesions. The lesions result from direct deposition of plasma cells within the bone, as well as osteoclast stimulation and osteoblast inhibition through cytokine production via interactions between the plasma cells and micro-environment. The most frequent sites of skeletal involvement include vertebrae, proximal humerus, sternum, ribs, pelvis and femur.4,5 Renal impairment may occur due to renal tubular damage by free light chains, hypercalcemia, infection, dehydration and chronic non-steroidal anti-inflammatory drugs use.5,6
The presentation of gastrointestinal (GI) plasmacytoma is non-specific, alternate to other sites of organ involvement, and the common symptom is abdominal pain. Other symptoms include lower GI bleeding, weight loss, change in bowel habits and large bowel obstruction.7
We present a case of an elderly patient that presented, not in MM, but with features of anemia suspecting lower GI malignancy or inflammatory bowel disease.
Case Report
A 78-year-old male presented with easy fatigue and awareness of heartbeat 2 weeks before admission. He reported multiple episodes of dizziness and weakness upon exertion. He did not report any history of bleeding from nose, mouth or stool. He did not complain of hematuria or reduced urine output. On examination, he had conjunctiva and palmar pallor with no palpable lymphadenopathy and no icterus. His chest and abdominal examination were relatively normal.
He was initially found to have normocytic, normochromic anemia of 5.3g/dL and platelet count of 49x109/L. The peripheral blood smear did not show any evidence of abnormal morphology. A stool sample showed positive occult blood suspecting the patient to have an upper gastrointestinal (GI) bleeding. An esophagogastroduodenoscopy showed diffuse mucosal pallor with minimal inflammation only. An abdominal ultrasound was relatively normal.
His control hemoglobin was 4.1g/dL after blood transfusion, suspecting ongoing bleeding. A colonoscopy showed clots under the superficial mucosa on different sites with superficial clots and fresh blood throughout the colon which could be explained by thrombocytopenia. Biopsy was not taken due to risk of bleeding. However, lesions in the caecum and terminal ileum could not be ruled out. At this point, he was suspected to have malignancy of the colon or inflammatory bowel disease. The carcinoembryonic antigen was 1.48ng/mL. Further workup showed him to have an INR of 1.3, repeat hemoglobin of 4.7g/dL and repeat platelets of 47x109/L.
His general condition was not improving and had a poor performance status. A C-reactive protein showed 25,005ng/mL (normal 0–700ng/mL) and lactate dehydrogenase of 217.3U/L (normal 240.0–480.0U/L). Part of his liver workup showed serum albumin of 27.4g/L and a serum total protein of 99.7g/L.
Following the elevated serum total protein levels, a serum creatinine and serum calcium were 485µmol/L and 3.91mmol/L, respectively. His skull X-ray showed multiple clear punched-out lesions in the skull bones (Figure 1). An abdominal computed tomography (CT) scan showed no obvious abdominal malignancy but detected osteolytic bone lesions on the thoracolumbar spine and pelvis (Figures 2, 3 and 4). All these lesions were present without reported bone pain. A serum protein electrophoresis showed M component 68g/L, kappa free light chain 16.0mg/L, lambda free light chain 974.0mg/L, kappa/lambda ratio of 0.016 and β2-microglobulin 33.0mg/L.
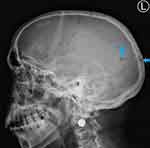 |
Figure 1 Lateral skull X-ray shows multiple well-circumscribed lytic punched-out round lesions (blue arrows). |
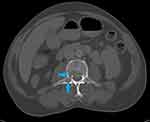 |
Figure 2 Axial CT abdomen bone window shows lytic lesions in the vertebral body (upper blue arrow) and right transverse process (lower blue arrow). |
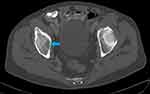 |
Figure 3 Axial CT pelvis bone window shows lytic lesion in the right ischial bone (blue arrow). |
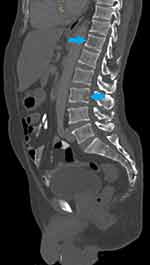 |
Figure 4 Sagittal CT abdomen and pelvis bone windows shows two lytic lesions in T12 (upper blue arrow) and L3 (lower blue arrow) vertebra. |
Given his poor performance status, he was initiated on cyclophosphamide 75% of the required dose and dexamethasone 20mg IV. Bortezomib was not given due to thrombocytopenia. Unfortunately, he was not fit to endure the next dose and passed away.
Discussion
Our case reported an elderly male who presented with lower GI bleeding, anemia and thrombocytopenia. Given his age, he was investigated for suspected lower GI malignancy as a first differential. With no obvious lesion found on endoscopic studies, a finding of serum hyperproteinemia prompted further investigation. He was found to have renal impairment, elevated serum calcium, skeletal involvement and elevated serum FLC highly suggestive of MM. Our case shows an example of extramedullary disease of the GI tract which is rare hence suspicion of GI malignancy first.
Extramedullary plasmacytoma occurs in around 3–20% of plasmacytomas in various studies, and involvement of the colon is rare.1,8 It is commonly seen in upper respiratory tract (82%) with the rest in the urinary bladder, brain, thyroid, skin, etc. Involvement of GI tract is seen in less than 10% of cases, frequent in the small intestinal tract, followed by the stomach colon and esophagus.8 Involvement of the GI tract in MM occurs later in disease progression or relapsing disease.9,10
Bleeding is more common in advanced stages of MM and likely an end-stage event.11,12 Bleeding in such conditions usually have multifactorial pathophysiology including thrombocytopenia, paraproteinemia, acquired bleeding disorders, amyloidosis, renal failure and vascular endothelial damage.9,13,14 Identifying these abnormalities is important, but the mainstay of therapy includes treatment of the underlying disease.13
The main cause of bleeding in MM is due to defective primary hemostasis.12 Our patient presented with high serum β2-microglobulin and serum free light chains suggestive of very active disease which may be reflective of increased bleeding tendencies relating to defective primary hemostasis.12
There is no definitive treatment for colon plasmacytoma. Studies have shown surgical intervention has good outcomes, and radiation therapy is used if surgery is not indicated.1 The use of adjuvant chemotherapy is recommended for patients with higher-grade disease, tumor size >5 cm, or refractory disease. The prognosis of GI involvement is unknown; however, studies have indicated a poor prognosis.1,8,9
Multiple myeloma is a systemic disease affecting multiple organ systems with varying atypical presentations. Presence of GI bleeding with inconclusive endoscopic and imaging studies may be related to hematopoietic malignancies which should be addressed further. But bleeding in MM is seen as a late complication suggestive of poor prognosis. Hence, early diagnosis and treatment are preferable in an attempt to alter the prognosis of MM.
Ethical Approval
The need for ethics approval for this case report was waived.
Consent
We obtained written informed consent from the patient’s son for the publication of this case report and the accompanying images.
Acknowledgment
We thank the patient and his son for their patience and co-operation.
Funding
There were no sources of funding.
Disclosure
The authors declare no conflicts of interest.
References
1. Fagkrezos D, Manes K, Paraskeva K, et al. Secondary extramedullary plasmacytoma of sigmoid colon in a patient with multiple myeloma: A case report. J Med Case Rep. 2018;12(1):1–7. doi:10.1186/s13256-018-1888-4
2. Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91(7):719–734. doi:10.1002/ajh.24402
3. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33. doi:10.4065/78.1.21
4. Bladé J, Rosiñol L. Complications of multiple myeloma. Hematol Oncol Clin North Am. 2007;21(6):1231–1246. doi:10.1016/j.hoc.2007.08.006
5. Hartley-Brown MA, Sullivan DM, Baz R. State-of-the-art management of complications of myeloma and its treatment. Adv Hematol. 2010;2010:1–8. doi:10.1155/2010/343089
6. King AJ, Eyre T, Sharpley F, Watson C, Ramasamy K, Willan J. Multiple myeloma in the very elderly patient: challenges and solutions. Clin Interv Aging. 2016;11:423. doi:10.2147/CIA.S89465
7. Gupta V, Nahak B, Sakhuja P, Agarwal AK, Kumar N, Mishra PK. Primary isolated extramedullary plasmacytoma of colon. World J Surg Oncol. 2007;5(1):1–5. doi:10.1186/1477-7819-5-47
8. George SM, Aljufairi EA, Chandran N, Almahari SAI. Plasmacytoma as a mimicker of colonic carcinoma in an elderly man. Case Rep Pathol. 2017;2017:1–5. doi:10.1155/2017/4846018
9. Suvannasankha A, Abonour R, Cummings OW, Liangpunsakul S. Gastrointestinal plasmacytoma presenting as gastrointestinal bleeding. Clin Lymphoma Myeloma. 2008;8(5):309–311. doi:10.3816/CLM.2008.n.044
10. Usmani SZ, Heuck C, Mitchell A, et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over-represented in high-risk disease even in the era of novel agents. Haematologica. 2012;97(11):1761–1767. doi:10.3324/haematol.2012.065698
11. Coppola A, Tufano A, Di Capua M, Franchini M. Bleeding and thrombosis in multiple myeloma and related plasma cell disorders. Semin Thromb Hemost. 2011;37(08):929–945. doi:10.1055/s-0031-1297372
12. Hinterleitner C, Pecher A, Kreißelmeier K, et al. Disease progression and defects in primary hemostasis as major cause of bleeding in multiple myeloma. Eur J Haematol. 2020;104:26–35. doi:10.1111/ejh.13331
13. Saif MW, Allegra CJ, Greenberg B. Bleeding diathesis in multiple myeloma. J Hematother Stem Cell Res. 2001;10(5):657–660. doi:10.1089/152581601753193869
14. Botin T, Oriol A, Ribera J-M. Paraprotein-related bleeding as a first symptom of multiple myeloma. J Leuk. 2015;03:2–4. doi:10.4172/2329-6917.1000183
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
